Abstract
Mutations in human prothrombin that generate a stable form of meizothrombin or meizothrombin(desF1) cause dysprothrombinemia in both the homozygous and heterozygous state, suggesting that meizothrombin has dominant anticoagulant effects in vivo. The enzymatic characterization of recombinant mouse meizothrombin, meizothrombin(desF1), and thrombin indicates that all 3 enzymes have similar activity toward the chromogenic substrate S-2238, that meizothrombin and meizothrombin(desF1) have less than 10% of the fibrinogen-clotting activity of thrombin, and that meizothrombin is more active than thrombin or meizothrombin(desF1) for thrombomodulin-dependent protein C activation. Thus, activated mouse prothrombin R157A/R268A is similar to human meizothrombin in activity toward S-2238, fibrinogen, and protein C. The time to occlusion after FeCl3-induced carotid artery injury was delayed (11.8 ± 3.6 minutes, n = 5) in Cf2+/- mice infused with prothrombin R157A/R268A compared with control mice infused with wild-type prothrombin (5.3 ± 1.5 minutes, n = 3; P = .006). In this model, prothrombin R157A/R268A has anticoagulant activity that reflects its decreased fibrinogen-clotting activity and preserved protein C-activating activity and is consistent with dominant inhibition of fibrinogen clotting. (Blood. 2004;104:415-419)
Introduction
Prothrombin is a blood-clotting factor that, when activated, generates thrombin, a protease with multiple biologic functions. Thrombin clots fibrinogen and activates platelets and it participates in the feedback regulation of several other procoagulant and anticoagulant enzyme pathways. For example, thrombin binds to thrombomodulin on endothelial cell surfaces, and the thrombin-thrombomodulin complex efficiently activates the serine protease zymogen protein C. Activated protein C cleaves blood-clotting factors Va and VIIIa, thereby inhibiting further thrombin generation. The anticoagulant protein C pathway limits intravascular coagulation associated with sepsis and inflammatory responses to endotoxin,1 and defects in the protein C pathway are common causes of venous thrombosis.2
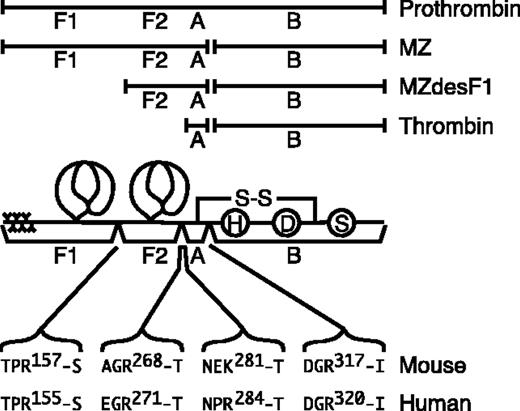
During prothrombin activation, at least 3 active species that may have distinct substrate specificity are produced by selected proteolytic cleavages (Figure 1). Meizothrombin is generated by the cleavage of the Arg320-Thr321 bond of prothrombin by factor Xa.3,4 Meizothrombin(desF1) is generated by autoproteolysis of meizothrombin atArg155-Ser156, which removes the membrane binding Gla domain and kringle 1. Thrombin is produced by a second factor Xa cleavage at Arg271-Thr272.5,6 Compared with thrombin, the ability of meizothrombin and meizothrombin(desF1) to clot fibrinogen or activate platelets is markedly reduced. However, both meizothrombin and meizothrombin(desF1) can bind thrombomodulin and activate protein C; in this reaction, meizothrombin(desF1) is approximately as active as thrombin, and human meizothrombin appears to be approximately 6-fold more potent than thrombin.6-8 Thus, meizothrombin and meizothrombin(desF1) have the potential to function as anticoagulants in vivo by selectively activating the protein C pathway. This possibility is supported by the properties of prothrombin Dhahran (Arg271His), which can be activated by factor Xa to generate a stable form of meizothrombin or meizothrombin(desF1) but not thrombin. Patients with prothrombin Dhahran have prolonged activated partial thromboplastin times in the homozygous or heterozygous condition.9 Although they do not have bleeding symptoms, the abnormal activated partial thromboplastin time in heterozygous individuals suggests that meizothrombin may have dominant inhibitory effects on prothrombin function, perhaps in part by favoring the activation of protein C or by competitively inhibiting the activation of wild-type prothrombin.
The activation of prothrombin. Prothrombin fragment 1 (F1) consists of the γ-carboxyglutamic acid domain and first kringle domain, and fragment (F2) consists of the second kringle domain. Thrombin consists of disulfide-linked A and B chains, where the B chain contains the His (H), Asp (D), and Ser (S) residues of the serine protease active site. Meizothrombin (MZa) is produced by factor Xa cleavage after Arg320 of human prothrombin or after Arg317 of mouse prothrombin. Thrombin is produced by factor Xa cleavage after Arg271 of human MZa or after Arg268 of mouse MZa. Meizothrombin(desF1) (MZdesF1a) is produced by autocatalytic cleavage after Arg155 of human MZa or after Arg157 of mouse MZa. Fragment F2 and the A chain of human thrombin also can be separated by cleavage after Arg284; this cleavage site is replaced by Lys281 in mouse prothrombin.
The activation of prothrombin. Prothrombin fragment 1 (F1) consists of the γ-carboxyglutamic acid domain and first kringle domain, and fragment (F2) consists of the second kringle domain. Thrombin consists of disulfide-linked A and B chains, where the B chain contains the His (H), Asp (D), and Ser (S) residues of the serine protease active site. Meizothrombin (MZa) is produced by factor Xa cleavage after Arg320 of human prothrombin or after Arg317 of mouse prothrombin. Thrombin is produced by factor Xa cleavage after Arg271 of human MZa or after Arg268 of mouse MZa. Meizothrombin(desF1) (MZdesF1a) is produced by autocatalytic cleavage after Arg155 of human MZa or after Arg157 of mouse MZa. Fragment F2 and the A chain of human thrombin also can be separated by cleavage after Arg284; this cleavage site is replaced by Lys281 in mouse prothrombin.
To assess the anticoagulant potential of meizothrombin, we have prepared and characterized recombinant mouse prothrombin R157A/R268A (aaII) and compared it with wild-type prothrombin having Arg residues at these positions (rrII). Prothrombin R157A/R268A (aaII) was activated to yield a meizothrombin-like species (aaMZa) that was converted slowly to a stable meizothrombin(desF1)-like product (aaMZdesF1a). As observed for the corresponding bovine and human prothrombin derivatives, aaMZa and aaMZdesF1a had markedly reduced ability to clot fibrinogen or activate platelets but preserved ability to activate protein C. The effects of mouse aaII in vivo were assessed in a model of acute carotid artery thrombosis.
Materials and methods
Construction, expression, and purification of prothrombin R157A/R268A
The full-length mouse prothrombin cDNA10 was mutated to alanine at the factor Xa cleavage sites following kringle 2 (Arg268) and at a predicted thrombin cleavage site between kringle 1 and kringle 2 (Arg157), and the product was cloned into the pNUT vector.11 Wild-type recombinant prothrombin with Arg residues at these positions (rrII) and the mutant prothrombin R157A/R268A (aaII) were expressed by stable transfection of baby hamster kidney (BHK) cells and purified as previously described.12,13 Briefly, conditioned medium containing recombinant protein was collected and prothrombin was precipitated by adding citric acid and 1 M BaCl2. The pellets were resuspended in 0.2 M sodium ethylenediaminetetraacetic acid (pH 8.0) and then dialyzed against 50 mM Tris-HCl (pH 7.5) and 150 mM NaCl (Tris-buffered saline [TBS]). After dialysis, proteins were purified by adsorption on Q Fast Flow Sepharose (Amersham Pharmacia Biotech, Piscataway, NJ) followed by gradient elution with 0 to 50 mM CaCl2 in TBS.
Activation, purification, active site titration, and characterization of aaMZa, aaMZdesF1a, and thrombin
Wild-type rrII (0.5 mg/mL) was incubated for 2 hours at room temperature with 20 μg/mL Taipan snake venom (Sigma, St Louis, MO) in the presence of rabbit brain cephalin, 4 mM CaCl2, 0.2 M NaCl, and 20 mM Tris-HCl (pH 7.4). Thrombin was purified by ion exchange chromatography on mono S or Sephadex SP6 (both from Amersham Pharmacia Biotech). Mutant rrII (0.5 mg/mL) was incubated for 45 minutes at 37°C with 10 μg/mL Echis carinatus venom (Haematologic Technologies, Essex Junction, VT), 5 mM CaCl2, 0.1 M NaCl, and 20 mM Tris-HCl (pH 7.4). Sufficient 5 M NaCl was gradually added to the activation mixture to achieve a concentration of 0.5 M NaCl. The mixture was chromatographed on a benzamidine-Sepharose column equilibrated with 0.5 M NaCl and 20 mM Tris-HCl (pH 7.4). The aaMZdesF1a was then eluted using 0.05 M glycine-HCl (pH 3.0) into tubes containing 150 μL of 1 M Tris-HCl (pH 7.4) and dialyzed against TBS. Recombinant aaMZa was generated by activation of aaII with agarose bead-conjugated E carinatus venom (Haematologic Technologies) and the products were recovered by centrifugation. Enzyme quantitation was done by active site titration using D-Phe-Pro-Arg-chloromethane (PPACK). Enzymes were diluted in amidolytic buffer (50 mM Tris-HCl, pH 8.0; 0.2 M NaCl; and 0.1% polyethylene glycol [PEG] 8000), and 75 μL of solution containing approximately 0.8 nM, 1.6 nM, or 4 nM protease was mixed with the same buffer containing serial dilutions of PPACK (4, 3.2, 2.4, 0.8, and 0 nM) and incubated for 5 minutes. D-Phe-pipecolyl-Arg-P-nitroanilide (S-2238) was added to a final concentration of 50 μM, and P-nitroanilide product was measured at 405 nm in a Spectra Max Plus plate reader (Molecular Devices, Sunnyvale, CA) for 30 minutes at 11-second intervals.
S-2238 hydrolysis
The enzyme activity was measured by adding 150 μL of 1 nM purified enzyme to 150 μL of amidolytic buffer containing various concentrations of S-2238. Absorbance at 405 nm was determined at 11-second intervals, and the initial rate of product formation was calculated.
Fibrinogen-clotting assay
A sample of 50 μL of 50 mg/mL bovine fibrinogen (Sigma) was added to 0.2 mL fibrinogen-clotting buffer (150 mM NaCl; 10 mM imidazole, pH 7.4; 10 mM CaCl2; and 6.6 g/L PEG 6000) containing approximately 2 pmol protease at 37°C, and the clotting time was determined with a fibrometer (BBL Fibro System; Becton Dickinson, Lincoln Park, NJ).
Protein C activation
The protein C-activation assay was done as previously described with slight modifications.14 Briefly, CV-1(18A) cells that express human thrombomodulin were harvested, washed twice, and resuspended in assay buffer (20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], pH 7.4; 150 mM NaCl; 5 mM CaCl2). Then, 1.5 pmol protease was added to 98.5 μL assay buffer containing 106 cells and 5 μg human recombinant protein C. The reaction mixture was incubated at 37°C for 30 minutes and the reaction was stopped by the addition of 7.5 μL assay buffer containing heparin (1 unit/μL), 0.32 μg/μL antithrombin, and 680 nM hirudin. The samples were centrifuged for 5 minutes at 16 000g, 10 μL supernatant was added to 290 μL of 100 μM pyroGlu-Pro-Arg-P-nitroanilide (S-2366) in 50 mM Tris (pH 8.6) and 50 mM CsCl in microtiter plates, and absorbance at 405 nm was determined as a function of time. The concentration of activated protein C was calculated based on standard curve generated using human activated protein C (Haematologic Technologies). The assay was also performed with 32 μg/mL soluble rabbit thrombomodulin (Haematologic Technologies).
Animals, injury, and monitoring of blood flow
Prothrombin null mice15 were backcrossed into the 129Svev background for at least 12 generations. Two- to 4-month old heterozygous Cf2+/- male mice were anesthetized by intraperitoneal injection of 70 mg/kg sodium pentobarbital. A coded sample of recombinant rrII or aaII in 150 μL TBS was infused in a blinded manner through the tail vein prior to surgery. The carotid artery was isolated and a small animal blood flow meter (Transonic Systems, Ithaca, NY) was applied. The carotid artery was injured as previously described16 with slight modification. A filter paper (1 mm × 2 mm) soaked with 10% FeCl3 solution was applied to the artery for 3 minutes and the cavity was filled with saline immediately.16 The flow rate was monitored and recorded and the time to occlusion was determined. The injured arteries were excised and fixed in 40% formaldehyde for morphologic studies. Stained tissue sections mounted in Permount (Biomeda, Foster City, CA) were photographed with a Nikon FDX-35 camera and Nikon Eclipse E800 microscope (both from Nikon, Tokyo, Japan) using a 40× objective lens (CF160 System) at an original magnification of × 400. The photomicrograph was scanned using Vuescan 7.6.21 (Hamrick Software, Tucson, AZ) and cropped in Adobe Photoshop CS (Adobe, San Jose, CA). All the animal experiments and care were done based on the Guide for Care and Use of Laboratory Animals (Department of Health, Education, and Welfare Publication No. NIH 78-23) and were approved by the Washington University Committee on Use and Care of Animals.
Western blotting analysis
To assess the intravascular distribution and recovery of injected proteins, Cf2+/- mice were injected with either 45 or 110 μg of aaII protein. After 20 minutes, the blood was collected by cardiocentesis, and plasma samples (0.1 μL) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting with rabbit polyclonal antimouse prothrombin antibodies.15
Statistics
Differences between groups were assessed for significance by Student t test.
Results
Design and characterization of a mouse precursor of stable meizothrombin
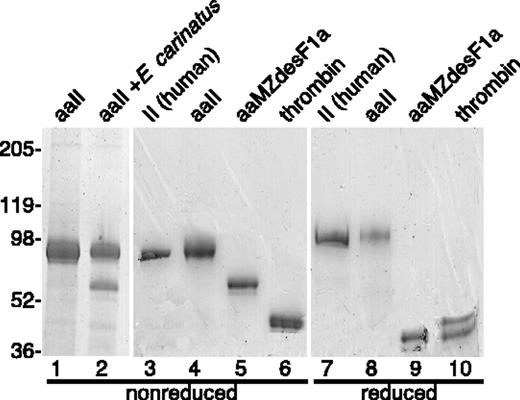
Human prothrombin is cleaved by factor Xa after Arg320 to yield meizothrombin and again after Arg271 to yield thrombin (Figure 1). Meizothrombin can also be cleaved by thrombin after Arg155 to generate meizothrombin(desF1) or after Arg284 to generate a second variant of thrombin. Based on sequence alignment,10 the corresponding cleavage sites in mouse prothrombin are Arg317, Arg268, and Arg157, respectively; the human Arg284 site is not conserved in mouse prothrombin. The mutations R157A and R268A were made in mouse prothrombin to produce prothrombin R157A/R268A (aaII), anticipating that these changes would prevent or retard the conversion of meizothrombin to smaller products, and therefore enable the preparation of a relatively stable, active, meizothrombin-like species. Mutation of Arg268 prevented the generation of thrombin upon activation by E carinatus venom, confirming that Arg268 is the only factor Xa cleavage site between F2 and the thrombin A chain of mouse prothrombin (Figure 2). Activation of aaII with either E carinatus or Taipan snake venom cleaved aaII quantitatively after Arg317, as shown by gel electrophoresis under reducing conditions (data not shown). Although the predicted thrombin cleavage site at Arg157 was mutated to alanine, either snake protease produced traces of meizothrombin(desF1)-like (aaMZdesF1a) molecules (Figure 2). The autolysis of aaMZa to aaMZdesF1a continued over several days (data not shown). These products may be generated by slow cleavage after Arg149 or possibly Lys162, residues that are not present in human prothrombin. No further processing of aaMZdesF1a to thrombin was observed. Thus, recombinant mutant aaII generates a relatively stable meizothrombin-like product that is susceptible to slow autocatalytic cleavage at sites near Arg157.
Recombinant mouse prothrombins and activated products. Recombinant aaII and rrII were proteolytically activated and the products aaMZa, aaMZdesF1a, and thrombin were purified and analyzed by gel electrophoresis. The gel was stained with Coomassie blue, and the positions of molecular weight standards (kDa) are indicated. Purified human plasma prothrombin was included for comparison. The major product of aaII activation with E carinatus venom is aaMZa (lane 2), which is converted slowly by autoproteolysis to aaMZdesF1a (lane 5). Activation of aaII to aaMZa (lane 2) was quantitative as shown by disappearance of the aaMZa band upon electrophoresis under reducing conditions.
Recombinant mouse prothrombins and activated products. Recombinant aaII and rrII were proteolytically activated and the products aaMZa, aaMZdesF1a, and thrombin were purified and analyzed by gel electrophoresis. The gel was stained with Coomassie blue, and the positions of molecular weight standards (kDa) are indicated. Purified human plasma prothrombin was included for comparison. The major product of aaII activation with E carinatus venom is aaMZa (lane 2), which is converted slowly by autoproteolysis to aaMZdesF1a (lane 5). Activation of aaII to aaMZa (lane 2) was quantitative as shown by disappearance of the aaMZa band upon electrophoresis under reducing conditions.
The concentrations of aaMZa, aaMZdesF1a, and thrombin were determined by active site titration with PPACK and kinetic constants for S-2238 cleavage were determined by an initial rate method. All 3 proteases had similar amidolytic activity, with similar values for Km and kcat (Table 1). In contrast, the fibrinogen-clotting activities of aaMZa and aaMZdesF1a were 5.8% ± 0.6% and 8.4% ± 1.4% that of wild-type thrombin, respectively. These results are consistent with the selective loss of activity toward fibrinogen reported previously for human and bovine meizothrombin and meizothrombin(desF1).6,8
Kinetic constants for cleavage of S-2238
Protease . | Km, μM . | kcat, s−-1 . |
|---|---|---|
| Thrombin | 1.79 ± 0.13 | 72.6 ± 1.4 |
| aaMZdesF1a | 1.41 ± 0.25 | 91.5 ± 3.8 |
| aaMZa | 2.0 ± 0.38 | 120 ± 5.5 |
Protease . | Km, μM . | kcat, s−-1 . |
|---|---|---|
| Thrombin | 1.79 ± 0.13 | 72.6 ± 1.4 |
| aaMZdesF1a | 1.41 ± 0.25 | 91.5 ± 3.8 |
| aaMZa | 2.0 ± 0.38 | 120 ± 5.5 |
Values are given as ± SD.
Products of aaII activation are potent thrombomodulin-dependent protein C activators
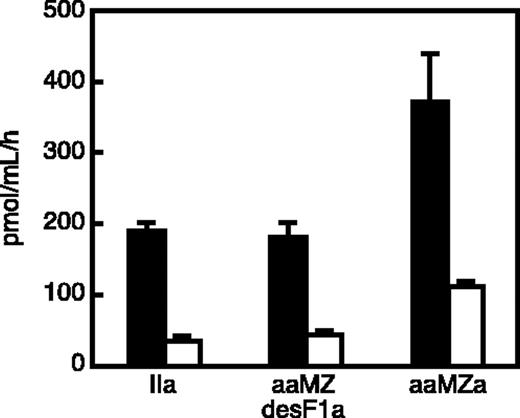
The ability of aaMZa and aaMZdesF1a to activate protein C was assessed with membrane-bound thrombomodulin (Figure 3). CV-1(18A) cells14 express full-length human thrombomodulin on the cell surface. In the presence of CV-1(18A) cells, protein C was rapidly activated by either aaMZa or aaMZdesF1a. Recombinant aaMZa was approximately 2-fold more active than an equal concentration of aaMZdesF1a or mouse thrombin. The control cell line IdE5 expresses a form of human thrombomodulin that lacks the fifth endothelial growth factor (EGF) domain and cannot bind thrombin,14 and IdE5 cells did not support protein C activation by these proteases (Figure 3). Soluble rabbit thrombomodulin also stimulated protein C activation by mouse aaMZa, aaMZdesF1a, or thrombin with approximately equal efficacy, and the reaction was dependent on calcium ions (data not shown). Therefore, the aaMZa and aaMZdesF1a species derived from aaII selectively activate the protein C anticoagulant pathway, and aaMZa was more efficient than aaMZdesF1 or thrombin when thrombomodulin was presented on a membrane surface. These properties are similar to those of aaMZa and aaMZdesF1a derived from a similar mutant human prothrombin.8
Thrombomodulin-dependent protein C activation. Samples of thrombin (IIa), meizothrombin(desF1) (aaMZdesF1a), and meizothrombin (aaMZa) were incubated for 30 minutes with protein C in the presence of membrane-associated recombinant human thrombomodulin (▪) or mutant thrombomodulin IdE5 lacking the fifth EGF domain (□). The activated protein C product was quantitated by hydrolysis of chromogenic substrate S-2366. Error bars represent standard error of the mean (SEM).
Thrombomodulin-dependent protein C activation. Samples of thrombin (IIa), meizothrombin(desF1) (aaMZdesF1a), and meizothrombin (aaMZa) were incubated for 30 minutes with protein C in the presence of membrane-associated recombinant human thrombomodulin (▪) or mutant thrombomodulin IdE5 lacking the fifth EGF domain (□). The activated protein C product was quantitated by hydrolysis of chromogenic substrate S-2366. Error bars represent standard error of the mean (SEM).
Prothrombin R157A/R268A protects against acute arterial thrombosis
Heterozygous Cf2+/- prothrombin-deficient mice were used to assess the effects of recombinant aaII on thrombosis after carotid artery injury. The level of plasma prothrombin in Cf2+/- mice is approximately 50% that of wild-type Cf2+/+ mice.15 Therefore, adequate supplementation with rrII or aaII would produce a phenotype similar to that of a mouse with a wild-type or heterozygous mutant prothrombin genotype, respectively. The blood volume of a mouse is 1 to 2 mL, and the concentration of prothrombin in human plasma is approximately 70 to 140 μg/mL. If the plasma prothrombin concentration is similar in human and mouse, then a heterozygous Cf2+/- mouse would have 35 to 70 μg total circulating prothrombin. The recovery of intravenously injected aaII was determined by Western blotting of plasma samples (Figure 4). Based on these results, approximately 50 μg of aaII approximately doubles the plasma concentration of prothrombin, achieving a level similar to that of wild-type Cf2+/+ mice. The injected aaII was not acutely toxic and the mice remained healthy for at least 2 weeks.
Detection of recombinant protein in mouse plasma. Plasma (0.1 μL) from mice of the indicated prothrombin genotype was analyzed by gel electrophoresis and Western blotting with polyclonal antimouse prothrombin antibodies. Two heterozygous Cf2+/- mice were injected with 45 μg or 110 μg of aaII 20 minutes before blood sampling. Purified rrII was included in the last lane as a size standard.
Detection of recombinant protein in mouse plasma. Plasma (0.1 μL) from mice of the indicated prothrombin genotype was analyzed by gel electrophoresis and Western blotting with polyclonal antimouse prothrombin antibodies. Two heterozygous Cf2+/- mice were injected with 45 μg or 110 μg of aaII 20 minutes before blood sampling. Purified rrII was included in the last lane as a size standard.
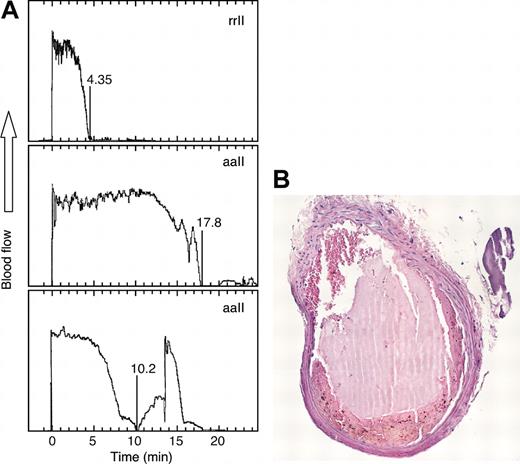
The effects of rrII and aaII on carotid artery thrombosis were compared in a model of ferric chloride-induced acute injury (Figure 5). Mice injected with rrII had total arterial occlusion after 5.3 ± 1.5 minutes (Table 2). Mice injected with aaII had a substantially longer time to occlusion of 11.8 ± 3.6 minutes (P = .006). The thrombi consisted of layers of platelets, fibrin, and red blood cells (Figure 5B), and the morphology did not differ between the mice injected with rrII and aaII. The antithrombotic effect of aaII was dose dependent with a maximal increase in the time to occlusion at doses of 50 μg and 100 μg, suggesting that aaII has a dominant inhibitory effect in vivo. The time to occlusion after injection of 25 μg aaII was significantly shorter than the time to occlusion after injection of 50 μg(P < .01). Increasing the dose to 100 μg did not further increase the time to occlusion compared with a dose of 50 μg.
Arterial occlusion after FeCl3-induced injury. (A) Heterozygous Cf2+/- mice were injected with 50 μg of rrII or aaII as indicated before carotid artery injury with FeCl3 and blood flow was recorded. (B) Cross section of a carotid artery thrombus stained with hematoxylin and eosin from a mouse injected with rrII.
Arterial occlusion after FeCl3-induced injury. (A) Heterozygous Cf2+/- mice were injected with 50 μg of rrII or aaII as indicated before carotid artery injury with FeCl3 and blood flow was recorded. (B) Cross section of a carotid artery thrombus stained with hematoxylin and eosin from a mouse injected with rrII.
Carotid artery occlusion times
Protein . | Dose, μg . | n . | Occlusion time ± SD, min . |
|---|---|---|---|
| rrll | 50 | 3 | 5.3 ± 1.5 |
| aall | 25 | 5 | 4.8 ± 1.7 |
| aall | 50 | 5 | 11.8 ± 3.6 |
| aall | 100 | 3 | 10.8 ± 2.9 |
Protein . | Dose, μg . | n . | Occlusion time ± SD, min . |
|---|---|---|---|
| rrll | 50 | 3 | 5.3 ± 1.5 |
| aall | 25 | 5 | 4.8 ± 1.7 |
| aall | 50 | 5 | 11.8 ± 3.6 |
| aall | 100 | 3 | 10.8 ± 2.9 |
Discussion
Thrombin generation usually proceeds through the intermediate MZa,4 which has potent anticoagulant activity in vitro. Compared with thrombin, MZa of either human or bovine origin has greatly reduced activity toward fibrinogen or platelets but increased ability to bind thrombomodulin and activate protein C on cell surfaces.6-8 Furthermore, kringle 2 of MZa shields the heparin binding site on the thrombin B chain17 so that MZa is resistant to inhibition by antithrombin and heparin.3,6,8 Our studies indicate that the properties of mouse aaMZa and aaMZdesF1a are similar to those reported previously for the corresponding bovine and human proteins, suggesting that their functions are shared among mammals. The biologic importance of regulating MZa, and also MZdesF1a, is supported by the conservation of the cleavage sites needed to convert them to thrombin in more distantly related vertebrates such as chicken and hagfish.18
The anticoagulant properties of MZa and MZdesF1a may increase the threshold for the full blood-clotting response by promoting sustained protein C activation when the rate of thrombin generation is relatively low. By the same mechanism, stable versions of these prothrombin activation intermediates could have antithrombotic activity in vivo, although this possibility has not been assessed previously. A mutant prothrombin might also competitively interfere with the activation of wild-type prothrombin, thereby inhibiting intravascular coagulation by an additional mechanism. In the present study, recombinant mouse aaII delayed carotid artery thrombosis in an acute injury model (Figure 5), confirming the predicted antithrombotic properties of MZa. In addition, these results suggest that a similar variant of human prothrombin could have therapeutic utility in settings where the increased activation of protein C may be beneficial.
Patients with bacterial sepsis often have acquired protein C deficiency, and mortality correlates inversely with protein C levels.19,20 A recent study also showed that heterozygous deficiency of protein C in mice was associated with increased mortality after challenge with bacterial endotoxin.21 These effects suggest that the anticoagulant and anti-inflammatory properties of activated protein C are beneficial in sepsis but can be limited by depletion of endogenous protein C. This deficiency can be bypassed by the intravenous administration of activated protein C, which is reported to improve survival and reduce the coagulopathic changes associated with bacterial sepsis in animal models22 and in human clinical trials.23 However, activated protein C also increases the incidence of serious bleeding.23
Bleeding caused by exogenous activated protein C might be circumvented by increasing the activation of endogenous protein C. For example, an anticoagulant human prothrombin similar to aaII would generate stable MZa and activate protein C mainly at local sites of ongoing blood coagulation where it may be most effective, possibly with reduced systemic effects.1 The plasma half-life of prothrombin is approximately 3 days, so that one dose of anticoagulant prothrombin may produce a therapeutic effect similar to that of a typical 3-day continuous infusion of activated protein C.23 The amount of prothrombin required may not be prohibitive. For example, approximately 4 mg prothrombin per kilogram body weight would double the plasma prothrombin concentration (≈1.5 μM), and this amount of protein is comparable to the total infusion of approximately 2.3 mg activated protein C per kilogram body weight during a course of treatment for septic shock. However, the interactions among protein C-pathway components are complex,1 and further studies will be required to address the therapeutic potential of anticoagulant prothrombins.
Prepublished online as Blood First Edition Paper, March 23, 2004; DOI 10.1182/blood-2004-02-0478.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

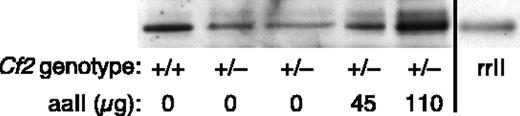
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal