Abstract
Fibrin is formed at sites of injury or inflammation and provides the temporary matrix to support vascular cell responses that are also mediated by cytokines including interleukin-1 (IL-1). We have shown previously that fibroblast growth factor 2 (FGF-2) binds with high affinity to fibrin(ogen). Because IL-1 has a structure similar to FGF-2, we have investigated the possible binding of IL-1 to fibrin(ogen). Experiments using IL-1 immobilized on Sepharose beads and soluble iodine 125 (125I)-labeled fibrinogen demonstrated no specific interaction of IL-1α with fibrinogen, but IL-1β showed saturable and specific binding. Scatchard analysis indicated a single binding site with an apparent Kd = 1.5 nM and a maximum molar binding ratio of IL-1β to fibrinogen of 1.8:1. Binding of 125I-IL-1β to Sepharose-immobilized fibrinogen also demonstrated a single binding site with an apparent Kd of 3.5 nM. IL-1β also bound specifically to fibrin monomer and polymerized fibrin with apparent Kds of 3.4 nM and 2.3 nM, respectively. IL-1β displaced FGF-2 for binding to fibrin, indicating an interaction with the same or a closely related site. Compared with free form, fibrinogen-bound IL-1β stimulated increased activation of endothelial cell nuclear factor κB (NF-κB), monocyte chemoattractant protein-1 (MCP-1) secretion, and nitric oxide (NO) synthesis. We conclude that IL-1β binds with high affinity to fibrin(ogen) and demonstrates increased activity in the bound form. (Blood. 2004; 104:409-414)
Introduction
Hemostatic activation and inflammatory responses both occur at sites of vascular injury, and coordination between these processes is needed for organized cell responses. Fibrinogen and fibrin are prominent at sites of injury, and increased vascular permeability results in movement of fibrinogen into the extracellular space where it may be converted to fibrin. Interactions of inflammatory cells with the endothelium are mediated by fibrinogen that acts as a bridge connecting endothelial cell receptors with inflammatory cells.1 Degradation products of fibrinogen and fibrin increase vascular permeability and induce chemotaxis at sites of inflammation.2,3 Fibrin that is formed at sites of injury is critical in hemostasis and also forms the provisional matrix to support cellular processes of repair. In this role, fibrin is not passive, but rather it actively directs cellular processes through specific receptor-mediated interactions to regulate permeability,4 endothelial cell adhesion,5 synthesis and secretion of tissue plasminogen activator and prostacyclin,6,7 plasminogen activator inhibitor-1,8 interleukin-8 (IL-8),9 and von Willebrand factor.10 Fibrinogen and fibrin are also implicated in the development, progression, and thrombotic complications of atherosclerosis, and they are found in increased amounts in atherosclerotic vessels.11
The vascular response to injury is also regulated by cytokines including those of the IL-1 family that are important in inflammation. The IL-1 polypeptides are pleiotropic cytokines that affect a wide variety of cells.12 IL-1α and IL-1β are 2 members that share many structural features and act on cells through the same receptors.12 However, they differ in their promoter regions, and IL-1α is primarily cell associated, whereas IL-1β is extracellular. In endothelial cells, IL-1 induces procoagulant activity,13 von Willebrand factor release,14 synthesis of plasminogen activator and plasminogen activator inhibitor-1,15 and inhibits the thrombomodulin-protein C anticoagulant pathway.16 On exposure to IL-1, endothelial cells increase synthesis of nitric oxide (NO)17 and chemoattractant cytokines18 and express the adhesion molecules intercellular adhesion molecule 1 (ICAM-1)19 and vascular cell adhesion molecule 1 (VCAM-1).20 These endothelial cell responses mediate inflammation and also influence hemostasis.
We have shown previously that fibrinogen and fibrin bind to the angiogenic growth factors fibroblast growth factor 2 (FGF-2) and vascular endothelial growth factor (VEGF)21,22 and that fibrinogen potentiates FGF-2 function23 and provides protection from proteolysis.24 IL-1α and IL-1β have structural features similar to FGF-2 including a β-barrel structure consisting of 12 β strands.25 They both are synthesized as intracellular proteins without a hydrophobic leader peptide but are released from cells and bind specific receptors that also share structural similarities in their extracellular binding domains26 with a similar mode of ligand recognition. Because of their similarities in structure to FGF-2, we hypothesized that IL-1α and IL-1β would bind to fibrin(ogen) to coordinate inflammatory and hemostatic responses at sites of injury. The results demonstrate that IL-1β but not IL-1α binds specifically and with high affinity to fibrin(ogen) and that this augments the effects of IL-1β on endothelial cells.
Materials and methods
Proteins
Plasminogen-free human fibrinogen was purchased from Enzyme Research Laboratories (South Bend, IN), and fibronectin in the preparation was depleted by chromatography on gelatin-Sepharose (Pharmacia, Piscataway, NJ). Fibrinogen eluting from the gelatin-Sepharose column was further depleted of fibronectin by immunoaffinity chromatography, and the final preparation contained less than 0.02 ng/mL of fibronectin as determined by enzyme-linked immunosorbent assay (ELISA; American Diagnostica, Greenwich, CT) at a fibrinogen concentration of 1 mg/mL. Radioiodination of fibrinogen to a specific activity of 1.9 × 108 counts per minute (cpm)/mg was performed using the iodogen method, and unbound iodine 125 (125I) was removed following chromatography on Sephadex G-10 (Pharmacia). Human thrombin (3250 NIH U/mg [National Institutes of Health Units/mg]) was obtained from Calbiochem-Novabiochem (San Diego, CA). Human recombinant IL-1α, IL-1β, and polyclonal antibody to human IL-1α and IL-1β were purchased from Peprotech (Princeton, NJ). 125I-IL-1α and 125I-IL-1β were obtained from NEN Life Science Products (Boston, MA) at a specific activity of 100 μCi/μg (3.7 MBq/μg). Purified immunoglobulin G (IgG) of monoclonal antibody J88B reactive with a site within the sequence Arg63-Met78 of the human fibrinogen γ chain was kindly provided by Dr P. J. Simpson-Haidaris (Rochester, NY).27
Binding of fibrinogen to immobilized IL-1α and IL-1β
IL-1 was immobilized on Affigel-15 beads (Biorad, Hercules, CA) as described previously.21 Briefly, 1 mL purified polyclonal IL-1α or IL-1β antibody (50 μg/mL) was incubated with 1 mL Affigel-15, which consists of a derivatized cross-linked agarose gel bead support with active N-hydroxysuccinimide esters, in 0.1 M sodium phosphate buffer (pH 7.4) containing 0.25 M NaCl and gently mixed at 25°C for 2 hours. More than 95% of the antibody was bound to the beads. Residual active ester sites were then blocked by the addition of 1 M ethanolamine (pH 8.0), and the suspension was washed several times with 0.1 M sodium phosphate buffer (pH 7.4) containing 0.25 M NaCl. IL-1α or IL-1β (10 μg/mL) was then added to this suspension and gently mixed at 25°C for 1 hour, following which unbound IL-1 was removed by washing with 0.1 M sodium phosphate buffer (pH 7.4) containing 0.25 M NaCl. The amount of IL-1α immobilized on the beads was 6.8 μg/mL and the amount of IL-1β was 7.7 μg/mL as determined by Bradford assay. For binding studies, 125I-fibrinogen at concentrations from 0.5 to 30 nM was incubated at 37°C with a 0.02-mL suspension of immobilized IL-1α or IL-1β in a final volume of 0.1 mL. Nonspecific binding was determined in concurrent parallel experiments using a 10-fold molar excess of unlabeled fibrinogen. Preliminary experiments demonstrated maximum-specific binding after 30 minutes incubation in 0.1 M sodium phosphate buffer (pH 7.4) containing 0.25 M NaCl, and these conditions were used for all subsequent experiments. Following incubation, the beads were separated by centrifugation at 5000g for 10 minutes after which the supernatant was removed, and the beads were then washed rapidly twice with 0.1 M sodium phosphate buffer (pH 7.4) containing 0.25 M NaCl at 4°C to minimize nonspecific association. The amount of bound fibrinogen was calculated from the radioactivity associated with the beads.
Binding of 125I-IL-1β and 125I-IL-1α to fibrinogen and fibrin monomer
A similar approach was used with 125I-IL-1α or 125I-IL-1β incubated with fibrinogen or fibrin monomer immobilized on Sepharose beads. Affigel-15 beads were first incubated with 1 mg/mL purified monoclonal J88B IgG in 0.2 M sodium bicarbonate buffer (pH 8.3) and gently mixed at 25°C for 2 hours. Residual sites were blocked by incubation in 1 M ethanolamine (pH 8.0), and the suspension was washed several times with 0.2 M sodium bicarbonate buffer (pH 8.3) containing 0.25 M NaCl. Gel with bound antibody was then incubated with 200 μg/mL fibrinogen in sodium phosphate buffer; it was then washed with sodium phosphate buffer (pH 7.4) containing 0.25 M NaCl to remove unbound fibrinogen. This was continued until no further fibrinogen was removed as determined by monitoring the optical density at 280 nm. To convert bound fibrinogen to fibrin monomer, beads were incubated with 0.5 U/mL thrombin at 37°C for 90 minutes. Characterization of binding of 125I-IL-1α or 125I-IL-1β to fibrin monomer was performed in the same way as 125I-fibrinogen binding to immobilized IL-1α or IL-1β. 125I-IL-1α or 125I-IL-1β at concentrations from 0.1 to 100 nM was incubated with a 0.02-mL suspension of beads containing 0.1 μg fibrin in a final volume of 0.1 mL. Nonspecific binding was determined in concurrent parallel experiments using a 10-fold molar excess of unlabeled IL-1α or IL-1β. Specificity of the binding of IL-1β to fibrin was confirmed by competition experiments in which 0.1 nM of 125I-IL-1β was incubated with 1 μg/mL immobilized fibrin monomer in a final volume of 0.1 mL, and the binding was competitively inhibited by unlabeled IL-1β at concentrations from 0.1 to 100 nM.
Binding of IL-1α or IL-1β to polymerized fibrin
125I-IL-1α or 125I-IL-1β at concentrations of 0.1 to 100 nM was added to 100 μg/mL fibrinogen in 0.1 M Tris buffer (pH 7.4) containing 0.25 M NaCl. Thrombin was then added to a final concentration of 0.5 U/mL, which resulted in clotting of the solution. Following incubation at 37°C for 30 minutes, the clot and supernatant were separated by vacuum filtration using GF/C glass microfiber filters (Sigma, St Louis, MO) previously soaked overnight in a solution of 0.5% polyvinyl pyrolidone and 0.1% Tween-20 to reduce nonspecific binding. The clot on the filter was washed quickly with cold 0.1 M Tris buffer (pH 7.4) containing 0.25 M NaCl, and associated radiolabel was measured. Nonspecific binding was determined in parallel experiments incorporating a 10-fold molar excess of unlabeled IL-1α or IL-1β.
Activation of nuclear factor κB (NF-κB)
Human umbilical vein endothelial cells (HUVECs) were grown in McCoy medium (Flow Laboratories, McLean, VA) supplemented with 10 mM l-glutamine, 20% fetal bovine serum (FBS; HyClone Laboratories, Logan, UT), and 100 U/mL of penicillin and streptomycin (GIBCO BRL, Grand Island, NY). Cells were passaged by detaching with 0.05% trypsin and maintained at 37°C with 5% CO2. For experiments, cells were used at passage 2 and were plated to achieve 80% to 90% confluence after 3 to 5 days in culture. Hirudin (0.5 U/mL; Sigma) was added to inhibit thrombin present in serum. HUVECs were incubated with 2 ng/mL IL-1β in the presence or absence of 1 μg/mL fibrinogen for 2 hours. Lipopolysaccharide (LPS; 1 μg/mL) was used as a positive control.
Preparation of nuclear extracts
Nuclear proteins were extracted as described previously.28 In brief, cells were scraped, washed twice in cold phosphate-buffered saline (PBS), and resuspended in 20 μL buffer A (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.9; 10 mM KCl; 1.5 mM MgCl2; 0.5 mM dithiothreitol [DTT]; and 0.5 mM phenylmethylsulfonyl fluoride [PMSF]) and allowed to swell on ice for 20 minutes. The nuclei were separated from the cell lysates by centrifugation at 10 000g for 5 minutes. The nuclear pellet was resuspended in 20 μL extraction buffer (20 mM HEPES, pH 7.9; 420 mM NaCl; 1.5 mM MgCl2; 0.2 mM ethylenediaminetetraacetic acid [EDTA]; 25% glycerol; 0.5 mM PMSF; and 0.5 mM DTT) and incubated for 5 to 10 minutes on ice. The samples were then centrifuged at 9000g for 5 minutes at 4°C. The supernatant was mixed with 30 μL dilution buffer (20 mM HEPES, pH 7.9; 50 mM KCl; 0.5 mM DTT; 0.5 mM EDTA; 20% glycerol; 0.2 mM PMSF). The nuclear extracts, thus obtained, were assayed for protein using the Bradford assay method. Aliquots of the extracts were stored at -70°C for subsequent use in gel-shift assays.
Electrophoretic mobility shift assay (EMSA)
Gel-shift assays were performed as described previously.28 A double-stranded oligonucleotide containing the consensus DNA-binding site for the NF-κB proteins (5′AGTTGAGGGGACTTTCCCAGGC 3′) was end-labeled using γ-32P-adenosine triphosphate (ATP) according to the manufacturer's protocol (Promega, Madison, WI) and purified using a Sephadex G-25 column (Pharmacia). The binding assay was performed as previously described.28 Labeled probe was mixed with 3 μg nuclear protein extract and incubated at 37°C for 20 minutes, following which the samples were electrophoresed on a 5% nondenaturing acrylamide gel at 100 V for 3 hours. The gels were dried, and bands were detected by autoradiography. Gel scanning was used to quantify and analyze changes in band intensities.
Measurement of NO
HUVEC monolayers were exposed to IL-1β (10 ng/mL) with or without fibrinogen (10 μg/mL) for 1 hour. Medium was then collected, and NO concentration was measured as nitrite using the Nitrate/Nitrite colorimetric assay kit (Cayman Chemicals, Ann Arbor, MI). Acetylcholine (3 mM; Sigma) was used as a positive control, and L-NAME (N(omega)-nitro-l-arginine methyl ester) (3 mM; Sigma) was used to inhibit NO synthesis.
Data analysis
Unless indicated otherwise, data are expressed as mean ± SD. Scatchard analysis of the data was performed using the Ligand program29 from Biosoft (Ferguson, MO). Each experiment was performed at least 3 times, and the significance of differences in means was determined using a 2-tailed Student t test.
Results
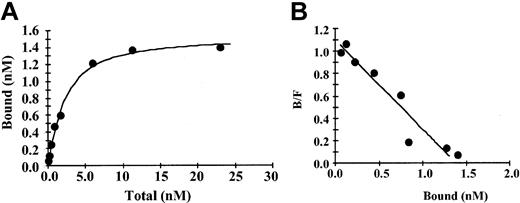
Binding of fibrinogen to immobilized IL-1β was saturable and specific with nonspecific binding representing less than 20% of the total (Figure 1A). Saturation of specific binding occurred at a fibrinogen concentration of approximately 10 nM, and an increase in nonspecific binding was observed at higher concentrations. In control experiments over the same range of concentrations there was a maximum of 5% binding of 125I-fibrinogen to beads with immobilized anti-IL-1β IgG only or to beads with no protein bound and active sites blocked with ethanolamine. A plot of bound versus bound/free fibrinogen (Figure 1B) was linear, suggesting the presence of a single binding site. This was confirmed by Scatchard analysis, which indicated that binding was best described by a single site model with an apparent Kd of 1.5 nM. Bmax (maximum specific binding) was 1.4 nM, and the maximum molar binding ratio of IL-1β to fibrinogen was 1.8:1. Using the same method, we observed no specific binding of IL-1α with fibrinogen (data not shown).
Binding of fibrinogen to immobilized IL-1β (A) 125I-fibrinogen was incubated with IL-1β immobilized on Sepharose beads, and the amount of bound protein was determined as radioactivity associated with the beads following centrifugation and washing. Nonspecific binding was determined in the same way in the presence of a 10-fold molar excess of unlabeled IL-1β. Specific binding was calculated by subtracting the nonspecific from the total bound. Each point represents the mean of 3 different experiments. (B) Scatchard plot. The best fit of the data was determined by analysis using the Ligand program29 and suggests a single binding site.
Binding of fibrinogen to immobilized IL-1β (A) 125I-fibrinogen was incubated with IL-1β immobilized on Sepharose beads, and the amount of bound protein was determined as radioactivity associated with the beads following centrifugation and washing. Nonspecific binding was determined in the same way in the presence of a 10-fold molar excess of unlabeled IL-1β. Specific binding was calculated by subtracting the nonspecific from the total bound. Each point represents the mean of 3 different experiments. (B) Scatchard plot. The best fit of the data was determined by analysis using the Ligand program29 and suggests a single binding site.
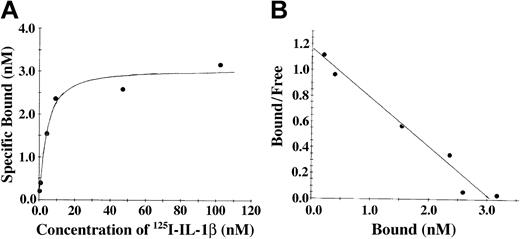
The association of IL-1β and fibrinogen was also characterized using soluble 125I-radiolabeled IL-1β and fibrinogen immobilized on Sepharose beads (Figure 2). With this system, saturable and specific binding was also observed, and nonspecific binding represented 20% or less of the total. Scatchard analysis (Figure 2B) indicated the presence of a single binding site with apparent Kd of 3.5 nM, and a maximum molar binding ratio of IL-1β to fibrinogen was 2:1. We observed no specific binding of IL-1α with fibrinogen using this system (data not shown).
The association of IL-1β and fibrinogen characterized using soluble 125I-radiolabeled IL-1β and fibrinogen immobilized on Sepharose beads. (A) Binding of IL-1β to fibrinogen. 125I-IL-1β was incubated with fibrinogen immobilized on Sepharose beads, and the amount of bound protein was determined as radioactivity associated with the beads following centrifugation and washing. Nonspecific binding was determined in the same way in the presence of a 10-fold molar excess of unlabeled IL-1β. Specific binding was calculated by subtracting the nonspecific from the total bound. (B) Scatchard plot. The best fit of the data was determined by analysis using the Ligand program29 and suggests a single binding site.
The association of IL-1β and fibrinogen characterized using soluble 125I-radiolabeled IL-1β and fibrinogen immobilized on Sepharose beads. (A) Binding of IL-1β to fibrinogen. 125I-IL-1β was incubated with fibrinogen immobilized on Sepharose beads, and the amount of bound protein was determined as radioactivity associated with the beads following centrifugation and washing. Nonspecific binding was determined in the same way in the presence of a 10-fold molar excess of unlabeled IL-1β. Specific binding was calculated by subtracting the nonspecific from the total bound. (B) Scatchard plot. The best fit of the data was determined by analysis using the Ligand program29 and suggests a single binding site.
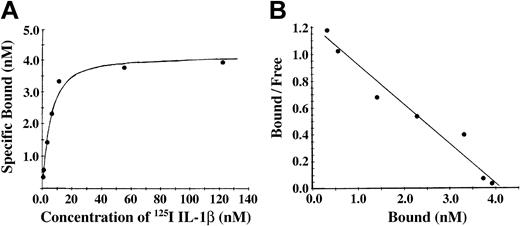
Conversion of fibrinogen to fibrin is mediated by thrombin, forming fibrin monomer, which then polymerizes to form a branching network of fibers. To characterize the association of IL-1β with fibrin, we incubated Sepharose-immobilized fibrinogen with thrombin. The antibody J88B mediated the immobilization of fibrinogen to the Sepharose beads, thereby limiting polymerization of the fibrin and forming a surface with immobilized fibrin monomer. Binding of 125I-IL-1β to fibrin monomer was found to be of high affinity with specific binding approaching saturation at a concentration of approximately 20 nM IL-1β (Figure 3A). Nonspecific binding was less than 20% at IL-1β concentrations below 100 nM and increased at higher concentrations. A plot of bound versus bound-free ligand was linear (Figure 3B), suggesting the presence of a single binding site, and this was confirmed by Scatchard analysis indicating the presence of one binding site with apparent Kd of 3.4 nM and Bmax of 4.1 nM and a maximum molar binding ratio of IL-1β to fibrin of 2.2:1. Using this method, we observed no specific binding of IL-1α with fibrin (data not shown).
Binding of 125I-IL-1β to fibrin monomer was found to be of high affinity. (A) Binding of IL-1β to fibrin monomer. Fibrinogen was immobilized on Sepharose beads and then converted to fibrin monomer by incubation with thrombin. 125I-IL-1β was incubated with the beads, and bound and free ligands were then separated by centrifugation. Nonspecific binding was measured in the presence of a 10-fold molar excess of unlabeled IL-1β, and specific binding was determined by subtraction of nonspecific from total binding. Each point represents the mean of 3 different experiments. (B) Scatchard plot. The best fit of the data was determined using the Ligand program29 and indicated the presence of single binding site.
Binding of 125I-IL-1β to fibrin monomer was found to be of high affinity. (A) Binding of IL-1β to fibrin monomer. Fibrinogen was immobilized on Sepharose beads and then converted to fibrin monomer by incubation with thrombin. 125I-IL-1β was incubated with the beads, and bound and free ligands were then separated by centrifugation. Nonspecific binding was measured in the presence of a 10-fold molar excess of unlabeled IL-1β, and specific binding was determined by subtraction of nonspecific from total binding. Each point represents the mean of 3 different experiments. (B) Scatchard plot. The best fit of the data was determined using the Ligand program29 and indicated the presence of single binding site.
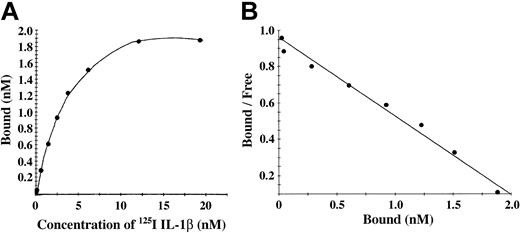
Characterization of binding to polymerized fibrin presents technical and interpretive problems because transport of IL-1α and IL-1β into the gel may be slow or incomplete, and access to potential binding sites within individual fibrin fibers may also be restricted. We chose, therefore, to add 125I-IL-1β or 125I-IL-1α to a solution of fibrinogen, which was then clotted with thrombin to limit problems of transport of IL-1 into a preformed gel. Total binding was measured with this clotting system in the absence of unlabeled competitor, whereas nonspecific binding was measured in the presence of 10-fold molar access of unlabeled competitor. No specific binding of IL-1α with fibrin was observed, whereas IL-1β showed a saturable and specific binding to polymerized fibrin (Figure 4A). A plot of bound versus bound-free 125I-IL-1β was linear (Figure 4B), and Scatchard analysis indicated a single binding site with an apparent Kd of 2.3 nM.
IL-1β showed a saturable and specific binding to polymerized fibrin. (A) Binding of 125I-IL-1β to polymerized fibrin. 125I-IL-1β was added to a solution of 100 μg/mL fibrinogen and clotted by the addition of 0.5 U/mL thrombin. Bound and unbound IL-1β were then separated by vacuum filtration, and nonspecific binding was determined in the presence of 10-fold molar excess of IL-1β. Specific binding was calculated by subtracting nonspecific from total binding. Each point represents the mean of 3 different experiments. (B) Scatchard plot. The best fit of the data was determined using the Ligand program29 and indicated the presence of single binding site.
IL-1β showed a saturable and specific binding to polymerized fibrin. (A) Binding of 125I-IL-1β to polymerized fibrin. 125I-IL-1β was added to a solution of 100 μg/mL fibrinogen and clotted by the addition of 0.5 U/mL thrombin. Bound and unbound IL-1β were then separated by vacuum filtration, and nonspecific binding was determined in the presence of 10-fold molar excess of IL-1β. Specific binding was calculated by subtracting nonspecific from total binding. Each point represents the mean of 3 different experiments. (B) Scatchard plot. The best fit of the data was determined using the Ligand program29 and indicated the presence of single binding site.
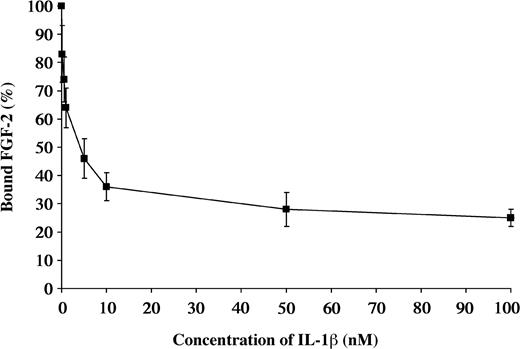
Due to the similarities in the structures of IL-1β and FGF-2, we performed competitive binding studies to determine if the same or related sites were involved. 125I-FGF-2 at a concentration of 0.2 nM was incubated with Sepharose-immobilized fibrin. Radioactivity associated with the beads after washing was defined as 100%. Varying concentrations of unlabeled IL-1β from 0.1 to 100 nM were then added to characterize the dissociation. The binding of 125I-FGF-2 was progressively reduced with increasing concentrations of unlabeled IL-1β (Figure 5).
Competitive inhibition of FGF-2 binding by IL-1β 125I-FGF-2 was incubated with the fibrinogen immobilized on Sepharose beads. Increasing concentrations of unlabeled IL-1β were used to competitively inhibit the binding of 125I-FGF-2 to fibrinogen. Each point represents mean ± SD of 3 separate experiments.
Competitive inhibition of FGF-2 binding by IL-1β 125I-FGF-2 was incubated with the fibrinogen immobilized on Sepharose beads. Increasing concentrations of unlabeled IL-1β were used to competitively inhibit the binding of 125I-FGF-2 to fibrinogen. Each point represents mean ± SD of 3 separate experiments.
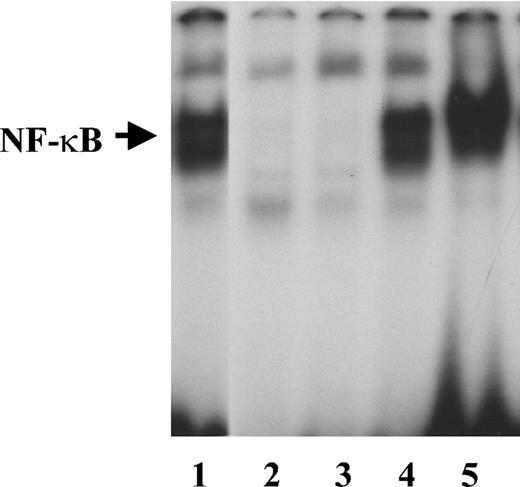
IL-1β induces NF-κB activity in endothelial cells.30 To determine whether it retains its activity when bound to fibrinogen, we measured NF-κB activation in HUVECs cultured for 2 hours in medium containing 2 ng/mL IL-1β in the presence or absence of 1 μg/mL fibrinogen. Fibrinogen alone did not cause activation of NF-κB as measured by EMSA (Figure 6). Gel scanning showed a 30.2-fold increase in NF-κB activation in the presence of IL-1β alone, which increased further to 43.4-fold with fibrinogen and IL-1β compared with medium alone.
NF-κB activation by free or fibrinogen-bound IL-1β Endothelial cells were plated on gelatin-coated wells in McCoy 5A medium supplemented with 20% FBS, 50 μg/mL endothelial cell growth supplement (ECGS), and 100 μg/mL heparin and grown to confluence. The cells were then washed twice with McCoy medium and incubated with 2 ng/mL of IL-1β in the presence or absence of 1 μg/mL fibrinogen for 2 hours. Nuclear protein extracts were then prepared, and EMSA was performed using a double-stranded 32P-labeled consensus NF-κB primer. LPS (1 μg/mL) was used as a positive control. Lanes are as follows: 1, LPS; 2, medium alone; 3, fibrinogen; 4, IL-1β; and 5, fibrinogen plus IL-1β.
NF-κB activation by free or fibrinogen-bound IL-1β Endothelial cells were plated on gelatin-coated wells in McCoy 5A medium supplemented with 20% FBS, 50 μg/mL endothelial cell growth supplement (ECGS), and 100 μg/mL heparin and grown to confluence. The cells were then washed twice with McCoy medium and incubated with 2 ng/mL of IL-1β in the presence or absence of 1 μg/mL fibrinogen for 2 hours. Nuclear protein extracts were then prepared, and EMSA was performed using a double-stranded 32P-labeled consensus NF-κB primer. LPS (1 μg/mL) was used as a positive control. Lanes are as follows: 1, LPS; 2, medium alone; 3, fibrinogen; 4, IL-1β; and 5, fibrinogen plus IL-1β.
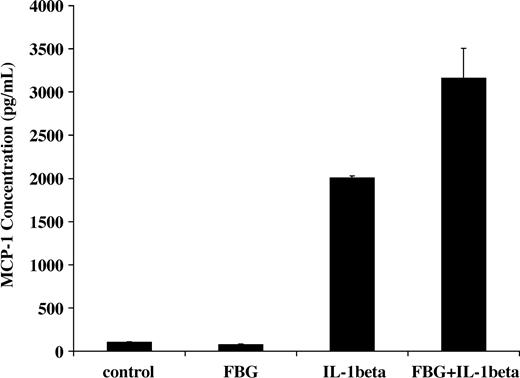
NF-κB activation induced by IL-1β in endothelial cells results in increased transcription of inflammatory mediators including monocyte chemoattractant protein-1 (MCP-1).31 Therefore, we measured the capacity of fibrinogen and IL-1β to stimulate MCP-1 secretion. IL-1β (2 ng/mL) was added to the culture medium in the presence or absence of 1 μg/mL fibrinogen, and MCP-1 concentration was measured by ELISA after 2 hours. The concentration with medium plus fibrinogen was 75 ± 7 pg/mL, and this was comparable to medium alone (101 ± 4 pg/mL). As expected, IL-1β significantly increased MCP-1 secretion (2005 ± 23 pg/mL), but the combination of both fibrinogen and IL-1β resulted in a further significant increase in MCP-1 in the medium to 3166 ± 340 pg/mL (P < .05; Figure 7)
Induction of MCP-1 secretion by fibrinogen-bound IL-1β Endothelial cells were plated on gelatin-coated wells in McCoy 5A medium supplemented with 20% FBS, 50 μg/mL ECGS, and 100 μg/mL heparin and allowed to reach confluence. The cells were washed twice with McCoy medium and incubated with 2 ng/mL of IL-1β in the presence or absence of 1 μg/mL fibrinogen for 2 hours. The medium was then collected, and MCP-1 was measured by ELISA. The results shown are the mean ± SD of 3 separate experiments.
Induction of MCP-1 secretion by fibrinogen-bound IL-1β Endothelial cells were plated on gelatin-coated wells in McCoy 5A medium supplemented with 20% FBS, 50 μg/mL ECGS, and 100 μg/mL heparin and allowed to reach confluence. The cells were washed twice with McCoy medium and incubated with 2 ng/mL of IL-1β in the presence or absence of 1 μg/mL fibrinogen for 2 hours. The medium was then collected, and MCP-1 was measured by ELISA. The results shown are the mean ± SD of 3 separate experiments.
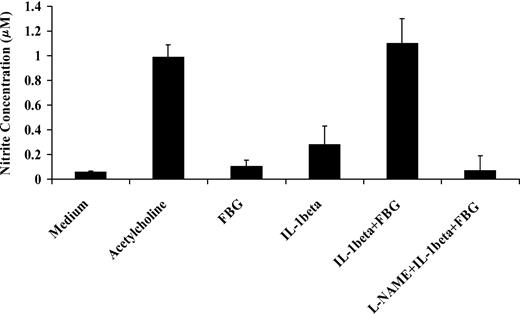
IL-1β activates NO synthase and increases NO secretion by endothelial cells.32 To determine the activity of IL-1β in the presence of fibrinogen, we exposed confluent HUVEC monolayers to IL-1β (10 ng/mL) with or without fibrinogen (10 μg/mL) for 1 hour. Medium was then collected, and the NO concentration in the medium was measured as nitrite levels using the Griess reaction. Fibrinogen alone did not induce NO production, but IL-1β caused a 4.5- ± 1.2-fold increase in NO production compared with medium (P < .03). Compared with IL-1β alone, however, fibrinogen and IL-1β stimulated 3.8- ± 0.6-fold greater secretion of endothelial cell NO (P < .002; Figure 8). This effect was comparable to the response with 3 mM acetycholine, a potent inducer of NO production. L-NAME, an inhibitor of NO synthase, completely blocked the effect. These results indicate that fibrinogen augmented the capacity of IL-1β to stimulate NO synthesis in endothelial cells.
Effect of fibrinogen on IL-1β induced nitric oxide synthesis by endothelial cells. Confluent HUVEC monolayers were exposed to IL-1β (10 ng/mL) with or without fibrinogen (10 μg/mL) for 1 hour. Medium was then collected, and NO production was measured as nitrite concentration in the medium using the Griess reaction. Acetylcholine (3 mM) was used as a positive control, and L-NAME (3 mM) was used to inhibit NO synthesis. The results shown are mean ± SD of 3 separate experiments.
Effect of fibrinogen on IL-1β induced nitric oxide synthesis by endothelial cells. Confluent HUVEC monolayers were exposed to IL-1β (10 ng/mL) with or without fibrinogen (10 μg/mL) for 1 hour. Medium was then collected, and NO production was measured as nitrite concentration in the medium using the Griess reaction. Acetylcholine (3 mM) was used as a positive control, and L-NAME (3 mM) was used to inhibit NO synthesis. The results shown are mean ± SD of 3 separate experiments.
Discussion
The results demonstrate that IL-1β binds specifically and with high affinity to fibrinogen and fibrin, whereas IL-1α does not. The apparent Kds were similar for fibrinogen, fibrin monomer, and polymerized fibrin with a maximal molar binding ratio of approximately 2:1 for each. Considering that fibrinogen is a dimerically symmetric molecule, the presence of a single binding site is consistent with the presence of one site on each half-molecule. We have shown previously that the angiogenic growth factors VEGF and FGF-2 also bind to fibrinogen and fibrin with high affinity, each demonstrating 2 high-affinity sites per fibrinogen molecule.21,22 FGF-1 showed no affinity,33 similar to the findings with IL-1α. Competition for binding to fibrinogen indicates that FGF-2 and IL-1β are likely to interact with the same or related sites. The site on FGF-2 that interacts with fibrinogen has been localized by mutational analysis to 5 residues from Phe95 to Arg109.34 Characterization of changes in binding of FGF-2 to fibrinogen during plasmic degradation suggests that the interactive site may be localized in the carboxyl-terminal region of the γ chain.35 Further studies will be required to fully characterize the sites involved in the IL-1β-fibrinogen interaction.
The significance of IL-1β binding to fibrinogen must be considered in relation to both its tissue distribution and also the availability of other sites for binding. Notably, IL-1α, which showed no affinity for fibrinogen, is primarily a cell-associated cytokine.12 IL-1β is normally present in blood with a plasma concentration of less than 0.6 pM, and, at a normal plasma fibrinogen concentration of approximately 7 μM and a Kd of 1.5 nM, nearly all IL-1β would be fibrinogen bound in the absence of other binding proteins. However, IL-1β also binds to other plasma proteins, including α2 macroglobulin, with which it interacts slowly, forming a covalent bond that inhibits receptor interaction.36 A specific IL-1β-binding protein was previously identified in human plasma, and it did not interact with IL-1α,37 similar to the pattern of fibrinogen binding presented. This protein was covalently cross-linked with IL-1β and had a molecular mass (Mr) of 43 kDa under reducing conditions, and this is consistent with that of the 46-kDa fibrinogen γ chain.35 IL-1β also binds to endothelial cells through IL-1 receptor (IL-R1) and exhibits Kds of 4.0 pM and 0.7 nM for high- and low-affinity interactions.38,39 Fibrin forms at sites of thrombosis, tissue injury, and inflammation, and IL-1β binding may serve to localize the activation of immune responses. In support of this concept, Perez and Roman40 have shown that fibrin colocalizes with IL-1β in lung granulomas using immunohistochemical methods.
In addition to localizing its activity, binding to fibrin(ogen) modifies the cellular effects of IL-1β. Our results show that fibrinogen-bound IL-1β not only retains activity but also has an increased capacity to activate NF-κB and to stimulate MCP-1 secretion and NO synthesis. These endothelial effects of IL-1β are mediated through binding to IL-R1,41 and the mechanisms responsible for augmented activity with fibrinogen could be coordinated at the receptor level. Endothelial cells interact with surface-immobilized fibrinogen to support adherence, but the fibrinogen-binding integrins are also expressed on the free, nonadherent cell surface.42,43 There are several other examples that demonstrate the importance of interactions between growth factors and integrins. For example, ligand-mediated integrin clustering induces aggregation of FGFR44 and stimulates phosphorylation of platelet-derived growth factor-β (PDGF-β) receptors.45 Schneller et al46 reported that highly tyrosine phosphorylated PDGF-β and insulin receptors coimmunoprecipitated with αvβ3. PDGFβ and αVβ3 coimmunoprecipitate in endothelial cells after activation and demonstrate synergistic stimulation.47 Rusnati et al48 demonstrated a direct interaction of surface-immobilized FGF-2 with αvβ3 that could mediate adhesion, mitogenesis, and urokinase-type plasminogen activator (u-PA) up-regulation. Tanghetti et al49 found that FGFR and αvβ3 cooperate in endothelial cell signaling, and combined growth factor and integrin activation may be required for sustaining prolonged cell activation.49 Together, this evidence indicates the specific interactions of adhesive proteins and that growth factor receptors mediate coordinated effects.
Fibrin(ogen) is always present in blood and extracellular fluids, and the high-affinity binding to IL-1β suggests that it will serve to both localize activity and act as a cofactor to augment cellular effects. We have shown that fibrinogen increases IL-1β-induced NF-κB activation and secretion of MCP-1 and NO. In addition, Perez et al50 demonstrated that fibrinogen stimulated production of IL-1β message and protein by binding to CD18 on human monocytes, and this was confirmed using the monocytic cell line U937. Further, fibrin suppresses production of IL-1Ra in blood cells.40 Together, these findings demonstrate several levels of coordination between the hemostatic and inflammatory systems mediated through fibrinogen and IL-1.
Prepublished online as Blood First Edition Paper, March 23, 2004; DOI 10.1182/blood-2004-01-0126.
Supported by grant HL-30616 from the National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank LiHua Rong for expert technical assistance in culturing primary endothelial cells and Jennifer Jodeksnis for excellent technical assistance. The assistance of Dawn Goetze in preparing this manuscript is acknowledged gratefully.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal