Abstract
Recently it was shown that, analogous to normal hematopoietic cells, the level of CXC chemokine receptor 4 (CXCR-4) expression on acute myeloid leukemia (AML) cells correlates with stromal cell derived factor-1 alpha (SDF-1)-induced chemotaxis. As we speculated that an anomalous organ distribution of AML cells could affect cell survival and thus result in an altered fraction surviving chemotherapy, we examined a possible correlation between patient prognosis and CXCR-4 expression in AML patients. We found that patients with a high CXCR-4 expression in the CD34+ subset had a significantly reduced survival and a higher probability of relapse, resulting in a median relapse-free survival (RFS) of only 8.3 months. CXCR-4 expression was significantly higher in fetal liver tyrosine kinase-3 (Flt3)/internal tandem duplication (ITD) AML than in Flt3/wild-type (wt) AML. Covariate analysis indicated that the prognostic significance of Flt3/ITDs with respect to RFS was no more apparent when analyzed in conjunction with the expression of CXCR-4 in the CD34+ subset, suggesting that the poor prognosis of Flt3/ITD AML might be subordinate to the increased CXCR-4 expression. Using a granulocyte colony-stimulating factor receptor (G-CSF-R)-expressing 32D cell line, we observed that SDF-1/CXCR-4 interaction is required for the survival of myeloid differentiating cells, and it also induces a block in G-CSF-induced myeloid differentiation. These data suggest that the SDF-1/CXCR-4 axis may influence therapy responsiveness and defines unfavorable prognosis in AML. (Blood. 2004;104:550-557)
Introduction
Stromal cell derived factor-1 alpha (SDF-1) is the major chemokine released by the bone marrow microenvironment,1,2 acting on lymphocytes and hematopoietic progenitors. SDF-1 has been shown to activate integrins on these precursor cells and induce their migration in vitro.3-5 In SDF-1 or CXC chemokine receptor 4 (CXCR-4; ie, the SDF-1 receptor) knock-out models, hematopoietic precursor cells fail to migrate to the bone marrow during fetal development.6,7 CXCR-4, also called LESTR or FUSIN,8,9 belongs to the G-protein-coupled receptors (GPCRs). Previous studies have suggested that this receptor might play a role in malignant hematopoiesis. CXCR-4 is overexpressed and functionally active in B-cell chronic lymphocytic leukemia (B-CLL) and might contribute to the bone marrow tropism of B-CLL cells.10 SDF-1 has also been implicated in influencing the localization of precursor B-cell acute lymphocytic leukemia (B-ALL) cells in niches in the bone marrow microenvironment that favor their survival and proliferation.11 In childhood ALL, a high expression of CXCR-4 has been shown to predict for extramedullary infiltration12 and might be associated with a poor outcome.13
In acute myeloid leukemia (AML) the immature malignant cells frequently leave the bone marrow, populate the blood, and lodge in extramedullary sites such as the spleen and liver.14 Recently it has been shown that analogous to normal hematopoietic cells also in AML, the level of CXCR-4 expression correlates with the SDF-1-induced chemotaxis of these cells.15,16 This indicates that SDF-1/CXCR-4 might be involved in the trafficking of the leukemic cells. We investigated whether the expression of CXCR-4 and/or the chemotactic response to SDF-1 correlates with the clinical outcome in patients with acute myeloid leukemia. We show that patients with a high expression of CXCR-4 in the CD34+ subset of cells have a significantly reduced overall survival and are at greater risk of recurrence of the leukemia. We also observed that fetal liver tyrosine kinase-3 (Flt3)/internal tandem duplication (ITD) AML has a significantly greater level of CXCR-4 expression compared with Flt3/wild-type (wt) AML, suggesting the possibility that the poor prognosis of Flt3/ITD AML might depend on the increased CXCR-4 expression.
Patients, materials, and methods
Patient samples
Bone marrow samples of patients with AML were obtained after informed consent at the time of diagnosis. Protocols were approved by the Erasmus Medical Center Institutional Review Board. Cases were classified according to the French-American-British (FAB) committee recommendations.17 Mononuclear cells were isolated by Ficoll separation followed by T-cell depletion, typically resulting in a population containing more than 95% blasts. After isolation, cells were subjected to controlled freezing and stored in liquid nitrogen. Before use, cells were thawed by stepwise dilution in Iscove modified Dulbecco medium (IMDM; Invitrogen, Merelbeke, Belgium) containing 1% bovine serum albumin (BSA). Postthawing viability varied between 70% and 90% as assessed by dye exclusion.
Patients were treated according to following studies of the adult Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON): AML-29 (44 patients, younger than 60 years),18 AML-4 (27 patients, younger than 60 years),19 AML-31 (11 patients, 60 years or older), or AML-11 (6 patients, 60 years or older).20 In these protocols, induction therapy included anthracyclin and cytarabine, and in patients younger than 60 years allogeneic and autologous stem cell transplantation or intensive chemotherapy was applied as postremission therapy. In the AML-29 study, induction therapy consisted of cytosine arabinoside (Ara-C; 200 mg/m2) for 7 days combined for the last 3 days with idarubicin (12 mg/m2) (cycle I) followed by a second cycle consisting of Ara-C (6 days, 1000 mg/m2) combined with amsacrine (days 3-5, 120 mg/m2). Patients were randomized during the first induction cycle to priming with granulocyte colony-stimulating factor (G-CSF, 150 μg/m2, subcutaneously) during both cycles. Within the AML-4 protocol, the induction therapy was similar to that of the AML-29 protocol, except that in cycle I daunomycin (45 mg/m2) was used instead of idarubicin. Induction therapy according to the AML-31 protocol consisted of Ara-C (200 mg/m2) for 7 days combined for the first 3 days with daunomycin (45 mg/m2) in arm 1 of the study, or daunomycin (35 mg/m2) + the multidrug-resistance modulator PSC833 (10 mg/kg) in arm 2. Finally, in the AML-11 study induction therapy consisted of Ara-C (200 mg/m2) for 7 days combined with daunomycin (30 mg/kg) on days 1 to 3.
Stratification according to karyotype risk groups
As cytogenetic aberrations are considered to be important prognostic factors for AML,21 patients were classified in 3 groups: (1) a favorable-risk group consisting of patients defined by a karyotype of t(8;21), t(15;17), or inv(16); (2) an unfavorable-risk group defined by del(5), del(7), or deletions of the q-arms of these chromosomes, t(6,9) alterations in the 11q23 region, or the presence of multiple aberrations (more than 3) in the karyotype; and (3) an intermediate-risk group that consisted of all other karyotypic aberrations and normal karyotypes.
Detection of Flt3 mutants
All AML samples were analyzed for internal tandem duplications (ITDs) in exons 14 and 15 (the exons formerly designated as exons 11 and 12; see Abu-Duhier et al22 for revised exon numbering) of the Flt3 gene. The samples were also screened for the presence of the D835 mutation in the activation loop.23 ITDs were detected using a modified polymerase chain reaction (PCR) procedure as described elsewhere.24 The use of exons 14 and 15 specific primers allowed us to cover the whole juxtamembrane (JM) and the first part of the first kinase domain (TK-1) where most of the reported ITDs are located. Genomic DNA was prepared using a standard procedure.25 Briefly, DNA from about 104 cells was amplified in a total of 50 μL reaction mixture, containing single-strength PCR buffer, 10 pmol of each primer, 10 mM deoxynucleoside triphosphates (dNTPs), and 0.5 U Taq polymerase (Supertaq; SpheroQ, Leiden, The Netherlands). Preheating of the samples at 95°C was followed by amplification for 35 cycles consisting of one minute at 43°C, one minute at 72°C, and one minute at 95°C. Following these cycles, a final extension at 72°C was performed for 10 minutes. PCR products were stained with SYBR green I (Molecular Probes, Leiden, The Netherlands) and resolved on 3% agarose gel. As a confirmation, samples positive for an Flt3 mutation were reanalyzed in a second PCR using the primers 14F and 15R.
The D835 mutation in the second tyrosine kinase domain (exon 20, previously exon 17) was detected as described by Yamamoto et al.23 Approximately 100 ng DNA was added to a reaction mix containing single-strength PCR buffer, 10 pmol of each primer (D835-forward: 5′-CGCCAGGAACGTGCTTG-3′, D835-reverse: 5′-GCAGCCTCACATTGCCCC-3′), 200 μM dNTPs, and 0.5 U Taq polymerase (Supertaq; SpheroQ). Preheating of the samples at 95°C was followed by amplification for 35 cycles consisting of one minute at 63°C, one minute at 72°C, and one minute at 95°C. Following these cycles, a final extension at 72°C was performed for 10 minutes. Amplified PCR products were digested with EcoRV and subsequently electrophoresed through 4% agarose. Alleles containing the D835 mutation were identified by an uncut 114-bp PCR fragment, while wild-type alleles were identified by a digested PCR product with fragments of 68 and 46 bp.
Migration assay
Migration assays were performed as previously described.26 Briefly, transwell plates (Costar Corning, Cambridge, MA) of 6.5-mm diameter and a pore size of 5 μm were coated overnight at 4°C with 100 μL of 20-μg/mL fibronectin16 (FN, fibronectin from bovine plasma; Sigma, St Louis, MO). Before addition of the cells, the transwells were washed 3 times with assay medium (IMDM + 0.5% bovine serum albumin [BSA fraction V; Sigma]). After this, 2 × 105 AML cells in 100 μL assay medium were added to the upper compartment of the transwell. To the lower well, 600 μL assay medium was added in the presence or absence of SDF-1 (100 ng/mL; R&D Systems, obtained through ITK-Diagnostics, Uithoorn, The Netherlands). The transwell plates were then incubated for 4 hours at 37°C and 10% CO2. After incubation, the number of cells that had migrated to the lower well was determined using a Sysmex cell counter (Toa Medical Electronics, Hamburg, Germany). The percentage of specific (ie, chemokine-induced) chemotaxis was calculated by dividing the number of input cells by the number of migrated cells, from which the number of cells that migrated in medium alone was subtracted.
As the process of cryopreservation and the subsequent thawing are very likely stressful events for cells, their responses may be altered when assayed immediately after thawing. We therefore compared the migratory abilities of a random selection of 7 AML samples after overnight incubation of the cells in assay medium (37°C and 10% CO2) with those of the same samples assayed directly after thawing. In all conditions assayed, the chemotaxis after overnight incubation was significantly increased (P ≤ .05, data not shown). Therefore, all subsequent experiments were performed after overnight incubation.
In vitro proliferation and differentiation of 32D/G-CSF-R cells
The 32D/G-CSF-R cell line27 was cultured in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 100 IU/mL penicillin, 100 ng/mL streptomycin, 10% fetal bovine serum, and 10 ng/mL murine interleukin-3 (IL-3; Chinese hamster ovary [CHO] supernatant). Differentiation was started by replacing the IL-3 with 100 ng/mL G-CSF (Amgen, Thousand Oaks, CA). Cells were counted and the cell density was readjusted to 2 × 105 cells daily. Morphologic analysis of differentiation was performed by microscopy of May-Grünwald-Giemsa-stained cytospins. Specificity of SDF-1 binding was tested by blocking SDF-1/CXCR-4 interactions using CXCR-4 blocking or SDF-1-neutralizing antibodies (5 μg/mL; R&D Systems).
Flow cytometry
To determine the antigen expression profile of both freshly thawed and overnight-incubated AML samples (see “Migration assay”), the cells were harvested and pelleted at 1600g for 5 minutes. Cells were washed and resuspended in PBS + 1% FCS + 0.1% sodium azide. Then, 50-μL aliquots of this suspension, containing 1 to 2 × 105 cells, were labeled for 30 minutes on ice with the appropriate dilutions of αCD34-allophycocyanin (APC) and αCXCR-4-phycoerythrin (PE) (both from Coulter Immunotech, Marseille, France). After staining, the samples were diluted to 300 μL with PBS + 1% FCS + 0.1% sodium azide, to which 2 μL of 7-amino-actinomycin D (7-AAD; Molecular Probes) was added to allow for the exclusion of dead cells. Cells were measured on a FACScalibur cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) and analyzed using the CellQuest software package (Becton Dickinson Immunocytometry Systems).
In the 32D cell system, the number of apoptotic cells was quantified by determining the number of annexin V-positive cells using the Apoptest kit (NeXins Research, Kattendijke, The Netherlands).
Transplantation of human AML cells in the NOD/SCID mouse
NOD/LtSz-Prkdcscid (nonobese diabetic-severe combined immunodeficiency [NOD/SCID]) mice were bred in the Erasmus animal facility and housed under specified pathogen-free conditions, using individually ventilated cages. Mice were supplied with sterilized food and acidified tap water to which 100 mg/L ciprofloxacine (Bayer, Leverkusen, Germany) was added. Both food and drink were available ad lib. Housing, care, and all animal experiments were done in accordance with Dutch legal regulations, which include approval by a local ethics committee.
Before transplantation of the AML cells, the mice received a total body irradiation of 3.5 Gy gamma radiation (137Cs source, Gammacell; Atomic Energy of Canada, Ottawa, ON). Transplantation of the AML cells was done by injecting groups of 3 to 5 mice with 3 × 107 AML cells in the lateral tail vein. After 6 weeks, the mice were killed by CO2 inhalation, after which both femora were isolated and bone marrow suspensions prepared.
To determine the percentage of AML cells in the bone marrow suspensions, cells were stained with antibodies against the human common leukocyte antigen (CD45), CD34, CD33, and CD38 (CD45-fluorescein isothiocyanate [FITC], CD38-FITC, CD33-PE, and CD34-APC; all obtained from Coulter Immunotech) and measured using a FACScalibur (Becton Dickinson Immunocytometry Systems). Analysis was done using the Cell Quest software package (Becton Dickinson). Erythrocytes were excluded from analysis by gating on forward and perpendicular light scatter, while dead cells were excluded by staining with 7-AAD.
Statistical analysis
Statistical analysis of the data was performed using the SPSS software package (SPSS, Chicago, IL). Differences between unrelated samples were analyzed using the Mann-Whitney U test. The Wilcoxon rank sum test was used for the analysis of paired samples.
Initial screening of the correlations between the CXCR-4 expression and patient prognosis was performed by multivariate Cox regression analysis using the rank numbers of the CXCR-4 expression as a variable to test for trend. Variables that showed a significant correlation in this initial screening were studied in further detail by stratification of the patients in high and low CXCR-4-expressing categories based on the optimal cut-point for the CXCR-4 expression. In this analysis, the optimal cut-point is defined as the percentage of cells expressing CXCR-4, at which the difference in patient prognosis between low- and high-expressing categories is largest. Patients with a CXCR-4 expression up to the cut-point were considered to be low expressing, while all others were considered to be high expressing. To generate these categories, half of the samples were used as a training set to determine the optimal cut-point of the CXCR-4 expression. The optimal cut-points determined in the training set were then used to categorize all samples in low- and high-expressing categories.
In the analysis for the coexpression of CD34 and CXCR-4, we observed that the differences between the 2 categories were significant if the cut-point was chosen in the range between 0.4% and 1.2% of cells coexpressing CD34 and CXCR-4 (optimal cut-point at 1.0%). For the analysis of the fraction of CD34+ cells expressing CXCR-4, we observed significant differences for cut-points in the range between 12% and 25% of CD34+ cells expressing CXCR-4 (optimal cut-point at 20%).
For the survival analysis, the Kaplan-Meier method of the SPSS software package was used. The log-rank test was used to check for equality of the survival distributions. The relapse-free survival (RFS) was calculated from date of complete remission (CR) to date of relapse. In this analysis, patients dying from non-leukemia-related causes during first CR were included until date of death. The overall survival (OS) was calculated from date of diagnosis to date of death. Multivariate Cox regression analysis was used to identify other predictor variables present in the study population. In all evaluations, values were considered significant if P values were less than .05.
Results
High percentages of CXCR-4 within the CD34+ fraction of cells predict for an increased relapse rate in primary AML
We analyzed the expression of CXCR-4 together with the expression of CD34 in a series of 90 samples of adult patients with AML. Patient details are given in Table 1. The results of the flow cytometric analysis, of which Figure 1 shows 3 representative examples, are summarized in Table 2. On average, around one third (median, 36.4%) of the Ficoll fraction of marrow cells from AML patients expressed CXCR-4. As indicated by the broad range of the mean fluorescent index (MFI), we observed a large variation in the level at which CXCR-4 was expressed. In most cases, the cells expressed relatively low levels of CXCR-4, but in certain cases the cells showed an excessively high expression. While a median of 22.6% of cells expressed CD34, only 3.9% (median) of cells coexpressed CD34 and CXCR-4, indicating that the percentages of cells expressing CXCR-4 within the CD34+ subset of cells were considerably less than that of the total cell population.
Clinical and hematologic characteristics of patients
Characteristic . | Value . |
|---|---|
| Total no. of patients | 90 |
| Age, y | |
| Median | 44 |
| Range | 16-88 |
| 60 y or younger, n | 72 |
| Sex, n | |
| Male | 44 |
| Female | 46 |
| WBC count, 109/L | |
| Median | 58 |
| Range | 1-319 |
| FAB class | |
| M0 | 3 |
| M1 | 18 |
| M2 | 19 |
| M3 | 5 |
| M4 | 23 |
| M5 | 22 |
| Cytogenetic abnormality* | |
| Favorable | 14 |
| Intermediate | 69 |
| Unfavorable | 7 |
| Initial therapy response | |
| CR | 62 |
| No CR | 28 |
Characteristic . | Value . |
|---|---|
| Total no. of patients | 90 |
| Age, y | |
| Median | 44 |
| Range | 16-88 |
| 60 y or younger, n | 72 |
| Sex, n | |
| Male | 44 |
| Female | 46 |
| WBC count, 109/L | |
| Median | 58 |
| Range | 1-319 |
| FAB class | |
| M0 | 3 |
| M1 | 18 |
| M2 | 19 |
| M3 | 5 |
| M4 | 23 |
| M5 | 22 |
| Cytogenetic abnormality* | |
| Favorable | 14 |
| Intermediate | 69 |
| Unfavorable | 7 |
| Initial therapy response | |
| CR | 62 |
| No CR | 28 |
WBC indicates white blood cell.
Favorable cytogenetic abnormalities included t(8;21), t(15;17), or inv(16); unfavorable cytogenetic abnormalities included del(5), Del(7), deletions of the long arms of chromosomes 5 or 7 (5q-, 7q-), t(6;9), 11q23 abnormalities, and complex karyotypes (> 3 abnormalities). Other karyotypic aberrations and normal karyotypes were included in the intermediate prognostic category.
Representative examples of the flow cytometric analysis of the CD34 and CXCR-4 expression. This figure shows the dot plots for the CD34 versus CXCR-4 expression of 3 representative AML samples. Quadrant setting was determined on the basis of labeled isotype controls. The numbers in the quadrants depict the percentages of cells in respective quadrants.
Representative examples of the flow cytometric analysis of the CD34 and CXCR-4 expression. This figure shows the dot plots for the CD34 versus CXCR-4 expression of 3 representative AML samples. Quadrant setting was determined on the basis of labeled isotype controls. The numbers in the quadrants depict the percentages of cells in respective quadrants.
Phenotypic analysis of the expression of CXCR-4 and CD34
Marker . | Median . | Range . |
|---|---|---|
| CXCR-4 | ||
| % CXCR-4 | 36.4 | 1.6-96.4 |
| MFI | 39.3 | 6.9-523.3 |
| % CD34+ | 22.6 | 0.2-96.3 |
| % CXCR-4+CD34− | 23.6 | 0.1-97.3 |
| % CXCR-4+CD34+ | 3.9 | 0.2-93.5 |
| % CXCR-4−CD34− | 18.8 | 0.3-97.0 |
| % CXCR-4−CD34+ | 8.6 | 0.0-92.0 |
| % CD34+ cells expressing CXCR-4 | 30.0 | 0.0-100.0 |
Marker . | Median . | Range . |
|---|---|---|
| CXCR-4 | ||
| % CXCR-4 | 36.4 | 1.6-96.4 |
| MFI | 39.3 | 6.9-523.3 |
| % CD34+ | 22.6 | 0.2-96.3 |
| % CXCR-4+CD34− | 23.6 | 0.1-97.3 |
| % CXCR-4+CD34+ | 3.9 | 0.2-93.5 |
| % CXCR-4−CD34− | 18.8 | 0.3-97.0 |
| % CXCR-4−CD34+ | 8.6 | 0.0-92.0 |
| % CD34+ cells expressing CXCR-4 | 30.0 | 0.0-100.0 |
Ninety marrow samples were labeled with fluorescent-labeled monoclonal antibodies and analyzed by flow cytometry. Values indicate the median percentages of cells expressing the indicated markers; MFI refers to the mean fluorescent index of the CXCR-4 expression.
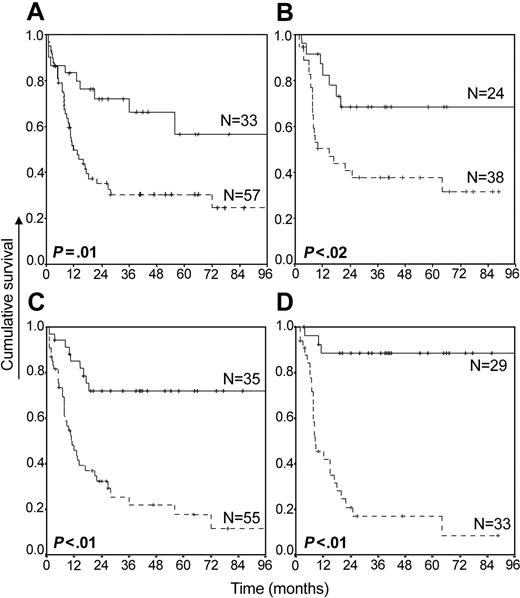
Initial screening using Cox regression analysis showed that the CXCR-4 expression proper only marginally correlated with patient prognosis (data not shown). Yet, the number of cells coexpressing CXCR-4 and CD34, showing a negative correlation with patient prognosis, was studied in further detail (Figure 2A-B). At the optimal cut-point of 1% of cells coexpressing CD34 and CXCR-4, patients with comparatively high percentages of double-positive cells had a reduced overall survival (OS) and a reduced relapse-free survival (RFS). The impact of the CXCR-4 expression on prognosis was even more prominent when the percentage of CXCR-4+CD34+ cells was expressed as a fraction of the total number of CD34+ cells (Figure 2C-D, optimal cut-point 20% of CD34+ cells expressing CXCR-4), resulting in a median OS and RFS of 11 and 8.3 months, respectively, for patients with a high proportion of CD34+ cells expressing CXCR-4. The latter values compared with a median OS and RFS of more than 96 months (P < .01) for patients with a small fraction of CD34+ cells expressing CXCR-4. At a median follow-up of 18 months, 22 of 33 patients with a high CXCR-4 expression had relapsed following complete remission (relative risk of relapse, 6.1; 95% confidence interval, 2.1-18.1).
Overall survival and relapse-free survival of patients with primary AML in relation to the coexpression of CXCR-4 and CD34. OS (A) and RFS (B) for AML are expressed in relation to the percentages of cells coexpressing CXCR-4 and CD34. Solid lines indicate patients in which less than 1% of all cells coexpressed CXCR-4 and CD34; dotted lines, patients in which 1% or more of cells coexpressed CXCR-4 and CD34. OS (C) and RFS (D) are given in relation to the fraction of CD34+ cells expressing CXCR-4. Solid lines indicate patients in which less than 20% of CD34+ cells expressed CXCR-4; dotted lines, patients in which 20% or more of the CD34+ cells expressed CXCR-4. Crosses refer to censored cases.
Overall survival and relapse-free survival of patients with primary AML in relation to the coexpression of CXCR-4 and CD34. OS (A) and RFS (B) for AML are expressed in relation to the percentages of cells coexpressing CXCR-4 and CD34. Solid lines indicate patients in which less than 1% of all cells coexpressed CXCR-4 and CD34; dotted lines, patients in which 1% or more of cells coexpressed CXCR-4 and CD34. OS (C) and RFS (D) are given in relation to the fraction of CD34+ cells expressing CXCR-4. Solid lines indicate patients in which less than 20% of CD34+ cells expressed CXCR-4; dotted lines, patients in which 20% or more of the CD34+ cells expressed CXCR-4. Crosses refer to censored cases.
Flt3/ITD AML shows an increased CXCR-4 expression
To be able to more accurately define the significance of the observed correlation between disease outcome and CXCR-4 expression, we performed a multivariate Cox regression analysis with the fraction of CD34+ cells expressing CXCR-4, the presence of Flt3 mutations, cytogenetic abnormalities, and age as variables in the regression analysis.
For the analysis of Flt3 mutations, all samples were analyzed by PCR for the presence of Flt3/ITDs and Flt3/D835 mutations. Of the 90 samples, 22 were shown to be positive for the presence of Flt3/ITDs and 3 contained the Flt3/D835 mutation. As the number of Flt3/D835 mutations was too low to allow for a separate statistical evaluation and the impact on patient prognosis is not as clear as for the Flt3/ITDs, the Flt3/D835 subset was not considered separately.
In multivariate Cox regression analysis, the percentage of CD34+CXCR-4+ cells and also age, unfavorable cytogenetics, and the presence of Flt3/ITDs were significant predictive factors as regards risk of relapse, resulting in a reduced OS and RFS (Table 3). Because of the variability in the fraction size of CXCR-4+ cells among the CD34 subset, we also evaluated its prognostic value in a multivariate analysis. When the fraction of CD34+ cells expressing CXCR-4 was considered for risk of relapse, the presence of Flt3/ITDs lost its impact on patient prognosis (Table 4). As this indicated that the presence of Flt3/ITDs was not an independent predictive factor in this analysis, we further analyzed this subtype of AML. Analysis of the flow cytometric data showed that the CXCR-4 expression (median expression, 72.1% vs 28.1%; P < .01) was increased in Flt3/ITD AML when compared with Flt3/wt AML. On the other hand, the number of CD34+ cells was reduced (median expression, 9.5% in Flt3/ITD AML compared with 42.1% in Flt3/wt AML; P = .03), resulting in a significantly greater fraction of CD34+ cells expressing CXCR-4 in Flt3/ITD AML (19 of 22 Flt3/ITD samples were above the cut-point of 20% of CD34+ cells expressing CXCR-4 compared with 36 of 66 for the Flt3/wt samples; P = .024, Fisher exact test).
Prognostic impact of the coexpression of CD34 and CXCR-4 and other prognostic variables as regards overall survival and relapse-free survival
. | Overall survival; n = 90 . | . | Relapse-free survival; n = 62 . | . | ||
|---|---|---|---|---|---|---|
| Variable . | Relative risk (95% CI) . | P . | Relative risk (95% CI) . | P . | ||
| Coexpression of CD34 and CXCR-4 in more than 1% of cells | 3.19 (1.62-6.27) | .001 | 3.92 (1.57-9.8) | < .001 | ||
| Presence of FIt3/ITDs | 3.01 (1.59-5.66) | .001 | 2.86 (1.2-6.7) | .015 | ||
| Unfavorable cytogenetic abnormalities* | 2.26 (0.93-5.47) | .014 | 3.0 (1.0-9.3) | .047 | ||
| Age | 1.02 (1.00-1.03) | .024 | 0.99 (0.98-1.02) | .490 | ||
. | Overall survival; n = 90 . | . | Relapse-free survival; n = 62 . | . | ||
|---|---|---|---|---|---|---|
| Variable . | Relative risk (95% CI) . | P . | Relative risk (95% CI) . | P . | ||
| Coexpression of CD34 and CXCR-4 in more than 1% of cells | 3.19 (1.62-6.27) | .001 | 3.92 (1.57-9.8) | < .001 | ||
| Presence of FIt3/ITDs | 3.01 (1.59-5.66) | .001 | 2.86 (1.2-6.7) | .015 | ||
| Unfavorable cytogenetic abnormalities* | 2.26 (0.93-5.47) | .014 | 3.0 (1.0-9.3) | .047 | ||
| Age | 1.02 (1.00-1.03) | .024 | 0.99 (0.98-1.02) | .490 | ||
To more accurately define the significance of the observed correlation between prognosis and the percentages of cells coexpressing CXCR-4 and CD34, we performed a multivariate Cox regression analysis with CXCR-4/CD34 coexpression, the presence FIt3 mutations, cytogenetics, and age as variables in the regression analysis. Results are presented as relative risks with the 95% confidence intervals and respective P values.
n = the number of samples included in the analysis.
See legend to Table 1.
The prognostic impact of FIt3/ITDs in relation to the fraction of CD34+ cells expressing CXCR-4
. | Relapse-free survival; n = 62 . | . | |
|---|---|---|---|
| Variable . | Relative risk (95% CI) . | P . | |
| CXCR-4 expression of 20% or more within the CD34+ subset | 13.4 (3.87-25.0) | < .001 | |
| Presence FIt3/ITDs | 1.54 (0.67-3.54) | .327 | |
| Unfavorable cytogenetic abnormalities* | 1.50 (0.81-2.63) | .048 | |
| Age | 0.57 (0.27-2.61) | .370 | |
. | Relapse-free survival; n = 62 . | . | |
|---|---|---|---|
| Variable . | Relative risk (95% CI) . | P . | |
| CXCR-4 expression of 20% or more within the CD34+ subset | 13.4 (3.87-25.0) | < .001 | |
| Presence FIt3/ITDs | 1.54 (0.67-3.54) | .327 | |
| Unfavorable cytogenetic abnormalities* | 1.50 (0.81-2.63) | .048 | |
| Age | 0.57 (0.27-2.61) | .370 | |
We performed a multivariate Cox regression analysis with fraction of CD34+ cells expressing CXCR-4, the presence FIt3- mutations, cytogenetics, and age as variables in the regression analysis. Results are presented as relative risks with 95% confidence intervals and respective P values. n = the number of samples included in the analysis.
See legend to Table 1.
The percentage of CXCR-4+ cells within the CD34+ cluster correlates with the outgrowth of AML cells in the NOD/SCID mouse model
It has been shown that SDF-1/CXCR-4 interactions may play an essential role in the homing and high-level multilineage engraftment of hematopoietic stem cells from healthy donors in the NOD/SCID human-mouse chimera model.28,29 We and others have also shown that Flt3/ITD AML has a significantly increased SCID repopulating ability when compared with Flt3/wt AML.30,31 As levels of CXCR-4 expression appear to be increased in Flt3/ITD AML, we set out to analyze whether the levels of CXCR-4 expression could be correlated with the NOD/SCID repopulating abilities of both Flt3/ITD and Flt3/wt AML. Due to the relatively high cell numbers needed to achieve reproducible and stable levels of chimerism in this model,32 we were able to analyze only 21 AML samples (15 Flt3/wt and 6 Flt3/ITD).
As shown in Table 5, at 6 weeks after transplantation there is a large variation in the percentages of AML cells repopulating the bone marrow of the NOD/SCID mice (median, 12.6% human cells; range, 0.5%-97.8%). Correlation analysis showed that the level of human chimerism in the NOD/SCID mice correlated with both the overall expression of CXCR-4 (correlation coefficient, 0.588; P = .01) and the expression of CXCR-4 within the CD34+ cluster of cells (correlation coefficient, 0.708; P = .001; Figure 3).
Outgrowth of human AML cells in NOD/SCID mice
. | % human AML cells . | . | . | Phenotype of the original AML sample . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | . | FAB . | FIt3 status . | % CXCR-4+ . | % CD34+ . | % CD34+CXCR-4+ . | % of CD34+expressing CXCR-4 . | |||
| 1 | 0.5 | M5 | ITD | 6.2 | 95.2 | 5.9 | 6.2 | |||
| 2 | 0.9 | M2 | wt | 27.0 | 86.8 | 11.3 | 13.0 | |||
| 3 | 0.9 | M2 | wt | 13.0 | 75.6 | 8.0 | 10.6 | |||
| 4 | 2.5 | M5 | wt | 5.9 | 1.7 | 0.2 | 12.0 | |||
| 5 | 3.1 | M4 | wt | 11.7 | 46.3 | 9.1 | 19.7 | |||
| 6 | 4.6 | M5 | wt | 51.2 | 76.0 | 28.7 | 37.7 | |||
| 7 | 8.3 | M1 | wt | 18.2 | 92.3 | 16.3 | 16.3 | |||
| 8 | 9.2 | M5 | wt | 33.4 | 82.9 | 28.8 | 34.8 | |||
| 9 | 9.7 | M2 | wt | 34.2 | 95.2 | 25.6 | 26.8 | |||
| 10 | 11.8 | M5 | wt | 39.3 | 11.4 | 9.6 | 84.5 | |||
| 11 | 13.4 | M5 | wt | 24.0 | 1.2 | 0.9 | 75.0 | |||
| 12 | 14.4 | M4 | wt | 51.2 | 52.6 | 26.9 | 51.2 | |||
| 13 | 15.1 | M4 | ITD | 98.5 | 1.0 | 0.3 | 26.8 | |||
| 14 | 26.6 | M4 | wt | 16.8 | 5.7 | 2.5 | 43.9 | |||
| 15 | 30.1 | M2 | ITD | 90.1 | 0.2 | 0.2 | 100 | |||
| 16 | 37.6 | M0 | wt | 4.6 | 1.6 | 0.6 | 40.5 | |||
| 17 | 38.5 | M1 | wt | 23.0 | 0.7 | 0.5 | 70.4 | |||
| 18 | 38.5 | M0 | wt | 92.6 | 96.3 | 83.7 | 86.9 | |||
| 19 | 48.6 | M2 | ITD | 56.2 | 1.5 | 1.0 | 66.7 | |||
| 20 | 81.7 | M2 | ITD | 59.3 | 26.5 | 8.0 | 30.3 | |||
| 21 | 97.8 | M5 | ITD | 73.2 | 0.3 | 0.2 | 72.0 | |||
. | % human AML cells . | . | . | Phenotype of the original AML sample . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | . | FAB . | FIt3 status . | % CXCR-4+ . | % CD34+ . | % CD34+CXCR-4+ . | % of CD34+expressing CXCR-4 . | |||
| 1 | 0.5 | M5 | ITD | 6.2 | 95.2 | 5.9 | 6.2 | |||
| 2 | 0.9 | M2 | wt | 27.0 | 86.8 | 11.3 | 13.0 | |||
| 3 | 0.9 | M2 | wt | 13.0 | 75.6 | 8.0 | 10.6 | |||
| 4 | 2.5 | M5 | wt | 5.9 | 1.7 | 0.2 | 12.0 | |||
| 5 | 3.1 | M4 | wt | 11.7 | 46.3 | 9.1 | 19.7 | |||
| 6 | 4.6 | M5 | wt | 51.2 | 76.0 | 28.7 | 37.7 | |||
| 7 | 8.3 | M1 | wt | 18.2 | 92.3 | 16.3 | 16.3 | |||
| 8 | 9.2 | M5 | wt | 33.4 | 82.9 | 28.8 | 34.8 | |||
| 9 | 9.7 | M2 | wt | 34.2 | 95.2 | 25.6 | 26.8 | |||
| 10 | 11.8 | M5 | wt | 39.3 | 11.4 | 9.6 | 84.5 | |||
| 11 | 13.4 | M5 | wt | 24.0 | 1.2 | 0.9 | 75.0 | |||
| 12 | 14.4 | M4 | wt | 51.2 | 52.6 | 26.9 | 51.2 | |||
| 13 | 15.1 | M4 | ITD | 98.5 | 1.0 | 0.3 | 26.8 | |||
| 14 | 26.6 | M4 | wt | 16.8 | 5.7 | 2.5 | 43.9 | |||
| 15 | 30.1 | M2 | ITD | 90.1 | 0.2 | 0.2 | 100 | |||
| 16 | 37.6 | M0 | wt | 4.6 | 1.6 | 0.6 | 40.5 | |||
| 17 | 38.5 | M1 | wt | 23.0 | 0.7 | 0.5 | 70.4 | |||
| 18 | 38.5 | M0 | wt | 92.6 | 96.3 | 83.7 | 86.9 | |||
| 19 | 48.6 | M2 | ITD | 56.2 | 1.5 | 1.0 | 66.7 | |||
| 20 | 81.7 | M2 | ITD | 59.3 | 26.5 | 8.0 | 30.3 | |||
| 21 | 97.8 | M5 | ITD | 73.2 | 0.3 | 0.2 | 72.0 | |||
There were 15 Fit3 wild-type and 6 FIt3/ITD samples analyzed in the NOD/SCID mouse model. Per group, 3 to 5 mice were injected with 3 × 107 AML cells. After 6 weeks, the mice were killed and bone marrow cells harvested. The percentage of human AML cells in the bone marrow was analyzed by flow cytometry and compared with the CXCR-4 and CD34 expression patterns of the samples before grafting.
Correlation between the percentages of CD34+ cells expressing CXCR-4 and the outgrowth in NOD/SCID. Samples from 21 AML patients were injected in NOD/SCID mice (3 × 107 cells per mouse, 3-5 mice per group). After 6 weeks the mice were killed and the number of human AML cells in the bone marrow was determined by flow cytometry. The outgrowth of AML cells in NOD/SCID was then correlated with the percentages of CD34+ cells expressing CXCR-4.
Correlation between the percentages of CD34+ cells expressing CXCR-4 and the outgrowth in NOD/SCID. Samples from 21 AML patients were injected in NOD/SCID mice (3 × 107 cells per mouse, 3-5 mice per group). After 6 weeks the mice were killed and the number of human AML cells in the bone marrow was determined by flow cytometry. The outgrowth of AML cells in NOD/SCID was then correlated with the percentages of CD34+ cells expressing CXCR-4.
Chemotaxis in response to SDF-1 does not correlate with patient prognosis or with the percentage of CXCR-4 expression in the CD34+ cluster of cells
Recently, Voermans et al16 reported that, similar to normal hematopoiesis, there is a linear correlation between chemotactic response and the expression levels of CXCR-4 in AML. To investigate if the migratory abilities correlated with patient prognosis, we analyzed the migratory abilities in response to SDF-1 in a subset of 60 AML samples.
After 4 hours of incubation, the median percentage of cells that had migrated in the presence of SDF-1 was 13.3%, which exceeded the comparative 2.5% value in medium alone (P < .01). Correlation analysis revealed a low but significant correlation between chemotaxis and the overall CXCR-4 expression as well as the percentage of CXCR-4+CD34+ cells (correlation coefficient, 0.326 [P = .01] and 0.368 [P = .004], respectively; data not shown). The SDF-1-induced chemotaxis did not correlate with patient prognosis.
SDF-1/CXCR-4 interactions are required for the survival of differentiating 32D cells and induce a block in myeloid differentiation
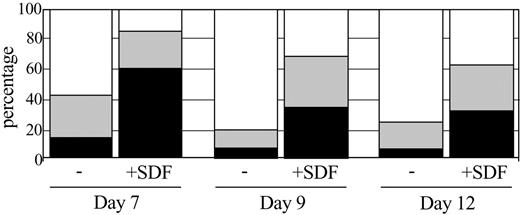
Low levels of SDF-1 may enhance myeloid progenitor cell survival in vitro.33,34 Further, the expression of CXCR-4 undergoes complex lineage-dependent regulation during myeloid differentiation.35 We therefore investigated the effect of SDF-1 on the survival and G-CSF-induced differentiation of the myeloid cell line 32D/G-CSF-R, a subclone of the 32D cell line known to express CXCR-4.36 To study whether SDF-1/CXCR-4 interactions influenced cell survival and neutrophil differentiation, 32D/G-CSF-R cells were cultured with G-CSF (100 ng/mL) and G-CSF + SDF-1 (100 ng/mL). G-CSF induced a rapid increase, which was suppressed in the presence of SDF-1, in the number of terminally matured neutrophils between days 6 and 12 (Figure 4). In order to check for the specificity of the SDF-1 inhibition through binding to CXCR-4, we performed the same experiment in the presence of a CXCR-4 blocking antibody. Instead of rescuing the impairment of differentiation, the CXCR-4 blocking antibody induced apoptosis in the 32D/G-CSF-R cells. As induction of apoptosis by the blocking antibody was also seen in the absence of exogenously added SDF-1 (data not shown), these data strongly suggest the presence of SDF-1 in the culture medium where it may provide a survival signal to the differentiating 32D cells. In order to exclude the possibility that the observed apoptosis was due to nonspecific toxicity, we also verified the effect of inhibiting SDF-1/CXCR-4 interactions by the addition of an SDF-1 neutralizing antibody. Both the SDF-1 neutralizing antibody and the CXCR-4 blocking antibody induced a strong increase in apoptotic cells during the 6 days of culture (Figure 5). Incubation of a CXCR-4-negative cell line (ie, KG-1) with the CXCR-4 and SDF-1 specific antibodies did not result in induction of apoptosis.
Effect of SDF-1 addition on G-CSF induced differentiation of 32D/G-CSF-R cells. Differential counts after 7, 9, and 12 days of culture in the presence of G-CSF (-) or G-CSF + SDF-1 (+ SDF). □ indicates the percentage of terminally matured neutrophils; ▦, the percentage of intermediately matured granulocytic cells; and ▪, the percentages of blast cells in the cultures.
Effect of SDF-1 addition on G-CSF induced differentiation of 32D/G-CSF-R cells. Differential counts after 7, 9, and 12 days of culture in the presence of G-CSF (-) or G-CSF + SDF-1 (+ SDF). □ indicates the percentage of terminally matured neutrophils; ▦, the percentage of intermediately matured granulocytic cells; and ▪, the percentages of blast cells in the cultures.
Blocking of SDF-1/CXCR-4 interactions induces apoptosis in 32D cells. On day 0, cultures were initiated with 2 × 105 32D/G-CSF-R cells/mL. Cells were counted and cultures were readjusted to 2 × 105 cells/mL on a daily basis. Cultures were either performed in the presence of G-CSF, G-CSF + a CXCR4-blocking antibody, or G-CSF + an SDF-1 neutralizing antibody. The percentage of cells in apoptosis (y-axis) was determined by a flow cytometric analysis of the number of annexin-V+ 7-AAD- cells. Error bars indicate ± 1 SD.
Blocking of SDF-1/CXCR-4 interactions induces apoptosis in 32D cells. On day 0, cultures were initiated with 2 × 105 32D/G-CSF-R cells/mL. Cells were counted and cultures were readjusted to 2 × 105 cells/mL on a daily basis. Cultures were either performed in the presence of G-CSF, G-CSF + a CXCR4-blocking antibody, or G-CSF + an SDF-1 neutralizing antibody. The percentage of cells in apoptosis (y-axis) was determined by a flow cytometric analysis of the number of annexin-V+ 7-AAD- cells. Error bars indicate ± 1 SD.
Discussion
In the past few years, our understanding of the presence and prognostic value of specific genetic defects (such as Flt3/ITD, AML-1-core binding factor β [CBFβ], promyelocytic leukemia-retinoic-acid receptor [PML-RAR], and various others) in human AML has increased. Notwithstanding this progress, there is a large subset of AML patients with prognostic heterogeneity and no detectable molecular or chromosomal aberrations. As it has been suggested that SDF-1/CXCR-4 interactions might be involved in the trafficking of leukemic cells, with possible prognostic implications, we investigated the expression of CXCR-4 and the chemotactic response to SDF-1 in relation to the clinical outcome of patients with newly diagnosed AML.
While the expression of CXCR-4 proper had a borderline prognostic value, the percentage of CD34+CXCR-4+ double-positive cells and the fraction of CD34+ cells expressing CXCR-4 appeared to be significant negative predictors of both overall survival and relapse-free survival. In multivariate analysis, the predictive value proved to be independent of other previously established prognostic markers such as age and cytogenetic abnormalities. A particularly interesting observation of the studies presented here is that Flt3/ITD AML was characterized by an increased expression of CXCR-4 (both in the number of cells expressing CXCR-4 and the level of CXCR-4 expression per cell). The difference in CXCR-4 expression between Flt3/ITD and Flt3/wt AML was also reflected in the fraction of CD34+ cells expressing CXCR-4, which with a median value of 52.7% was significantly more in Flt3/ITD AML when compared with the median value of 19.8% in Flt3/wt AML (P < .01). We also observed that in Flt3/ITD AML the expression of CXCR-4 correlated with the mutant-wild-type ratio present in the respective samples and that activation of the wild-type Flt3 resulted in an up-regulation of the CXCR-4 expression (data not shown), suggesting a link between the expression of CXCR-4 and Flt3/ITDs. Furthermore, while Flt3/ITDs had a significant negative impact on the RFS when analyzed as a single variable (data not shown), the impact of Flt3/ITDs was no longer apparent when it was analyzed in conjunction with the fraction of CD34+ cells expressing CXCR-4. Taken together these data might suggest that the poor prognosis of Flt3/ITD AML depends on the increased CXCR-4 expression in this type of AML.
In order to investigate whether the correlations between the CXCR-4 expression and prognosis could be explained by a different organ distribution of AML cells, we analyzed the in vitro migratory behavior of these cells in response to SDF-1, the ligand of CXCR-4. We observed that AML with a high percentage of cells expressing CXCR-4 showed an increased chemotactic response in culture. However, the low correlation coefficient between CXCR-4 expression and chemotactic response would suggest that factors other than the expression of CXCR-4, such as the expression of adhesion molecules and cell cycle induction, might play a role in determining the migratory abilities.
Ponomaryov et al37 observed an increased expression of SDF-1 in the bone marrow following conditioning with DNA-damaging agents (ionizing radiation, cyclophosphamide, and 5-fluorouracil [5-FU]); this resulted in an increase in CXCR-4-dependent homing to the bone marrow, which consequently facilitated engraftment of hematopoietic stem cells. Although not shown to be true for leukemic stem cells, these observations on normal bone marrow cells fit well with our observation of a significant correlation between the expression of CXCR-4 and the outgrowth of AML cells in the NOD/SCID mouse model.
Apart from its chemotactic activity, SDF-1 has also been suggested to play a role in hematopoietic (stem) cell survival and function by suppression of apoptosis and promoting the G0/G1 transition.37-40 The observation that blocking of CXCR-4 induces apoptosis in the 32D system may suggest that also in myeloid cells SDF-1/CXCR-4 interactions provide strong antiapoptotic signals. However, in the 32D cell system, high levels of SDF-1 induced a partial block in differentiation. This raises the possibility that increased expression of CXCR-4 contributes to leukemogenesis by inducing a block in differentiation. Recently Jorda et al41 have observed that Cb2, another Gαi-GPCR and a frequent proviral target in Cas-Br-M-MuLV-induced myeloid leukemias, produces an arrest in myeloid differentiation in the 32D system, suggesting a more general role of Gαi-GPCRs in leukemogenesis.
In conclusion, our data indicate that a high expression of CXCR-4 in the CD34 cluster of cells defines unfavorable prognosis of AML. Little is known about the role of CXCR-4/SDF-1 in the development, therapy responsiveness, and disease progression of AML. Which of the 3 possible mechanisms (ie, an increased homing, a block in differentiation, and antiapoptotic signaling) by itself influences disease prognosis remains to be seen. To this end, these data warrant a more extensive study into the role of CXCR-4 in the pathobiology of acute myeloid leukemia.
Prepublished online as Blood First Edition Paper, March 30, 2004; DOI 10.1182/blood-2004-02-0566.
Supported by the Dutch Platform for Alternatives in Animal Research (PAD) grant no. VWS/PAD 9625.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank the staff of the bone marrow transplantation unit of the Department of Hematology for their help in obtaining the patient samples, Meritxell Alberich Jorda for her help with the 32D differentiation assays, and Prof Dr Ivo Touw for critically reviewing this manuscript.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal