Abstract
The current World Health Organization (WHO) classification of hematopoietic malignancies defines several types of mature T-cell leukemia including T-cell prolymphocytic leukemia (T-PLL), Sezary syndrome (SS), and T-cell large granular lymphocytic (T-LGL) leukemia. These neoplasms can show overlapping features with each other and with T-cell lymphomas involving peripheral blood (PB). We analyzed the spectrum of clinicopatho-logic features in 102 mature T-cell leukemias and compared them to 10 hepatosplenic T-cell lymphomas that involved PB. T-PLL, defined as a T-cell leukemia showing rapidly rising PB lymphocyte counts, was the only tumor type expressing the oncoprotein TCL1 (71% of cases) and could present with relatively low lymphocyte levels or small tumor cell morphology. SS, defined by accompanying erythrodermic skin disease, was frequently associated with peripheral eosinophilia but could also develop high numbers of prolymphocytes, especially late in the disease course. T-LGL leukemia, defined by accompanying cytopenias or autoimmune phenomena (or both), had the best clinical outcome and generally showed the lowest circulating lymphocyte levels with only a few cases developing marked lymphocytosis. Using the dominant clinical or phenotypic feature, we describe here the degree of overlap among currently recognized WHO categories and identify areas where further clarification is needed. Our results indicate that incorporation of additional criteria, such as TCL1 expression status and hematologic parameters, can assist in a more accurate classification. (Blood. 2004;104:328-335)
Introduction
The World Health Organization (WHO) classification of hematopoietic and lymphoid tumors recognizes 5 types of mature T-cell tumors that commonly involve the peripheral blood (PB) and bone marrow at presentation.1 Of these, only adult T-cell leukemia/lymphoma (ATLL), a tumor defined by infection with human T-cell lymphotropic viruses (HTLV), has a single defining diagnostic criterion, but it is extremely rare in the United States.
The entity of T-cell prolymphocytic leukemia (T-PLL) currently encompasses most T-cell leukemias formerly diagnosed as T-cell chronic lymphocytic leukemia (CLL)2 as well as those cases with prolymphocytoid morphology.3-5 Most cases categorized as T-PLL have a CD4+CD8- T-cell immunophenotype but CD4+CD8+ and CD4-CD8+ tumors are also commonly seen.5 The majority of T-PLL tumors show chromosomal rearrangements involving chromosome 14 that transcriptionally activate the T-cell leukemia-1 (TCL1) gene at 14q32 through juxtaposition of T-cell receptor (TCR) enhancer/promoter sequences.4 However, absence of TCL1 chromosomal rearrangements have been reported by some groups in a substantial number of leukemias classified as T-PLL.6,7
Sezary syndrome (SS) is a T-cell tumor defined by the presence of generalized erythroderma and lymphadenopathy, with tumor cells usually having an irregular or cerebriform nuclear morphology.8 Almost all such tumors have a CD4+ T-cell immunophenotype. Several studies have suggested that some cases of SS may overlap morphologically with T-PLL.9 The current WHO classification does not provide criteria to distinguish leukemias that present initially with erythroderma (primary SS or Sezary leukemia)10 from secondary SS that represents extracutaneous progression of mycosis fungoides (MF) or define the levels of PB tumor involvement required for diagnosis. Few studies have addressed the significance of these different patterns or the degree of disease in SS.11,12
Large granular lymphocyte (LGL) leukemia is usually defined as an indolent lymphoproliferative disorder composed of lymphocytes with cytoplasmic cytotoxic granules. LGL leukemia can be derived from natural killer cells, CD8+ T cells, or less commonly CD4+ T cells.13 LGL leukemia is frequently associated with autoimmune phenomena and suppression of hematopoiesis resulting in peripheral neutropenia with or without anemia. The clinical spectrum of T-cell LGL (T-LGL) includes polyclonal and oligoclonal proliferations and the severity of symptoms and clinical outcome have been shown to be related both to tumor cell immunophenotype and comorbid conditions. T-LGL leukemia cases that express CD8 and γ/δ TCR overlap immunophenotypically with hepatosplenic T-cell lymphoma (HSTCL), which also frequently involves the bone marrow and PB at low levels. Variant cases of HSTCL expressing the α/β TCR are also well described.14
We and others15 have encountered a number of cases of leukemic T-cell tumors with overlapping clinical and pathologic features. In this study, we systematically examine the degree of heterogeneity among HTLV- mature T-cell tumors presenting in PB. Our approach is to base the diagnosis on the dominant presenting clinical feature and then assess the patterns of heterogeneity observed in regard to presenting features, disease course, treatment response, and clinical outcome.
Patients, materials, and methods
Patients and diagnostic groups
This study includes patients with T-PLL, SS, and T-LGL leukemia seen at The University of Texas M.D. Anderson Cancer Center between 1996 and early 2003 and was performed in accordance with an M.D. Anderson Cancer Center institutional review board-approved protocol and with the provisions of the Helsinki protocol. Patients signed the institutional consent forms for use of tissue for research. We included patients with fully characterized mature T-cell lymphoproliferative disorders diagnosed in PB or bone marrow or both. All patients were serologically negative for HTLV-I/II, and 5 cases of ATLL seen during this period were excluded. Only cases with full clinical presenting features, PB data, and a T-cell flow cytometric panel were included. For example, 38 patients with T-LGL leukemia were seen during this period, but complete clinical and diagnostic information or follow-up was available in only 15 cases.
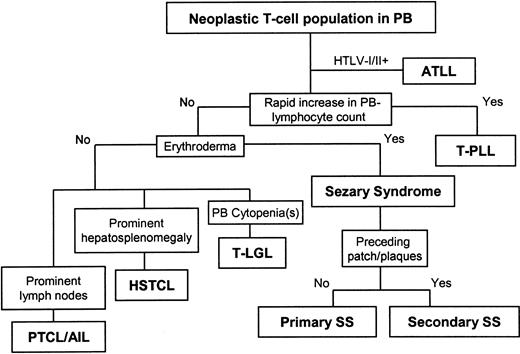
We used the classification scheme shown in Figure 1, assigning the diagnosis on each case based on the dominant clinical finding at presentation. By flow cytometry analysis, all cases had an immunophenotypically discrete T-cell tumor population in PB that represented at least 200 tumor cells/μL.16 Thirty-eight cases with rapidly rising white blood cell (WBC) counts (ie, doubling over a 1-year period or less) were diagnosed as T-PLL. T-cell tumors in PB that presented with erythroderma were diagnosed as primary SS (32 cases) and those with antecedent patch or plaque MF (or both) before PB spread were classified as secondary SS (17 cases). Cases with associated cytopenias or identifiable autoimmune phenomena or both were diagnosed as T-LGL leukemia (15 cases). For comparison, we also included 10 cases classified as HSTCL based on the presence of splenomegaly or hepatomegaly (or both) out of proportion to the level of PB involvement. Tumors with predominantly lymph node-based disease and low levels of PB tumor involvement were classified as nodal peripheral T-cell lymphoma (PTCL) and not further studied here.
Algorithm for classification of T-cell neoplasms involving peripheral blood. Cases were classified by sequential application of the most prominent presenting feature, beginning with adult T-cell leukemia-lymphoma (ATLL, excluded from study), T-cell prolymphocytic leukemia (T-PLL), primary or secondary Sezary syndrome (SS), T-cell large granular lymphocyte (T-LGL) leukemia, and hepatosplenic T-cell lymphoma (HSTCL). Nodal peripheral T-cell lymphoma (PTCL) and angioimmunoblastic T-cell lymphoma (AIL) involving peripheral blood (PB) were not included in this analysis.
Algorithm for classification of T-cell neoplasms involving peripheral blood. Cases were classified by sequential application of the most prominent presenting feature, beginning with adult T-cell leukemia-lymphoma (ATLL, excluded from study), T-cell prolymphocytic leukemia (T-PLL), primary or secondary Sezary syndrome (SS), T-cell large granular lymphocyte (T-LGL) leukemia, and hepatosplenic T-cell lymphoma (HSTCL). Nodal peripheral T-cell lymphoma (PTCL) and angioimmunoblastic T-cell lymphoma (AIL) involving peripheral blood (PB) were not included in this analysis.
Immunophenotyping
T-cell lineage required expression of surface CD3, as assessed by flow cytometry, or the presence of a clonal T-cell receptor (TCR) gene rearrangement by Southern blot or polymerase chain reaction analysis. We used a standard T-cell antigen panel (CD3, CD4, CD5, CD7, CD8) to assess the immunophenotype of tumor cells by flow cytometry in 189 PB samples from the 102 study patients, including more than one sample from 53 of the patients. TCR-α/β and TCR-γ/δ expression levels were assessed in a subset of cases. Cluster analysis was used to identify tumor cells by their variations in the expression level of analyzed markers as compared to the admixed nonneoplastic T cells.16 In cases diagnosed after the year 2000, we have also used uniform absence or positivity for CD26 staining to aid in identification of the neoplastic T-cell populations.17
Immunohistochemical stains for TCL1 were performed on formalinfixed, paraffin-embedded tissue sections of skin or bone marrow aspirate clot or core biopsy specimens. Following heat-induced epitope retrieval with citrate buffer solution (pH 6.0), slides were incubated with polyclonal TCL1 antisera (gift from Dr M. Teitell, University of California, Los Angeles) for 2 hours at room temperature and then developed using the avidin-biotin-peroxidase complex method (LSAB+ kit; Dako, Carpinteria, CA), with 3-3′diaminobenzidine (Dako) as the chromogenic substrate. Slides were counterstained with Mayer hematoxylin. Photomicrographs were taken using a Nikon Labophot 2 microscope (Nikon, Melville, NY) with an Olympus DP11 camera (Olympus, Melville, NY). Pictures were assembled into composites using Adobe Photoshop 7.0 software (Adobe, San Jose, CA). Western blot analysis for TCL1 protein was performed on a subset of cases, as previously described.18 Briefly, tumor cells were isolated by density centrifugation (Histopaque; Sigma, St Louis, MO) and cell lysates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot using TCL1 polyclonal antisera (1:15 000 dilution), secondary antirabbit IgG horseradish peroxidase (HRP)-linked antisera, and the enhanced chemiluminescence (ECL) detection system (Amersham Biosciences, Piscataway, NJ).
Statistical analysis
The Kruskal-Wallis test was used to detect differences in medians of clinical or phenotypic features between all diagnostic groups, with the Fisher protected least significant difference (LSD) test used to assign P values. We used the Mann-Whitney U test for comparisons between 2 groups, and P values assigned using the χ2 test. Overall survival (OS) was measured from the time of pathologic diagnosis to the time of last follow-up or death. Actuarial survival was determined using Kaplan-Meier analysis with censoring of patients lost to follow-up. Differences in OS between the diagnostic groups were estimated by Cox proportional hazard regression.
Results
Demographics
Table 1 compares the demographic data and presenting features of cases classified as T-PLL, primary SS, secondary SS, and T-LGL leukemia, respectively. The majority of patients with T-PLL, SS, and T-LGL leukemia presented in their sixth to eighth decades with no significant differences in median age or sex. Patients with T-PLL, primary and secondary SS as well as T-LGL leukemia were significantly older than those diagnosed with HSTCL (median ages of 63, 61, 62, 53 versus 47 years, respectively, P = .037). Excluding secondary SS, 62% (53 of 85) of the leukemias and 60% (6 of 10) of HSTCLs were untreated at the time of presentation to our institution.
Clinical and hematologic features of mature T-cell leukemias
. | T-PLL; n = 38 . | Primary SS; n = 32 . | Secondary SS; n = 17 . | T-LGL leukemia; n = 15 . | P . |
|---|---|---|---|---|---|
| Median age, y (range) | 63 (41-83) | 61 (37-84) | 62 (30-80) | 53 (24-86) | .26 |
| No. male patients (%) | 19 (50) | 19 (59) | 7 (41) | 10 (67) | .44 |
| No. previously untreated (%) | 19 (50) | 17 (53) | 2 (12) | 11 (73) | .3‡ |
| Median mo of prior symptoms (range) | 2 (0-34) | 24 (0-120) | 38 (0-444) | 9 (1-168) | < .001 |
| Median presenting lymphocyte count, × 109/L (range) | 31.8 (4.7-614.8) | 3.8 (0.2-68.3) | 2.0 (0.5-33.8) | 2.5 (0.35-32.9) | < .001 |
| Median lymphocytes as percentage of total PB WBCs at presentation (range) | 82.5 (43-98) | 40.5 (6-93) | 27.0 (5-86) | 64.0 (52-90) | < .001 |
| Median peak lymphocyte count, × 109/L (range) | 171.3 (20.9-762.0) | 5.9 (0.6-121.9) | 5.7 (1.1-33.9) | 3.6 (0.88-94.1) | < .001 |
| Median lymphocytes as percentage of total PB WBCs at peak (range) | 93 (60-99) | 43 (5-99) | 33 (12-89) | 74 (54-99) | < .001 |
| Median presenting PB neutrophils, × 109/L (range) | 5.2 (0.0-32.7) | 3.8 (0.74-12.0) | 4.3 (0.8-11.1) | 0.65 (0.0-3.5) | < .001 |
| No. with neutropenia at presentation (%)* | 7 (18) | 4 (12) | 2 (12) | 12 (80) | < .001 |
| Median pretreatment PB eosinophils, × 109/L (range) | 0 (0.0-1.1) | 0.81 (0.03-14.6) | 0.7 (0.0-20.6) | 0.14 (0.0-0.9) | < .001 |
| No. with pretreatment PB eosinophilia (%)† | 5 (13) | 21 (66) | 9 (53) | 2 (13) | < .001 |
| No. with palpable lymph nodes at diagnosis (%) | 19 (50) | 20 (63) | 14 (82) | 1 (7) | .0019 |
| No. with hepatosplenomegaly at diagnosis (%) | 25 (66) | 3 (9) | 0 (0) | 8 (53) | < .001 |
| No. with skin findings at diagnosis (%) | 10 (26) | 32 (100) | 17 (100) | 4 (27) | < .001 |
| No. with presence of effusions at diagnosis (%) | 8 (21) | 1 (3) | 0 (0) | 0 (0) | .01 |
| Survival | < .001 | ||||
| No. deaths from disease (%) | 29 (76) | 10 (31) | 10 (59) | 1 (7) | |
| Median OS, mo | 29.5 | NA | 60 | NA | |
| 3-year survival rate, % | 46 | 73 | 64 | 100 |
. | T-PLL; n = 38 . | Primary SS; n = 32 . | Secondary SS; n = 17 . | T-LGL leukemia; n = 15 . | P . |
|---|---|---|---|---|---|
| Median age, y (range) | 63 (41-83) | 61 (37-84) | 62 (30-80) | 53 (24-86) | .26 |
| No. male patients (%) | 19 (50) | 19 (59) | 7 (41) | 10 (67) | .44 |
| No. previously untreated (%) | 19 (50) | 17 (53) | 2 (12) | 11 (73) | .3‡ |
| Median mo of prior symptoms (range) | 2 (0-34) | 24 (0-120) | 38 (0-444) | 9 (1-168) | < .001 |
| Median presenting lymphocyte count, × 109/L (range) | 31.8 (4.7-614.8) | 3.8 (0.2-68.3) | 2.0 (0.5-33.8) | 2.5 (0.35-32.9) | < .001 |
| Median lymphocytes as percentage of total PB WBCs at presentation (range) | 82.5 (43-98) | 40.5 (6-93) | 27.0 (5-86) | 64.0 (52-90) | < .001 |
| Median peak lymphocyte count, × 109/L (range) | 171.3 (20.9-762.0) | 5.9 (0.6-121.9) | 5.7 (1.1-33.9) | 3.6 (0.88-94.1) | < .001 |
| Median lymphocytes as percentage of total PB WBCs at peak (range) | 93 (60-99) | 43 (5-99) | 33 (12-89) | 74 (54-99) | < .001 |
| Median presenting PB neutrophils, × 109/L (range) | 5.2 (0.0-32.7) | 3.8 (0.74-12.0) | 4.3 (0.8-11.1) | 0.65 (0.0-3.5) | < .001 |
| No. with neutropenia at presentation (%)* | 7 (18) | 4 (12) | 2 (12) | 12 (80) | < .001 |
| Median pretreatment PB eosinophils, × 109/L (range) | 0 (0.0-1.1) | 0.81 (0.03-14.6) | 0.7 (0.0-20.6) | 0.14 (0.0-0.9) | < .001 |
| No. with pretreatment PB eosinophilia (%)† | 5 (13) | 21 (66) | 9 (53) | 2 (13) | < .001 |
| No. with palpable lymph nodes at diagnosis (%) | 19 (50) | 20 (63) | 14 (82) | 1 (7) | .0019 |
| No. with hepatosplenomegaly at diagnosis (%) | 25 (66) | 3 (9) | 0 (0) | 8 (53) | < .001 |
| No. with skin findings at diagnosis (%) | 10 (26) | 32 (100) | 17 (100) | 4 (27) | < .001 |
| No. with presence of effusions at diagnosis (%) | 8 (21) | 1 (3) | 0 (0) | 0 (0) | .01 |
| Survival | < .001 | ||||
| No. deaths from disease (%) | 29 (76) | 10 (31) | 10 (59) | 1 (7) | |
| Median OS, mo | 29.5 | NA | 60 | NA | |
| 3-year survival rate, % | 46 | 73 | 64 | 100 |
Neutropenia was defined as absolute neutrophil count (ANC) less than 1800 neutrophils/μL peripheral blood.
Eosinophilia was defined as absolute eosinophil count (AEC) greater than 0.5 × 103 eosinophils/μL peripheral blood.
Comparison did not include secondary SS because MF patients were nearly always treated prior to leukemic spread.
Comparison of presenting features
Among T-PLL, 14 patients (37%) were initially diagnosed following complete blood count (CBC) done at time of routine examination or during workup for conditions such as pneumonia, gastrointestinal bleed, chest pain, and prostate carcinoma. The other 63% of T-PLL patients reported symptoms more likely related to leukemia for a median of 2 months prior to diagnosis, which included weight loss or increasing fatigue in 12, fever/night sweats in 3, palpable lymphadenopathy in 3, shortness of breath in 3, and skin edema or bruising in 3 patients. This short prodromal period in T-PLL differed significantly from that seen in patients with primary and secondary SS (median, 24 and 38 months, respectively) and T-LGL leukemia (median, 9 months; P < .001). Similar to T-PLL, patients with HSTCL also had a comparably short symptomatic phase before diagnosis (median, 2.5 months).
By definition, all patients with primary SS presented with increasing erythroderma over the course of weeks to months. In cases with detailed histories, patients usually had localized erythroderma that initially spread locally or became generalized over a period of several months. Nine patients with primary SS had intense pruritus for months to several years before diagnosis. In 4 patients, erythroderma was preceded by long periods of xerosis and scaling.
The T-LGL leukemia group showed the most variability in presenting signs and symptoms, with the symptomatic phase prior to diagnosis ranging from 1 month to 14 years. The average prodromal period was 37.0 months (SD ± 13.0) and included arthritis in 7, recurrent infections in 7, and fatigue in 6 patients. Single patients with T-LGL leukemia had documented histories of ulcerative colitis, lupus erythematosus, and psoriasis. One patient showed a Coombs-positive hemolytic anemia, one patient had polyclonal gammopathy, and one patient had elevated rheumatoid arthritis factor.
Sites of involvement noted on initial examination
Hepatosplenomegaly, noted usually by physical examination, was the characteristic feature of HSTCL (9 of 10 cases, 90%). Within the study group (Table 1) this feature was significantly more common at presentation in T-PLL (66%) and T-LGL leukemia (53%) than in patients with primary or secondary SS (P < .001). In contrast, palpable peripheral lymphadenopathy at presentation (usually axillary or inguinal) was more common in primary and secondary SS (63% and 82%, respectively) than in T-PLL (50%) and far less frequent in T-LGL leukemia (7%).
Skin lesions were present at initial examination in 10 of 38 (26%) T-PLL patients with 4 showing edema, 3 showing maculopapular lesions, and one each showing focal erythroderma, excoriation, and bruising. Three patients with T-LGL leukemia had petechial skin lesions and one had dermal edema. In addition to generalized erythroderma, 11 of the patients with primary SS also had palmar or plantar keratoderma on initial examination.
Presenting peripheral blood counts
T-PLL, primary and secondary SS as well as T-LGL leukemia differed significantly with respect to presenting PB lymphocyte counts (medians and ranges are given in Table 1). In T-PLL, counts were overall lower in patients diagnosed at an asymptomatic stage following incidental PB analysis. In primary SS, 3 cases presented with counts higher than 30 × 109/L (30 000/μL). The median count for PB lymphoma cells at presentation in HSTCL was 1.5 × 109/L (1500/μL). Absolute PB eosinophilia (> 0.5 × 109/L [> 500/μL]) was seen in 66% of primary SS cases (median eosinophil count, 0.81 × 109/L [810/μL]) with a comparable prevalence only found in patients with secondary SS (53%; median eosinophil count, 0.7 × 109/L [700/μL]). In contrast, eosinophilia was seen in only 13% of cases of each T-PLL and T-LGL leukemia, a difference that was statistically significant (P < .001).
T-LGL leukemia cases usually (80%) presented with neutropenia, which, however, was also seen in 18% of T-PLL and in 50% of HSTCL cases. Also, T-LGL leukemia presented with multiple cytopenias more often than other mature T-cell leukemias, including pancytopenia in 6, anemia in 7, thrombocytopenia in 1, and generalized leukopenia in 1 patient. Although characterized by low absolute counts, PB lymphocyte percentages in T-LGL leukemia at presentation and over the course of disease were higher than in all non-T-PLL cases.
Tumor cell morphology and pattern of bone marrow involvement
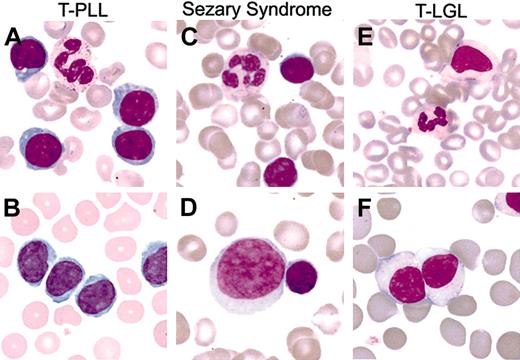
Table 2 summarizes tumor cell morphology and pattern of bone marrow involvement. Although T-PLL usually contained classical nucleolated prolymphocytes (Figure 2A), there was a considerable proportion of cases with the predominance of small lymphocytes or tumor cells with cerebriform nuclear contours (Figure 2B). The primary SS cases revealed the greatest morphologic variability, including classical cases with cerebriform nuclei (Figure 2C) and those showing a predominance of large prolymphocytoid forms (Figure 2D). The range of bone marrow infiltration patterns in T-PLL and SS, as assessed on core biopsies, was similar (Table 2). T-LGL leukemia (Figure 2E-F) much more frequently showed benign, lymphoid aggregates rich in B cells (11 of 15 cases) as well as interstitial tumor cell infiltrates. None of the cases showed a predominantly intrasinusoidal pattern of infiltration, which was otherwise characteristic for HSTCL (6 of 10, 60%; with the other 4 cases showing an interstitial to diffuse pattern).
Morphologic and immunophenotypic features of mature T-cell leukemias
. | T-PLL; n = 38 . | Primary SS; n = 32 . | T-LGL leukemia; n = 15 . |
|---|---|---|---|
| Tumor cell morphology, no. (%) | |||
| Mostly prolymphocytoid | 20 (53) | 7 (22) | 1 (7) |
| Mostly small cerebriform | 8 (21) | 14 (44) | 2 (14) |
| Mixed small and large forms | 10 (26) | 11 (34) | 12 (80) |
| Cytoplasmic granules | 3 (8) | 4 (13) | 12 (80) |
| Pattern in bone marrow biopsy, no. (%) | |||
| Nodular | 0 (0) | 1/30 (3) | 1 (7)† |
| Nodular/interstitial | 5 (13) | 6/30 (20) | 10 (67)† |
| Interstitial | 30 (79) | 23/30 (77) | 4 (26) |
| Diffuse | 3 (8) | 0/30 (0) | 0 (0) |
| Sinusoidal | 0 (0) | 0/30 (0) | 0 (0) |
| Tumor immunophenotype by flow cytometry, no. (%) | |||
| CD4+ CD8− | 24 (63) | 30 (94) | 2 (13) |
| CD4+ CD8+ | 12 (32) | 1 (3) | 0 (0) |
| CD4− CD8+ | 2 (5) | 0 (0) | 13 (87) |
| CD4− CD8− | 0 (0) | 1 (3) | 0 (0) |
| CD5+ | 38 (100) | 18/18 (100) | −/dim 9/12 (75) |
| CD7+ | 35/37 (95) | 19/31 (61) | 10/13 (77) |
| CD26+ | 20/26 (77) | 1/32 (3) | 2/11 (18) |
| TCR-α/β+ | 12/14 (86)‡ | 4/4 (100) | 12/14 (86) |
| TCR-γ/δ+ | 0/14 (0)‡ | 0/2 (0) | 2/14 (14) |
| TCL1 expression* | 27/38 (71) | 0/17 (0) | 0/5 (0) |
. | T-PLL; n = 38 . | Primary SS; n = 32 . | T-LGL leukemia; n = 15 . |
|---|---|---|---|
| Tumor cell morphology, no. (%) | |||
| Mostly prolymphocytoid | 20 (53) | 7 (22) | 1 (7) |
| Mostly small cerebriform | 8 (21) | 14 (44) | 2 (14) |
| Mixed small and large forms | 10 (26) | 11 (34) | 12 (80) |
| Cytoplasmic granules | 3 (8) | 4 (13) | 12 (80) |
| Pattern in bone marrow biopsy, no. (%) | |||
| Nodular | 0 (0) | 1/30 (3) | 1 (7)† |
| Nodular/interstitial | 5 (13) | 6/30 (20) | 10 (67)† |
| Interstitial | 30 (79) | 23/30 (77) | 4 (26) |
| Diffuse | 3 (8) | 0/30 (0) | 0 (0) |
| Sinusoidal | 0 (0) | 0/30 (0) | 0 (0) |
| Tumor immunophenotype by flow cytometry, no. (%) | |||
| CD4+ CD8− | 24 (63) | 30 (94) | 2 (13) |
| CD4+ CD8+ | 12 (32) | 1 (3) | 0 (0) |
| CD4− CD8+ | 2 (5) | 0 (0) | 13 (87) |
| CD4− CD8− | 0 (0) | 1 (3) | 0 (0) |
| CD5+ | 38 (100) | 18/18 (100) | −/dim 9/12 (75) |
| CD7+ | 35/37 (95) | 19/31 (61) | 10/13 (77) |
| CD26+ | 20/26 (77) | 1/32 (3) | 2/11 (18) |
| TCR-α/β+ | 12/14 (86)‡ | 4/4 (100) | 12/14 (86) |
| TCR-γ/δ+ | 0/14 (0)‡ | 0/2 (0) | 2/14 (14) |
| TCL1 expression* | 27/38 (71) | 0/17 (0) | 0/5 (0) |
Determined by Western blot or immunohistochemistry or both.
Four of these 11 cases showed an exclusively interstitial pattern in subsequent biopsy specimens.
Two analyzed cases did not show expression of surface TCR.
Typical and variant PB tumor cell morphology among mature T-cell leukemias. (A) T-PLL with typical prolymphocytoid morphology. (B) T-PLL with tumor cells having cerebriform nuclear morphology. (C) SS with typical cerebriform tumor morphology. (D) SS with large transformed nucleolated tumor cells (large cell, left) associated with a minor population of lower-grade tumor cells with cerebriform morphology (small cell, right). (E) T-LGL leukemia with monocytoid appearance. (F) T-LGL leukemia with high white blood cell count and variably nucleolated tumor cells. Original magnification, × 500 (Nikon Plan 50 0.5-0.85 oil lens).
Typical and variant PB tumor cell morphology among mature T-cell leukemias. (A) T-PLL with typical prolymphocytoid morphology. (B) T-PLL with tumor cells having cerebriform nuclear morphology. (C) SS with typical cerebriform tumor morphology. (D) SS with large transformed nucleolated tumor cells (large cell, left) associated with a minor population of lower-grade tumor cells with cerebriform morphology (small cell, right). (E) T-LGL leukemia with monocytoid appearance. (F) T-LGL leukemia with high white blood cell count and variably nucleolated tumor cells. Original magnification, × 500 (Nikon Plan 50 0.5-0.85 oil lens).
Immunophenotypic features
Expression of CD4 in the absence of CD8 was the most frequent phenotype in T-PLL (63%) and primary or secondary SS cases (94% each). A proportion of 32% of T-PLL showed coexpression of CD4 and CD8. The CD4-CD8+ immunophenotype was predominant in T-LGL leukemia. Whereas tumor cells in all cases of T-PLL and primary SS showed CD5 expression, the neoplastic cells in the majority of T-LGL were dimly positive or negative for CD5. Although expression of TCR-γ/δ was seen in 6 (60%) of 10 cases diagnosed as HSTCL, it was also seen in 2 (14%) of 14 cases classified as T-LGL leukemia but not in any case diagnosed as T-PLL or SS. Expression of CD7 was most common in tumor cells of T-PLL and less often but frequently found in the other categories (Table 2; 8 [47%] of 17 in secondary SS and 6 [67%] of 9 in HSTCL). The majority of cases of T-PLL (77%) showed CD26+ tumor cells, whereas this was infrequent in all other categories (Table 2; 3 [18%] of 17 in secondary SS and 0 of 3 in HSTCL).
TCL1 expression
Western blot analysis using a polyclonal TCL1 antiserum revealed TCL1 expression in 10 of 13 T-PLL cases, 0 of 2 primary SS cases, and in 0 of 1 T-LGL leukemia case. Immunostaining on paraffin sections confirmed TCL1 expression seen by Western blot. Overall, TCL1 protein was detected in 27 (71%) of 38 T-PLL, 0 of 17 primary SS, 0 of 13 secondary SS, 0 of 5 T-LGL leukemia, and 0 of 4 HSTCL.
Course of disease
The median peak PB lymphocyte count in T-PLL cases over the course of disease was 171.3 × 109/L (171 300/μL) (Figure 3). The doubling time of PB lymphocytes in T-PLL ranged from 2 weeks to 4 months in the period immediately prior to treatment. In contrast (Figure 3), the median peak PB lymphocyte counts in primary SS, T-LGL leukemia, and HSTCL were 5.9 × 109/L (5900/μL), 3.6 × 109/L (3600/μL), and 2.4 × 109/L (2400/μL), respectively. PB lymphocyte counts of most primary SS and T-LGL leukemias were relatively stable before initial treatment and in therapy-free intervals. However, 2 patients with primary SS developed rapidly rising lymphocyte counts (exceeding 100 × 109/L [100 000/μL] in each case) in the terminal phase in a pattern similar to T-PLL. The PB lymphocytes in both of these cases were predominantly prolymphocytoid forms.
Comparison of peak absolute lymphocyte counts among categories of leukemic mature T-cell tumors. Shown are peak levels of PB lymphocytosis that developed over the course of disease among the 5 groups of T-cell leukemia/lymphoma. The boxes around the median represent the 10th and 90th percentiles of values graphed on a logarithmic scale. The whiskers cover the range of minimum and maximum values. Patients with T-PLL developed significantly higher absolute lymphocyte counts (median, 171.3 × 109/L [171.3 × 103/μL]) compared with the other categories.
Comparison of peak absolute lymphocyte counts among categories of leukemic mature T-cell tumors. Shown are peak levels of PB lymphocytosis that developed over the course of disease among the 5 groups of T-cell leukemia/lymphoma. The boxes around the median represent the 10th and 90th percentiles of values graphed on a logarithmic scale. The whiskers cover the range of minimum and maximum values. Patients with T-PLL developed significantly higher absolute lymphocyte counts (median, 171.3 × 109/L [171.3 × 103/μL]) compared with the other categories.
The development of effusions was significantly more common in T-PLL (37%) as compared with non-T-PLL cases (SS and T-LGL leukemia combined, 17%, P = .02). Even at time of presentation, pleural or pericardial effusions were common in T-PLL (8 of 38, 21%) but were rare (1 of 32, 3%) in primary SS or absent in both secondary SS and T-LGL leukemia (P = .01). On progression, 6 additional T-PLL patients developed effusions, which were also seen in 5 primary SS (19%), 3 secondary SS (18%), and 2 T-LGL leukemias (13%). Development of skin findings on disease progression was also relatively common in T-PLL. In addition to the 10 patients with initial skin findings, 4 T-PLL patients developed new skin lesions, including erythroderma in 3 and a diffuse maculopapular eruption in 1.
Degree of overlap in disease features
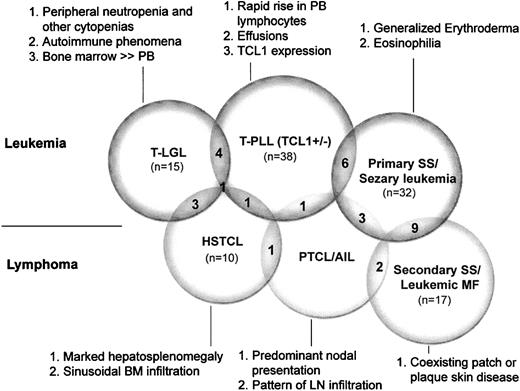
Figure 4 illustrates the degree of diagnostic overlap indicating cases in which the primary feature of 2 different categories was each present in a given tumor. Other features represent the most helpful secondary parameters in assigning a definitive diagnosis to overlap cases. For example, 4 cases classified as T-PLL based on rapidly rising PB lymphocyte counts developed maculopapular or localized erythrodermic skin rashes that resembled SS, and 2 cases presenting with erythroderma that were classified as primary SS had high PB lymphocyte counts with a predominance of larger prolymphocytoid forms as is typically seen in T-PLL.
Overlap in primary presenting features among leukemic T-cell tumors and secondary features that assist in more definitive classification. The number of overlapping cases is given in the convex, shaded areas between the overlapping circles, with each overlap case manifesting the primary feature from both categories. Indicated are the primary (no. 1) as well as secondary features (nos. 2 and 3) identified in this study that correspond to particular WHO diagnostic categories. Abbreviations are explained in the legend to Figure 1.
Overlap in primary presenting features among leukemic T-cell tumors and secondary features that assist in more definitive classification. The number of overlapping cases is given in the convex, shaded areas between the overlapping circles, with each overlap case manifesting the primary feature from both categories. Indicated are the primary (no. 1) as well as secondary features (nos. 2 and 3) identified in this study that correspond to particular WHO diagnostic categories. Abbreviations are explained in the legend to Figure 1.
The overlap between T-PLL and T-LGL leukemia encompassed several different types of cases. A single case presenting with extensive marrow involvement by a CD4+ T-cell tumor with prolymphocytoid morphology also had a very low PB lymphocyte count (0.0005 × 109/L [0.5/μL]) and pancytopenia as seen in T-LGL leukemias. On the other hand, another patient presented with splenomegaly, high PB lymphocyte count (48 × 109/L [48 000/μL]), and intermediate-sized lymphocytes with some cytoplasmic granules that shared features of T-LGL leukemia and T-PLL.
Cases of T-PLL, primary SS, and HSTCL that raised the differential diagnosis of nodal PTCL with PB involvement showed lymphocytosis or hepatosplenomegaly or both but also had generalized lymphadenopathy. The largest diagnostic overlap occurred between cases of primary and secondary SS where distinguishing the pattern of skin disease prior to systemic dissemination (ie, erythroderma versus patch/plaque disease) was difficult.
Response to treatment
The range of treatments used for T-PLL and responses observed are summarized in Table 3.19,20 In all but 5 patients with T-PLL, therapy was not initiated until PB lymphocyte counts were above 100 × 109/L (100 000/μL) and included both single-agent and multiagent regimens. Therapeutic response to an agent was usually readily assessable in T-PLL and SS by observing the precipitous drop in PB lymphocyte counts within several days of initiation of treatment. There was usually variable response of any given tumor to different agents; for example, 9 patients who responded to either alemtuzumab (Campath), arabinosyl guanine (compound 506U), or cyclophosphamide/fludarabine regimens were resistant to at least one of the other 2 regimens. The 2 most long-standing complete responses (up to 36 months) occurred after alemtuzumab monotherapy and after allogeneic stem cell transplantation, respectively.
Summary of treatments used for T-PLL
Therapeutic agent . | No. of patients treated . | Overall response, % . |
|---|---|---|
| Alemtuzumab | 22 | 64 |
| 2-deoxycoformycin (2-DCF, pentostatin) | 6 | 0 |
| 2-chlorodeoxyadenosine (2-CDA) | 10 | 33 |
| Fludarabine | 5 | 40 |
| Arabinosyl guanine | 12 | 58 |
| Regimens with alkylating agents and fludarabine | 11 | 46 |
| Regimens with alkylating agents and anthracycline | 12 | 50 |
Therapeutic agent . | No. of patients treated . | Overall response, % . |
|---|---|---|
| Alemtuzumab | 22 | 64 |
| 2-deoxycoformycin (2-DCF, pentostatin) | 6 | 0 |
| 2-chlorodeoxyadenosine (2-CDA) | 10 | 33 |
| Fludarabine | 5 | 40 |
| Arabinosyl guanine | 12 | 58 |
| Regimens with alkylating agents and fludarabine | 11 | 46 |
| Regimens with alkylating agents and anthracycline | 12 | 50 |
Twenty-eight of the patients with primary SS were treated for variable periods by photopheresis with addition of systemic interferon or bexarotene if remission did not occur within 6 months.21,22 Other regimens given in more than 2 patients included psoralen/UV-A in 12, retinoids/receptor ligands in 21, pentostatin in 8, corticosteroids in 7, multiagent chemotherapeutic regimens in 13, gemcitabine in 6, interferon in 13, denileukin diftitox in 6, cyclosporine in 3, alemtuzumab in 3, and allogeneic PB stem cell or bone marrow transplantation in 4. The spectrum of treatment in secondary SS was comparable to the one in primary SS with a higher rate of systemic single or multiagent, conventional chemotherapies (13 of 17, 76%) including allogeneic PB stem cell transplantation in 2 patients.
The initial therapeutic strategy in cases classified as T-LGL leukemia was largely aimed at improving the cytopenias and included growth factors (eg, granulocyte-macrophage colony-stimulating factor [GM-CSF] or erythropoietin) in 7 patients. Subsequent treatments included cyclosporine in 5, splenectomy in 4, systemic corticosteroids in 4, γ-globulin in 2, and polychemotherapy with alkylating agents or purine analogues in 3. Symptomatic improvement and partial resolution of cytopenias was achieved in 83% of patients, often for prolonged periods. A confirmed complete remission was obtained in a 24-year-old patient after allogeneic stem cell transplantation. Two patients who did not receive any therapy were alive with disease 6.0 and 49 months after diagnosis.
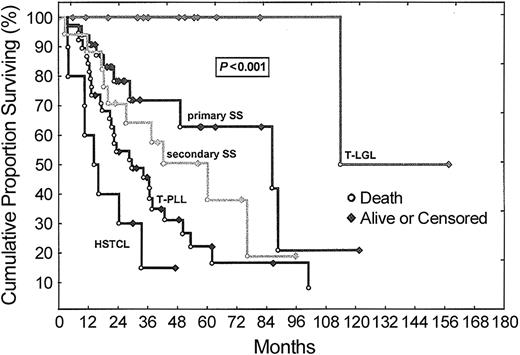
Comparison of OS
Follow-up times were calculated from the time of initial diagnoses and did not differ between categories. There were no deaths due to unknown or disease-unrelated causes. Figure 5 displays a Kaplan-Meier analysis of OS, which showed statistically significant differences (P < .001) between all tumor groups (Table 1). In T-PLL, there was no statistically significant difference in outcomes between cases that were positive or negative for the oncoprotein TCL1 (not shown). After a maximum follow-up of 158 months, 94% of the patients with T-LGL leukemia were still alive, with (80%) or without (14%) evidence of disease (median follow-up for T-LGL leukemia was 48.7 months). The only observed death among T-LGL leukemia patients occurred after 113.9 months. Finally, 8 (80%) of 10 patients with HSTCL died, resulting in a median OS of 16.0 months and a 3-year OS rate of 17%.
OS according to WHO category among leukemic T-cell tumors. Kaplan-Meier analysis demonstrates a significant difference in disease-specific OS between patients with T-PLL, primary SS, secondary SS, T-LGL leukemia and HSTCL (P = .0002). Ten of 32 patients with primary SS and 1 of 15 patients with T-LGL leukemia had died at last follow-up but some follow-up times were short compared with OS, precluding calculation of a meaningful median survival. Abbreviations are explained in Figure 1.
OS according to WHO category among leukemic T-cell tumors. Kaplan-Meier analysis demonstrates a significant difference in disease-specific OS between patients with T-PLL, primary SS, secondary SS, T-LGL leukemia and HSTCL (P = .0002). Ten of 32 patients with primary SS and 1 of 15 patients with T-LGL leukemia had died at last follow-up but some follow-up times were short compared with OS, precluding calculation of a meaningful median survival. Abbreviations are explained in Figure 1.
To assess for selection bias due to referral patterns, we performed a separate analysis only on those patients who were previously untreated at the time of presentation to our institution (Table 1; 6 of 10 [60%] patients with HSTCL were untreated). Patients with leukemic stage of MF (secondary SS) were almost exclusively pretreated (15 of 17, 88%) and therefore not included in this subanalysis. The observed difference in OS between the groups (P = .01) was similar to that seen in the overall series (P < .001). Only in T-PLL did the previously untreated patients have a slightly longer median survival (30 months versus 22 months) and higher 3-year OS rate (50% versus 42%) than the pretreated group. However, this difference was not significant in the log-rank statistics (P = .4).
Discussion
Using the dominant presenting clinical feature for classification of leukemic T-cell tumors, we assessed how the current WHO diagnostic categories match the clinical spectrum of tumors we have observed at our institution. We took this approach because, with the exception of HTLV-I/II seropositivity, currently no defining molecular or pathogenetic criteria are included in the WHO scheme for the diagnosis of mature T-cell leukemia types. Using this classification approach, we were then able to estimate the degree of disease variability present both within and between each WHO category and to identify the most helpful secondary diagnostic features for each tumor type, as summarized in Figure 4. This analysis uncovers considerable overlap between certain diagnostic categories and identifies areas where further work on classification needs to be focused.
The entity that is closest to having a uniform molecular abnormality that could be used for classification purposes is T-PLL, which frequently shows overexpression of the kinase coactivator TCL1 due to chromosomal rearrangements,23 with TCL1 expression not found in other T-cell tumor types or in nonneoplastic mature T cells. We found, using rapidly rising PB lymphocyte counts as the classification criterion, that 71% of T-PLL cases showed expression of TCL1, whereas tumors in the other categories lacked TCL1 protein. Thus defined, T-PLL cases were associated with higher presenting lymphocyte counts and had more frequent splenomegaly compared with primary or secondary SS. The presence of malignant effusions or dermal edema at presentation were helpful (although not specific) secondary diagnostic features in T-PLL. Although prolymphocytoid tumor cell morphology was more common in T-PLL, it was also seen in SS and less commonly in T-LGL leukemia.
We also note that approximately 50% of TCL1+ T-PLL were diagnosed incidentally, in some cases when PB lymphocyte counts were barely elevated. Furthermore, all but one T-PLL case eventually terminated in a phase of exponential growth with lymphocyte doubling times of several days to weeks. This fits with the pattern of leukemogenesis seen in TCL1 transgenic mice where T-cell tumors develop only after long latency.24,25 Both of these findings also suggest that secondary transforming events subsequent to TCL1 deregulation are required before development of overt leukemia.
We did not identify obvious differences in the pattern of disease presentation, tumor spread, or OS between TCL1+ T-PLL and the 29% of T-PLL cases that lacked TCL1 expression. As suggested by previous investigators, we did find a number of cases of TCL1+ T-PLL that had small cell morphology and would have been previously classified as T-CLL.4 There were, however, also cases of TCL1- small cell T-PLL, indicating that morphologic criteria alone cannot be used to subdivide this category. We did note a slightly higher number of cases with skin infiltration in the TCL1- T-PLL group, suggesting a possible relationship of these cases to SS.
Regardless of TCL1 status, nearly all cases of T-PLL had an aggressive clinical course. However, the heterogeneous response of TCL1+ T-PLL to different therapeutic agents suggests that the secondary transforming events may differ widely among individual T-PLL tumors. Thus, although TCL1 activation is the primary initiating event in most T-PLL cases, dissection of the subsequent oncogenic events will likely be necessary to define prognostic groups and identify therapy-response predictors.
We separately analyzed SS that arose in patients with longstanding patch/plaque MF (secondary) as compared to those that presented with rapidly progressive erythroderma (primary). The presence of PB eosinophilia (eosinophil count > 0.5 × 109/L [> 500/μL]) was a highly characteristic and diagnostically useful feature seen in both primary and secondary SS but not other disease categories. The category of primary SS has been referred to as Sezary cell leukemia in some previous publications and encompasses cases most likely to be confused with T-PLL.9,26 Here, using erythroderma as the distinguishing neoplastic feature, primary SS tumor cells more often showed cerebriform nuclear morphology and had more frequent and prominent palpable lymphadenopathy than T-PLL and T-LGL leukemia. However, some cases of T-PLL had prominent peripheral lymphadenopathy and prominent skin findings (usually edema) but only rarely erythroderma.27 The majority of SS cases showed at least a minor population of large nucleolated tumor cells in PB and could have prominent prolymphocytoid tumor cell morphology, especially late in the disease course. Primary and secondary SS (as well as T-PLL) also overlap diagnostically with peripheralized PTCL in patients with large-volume nodal disease.
Using the presence of cytopenias with low numbers of circulating tumor T cells as the defining diagnostic criteria, typical LGL morphology and prominent cytoplasmic granules were seen in the majority of cases of T-LGL leukemia. Here, overlap features with HSTCL was the primary diagnostic consideration. The pattern of bone marrow infiltration could be helpful in this distinction as reactive B-cell-rich lymphoid aggregates were present in the marrow along with interstitial tumor cell infiltration in nearly all T-LGL leukemias. In contrast, a partially or completely intrasinusoidal tumor growth pattern of marrow infiltration was seen in the majority of HSTCL cases.28 In most T-LGL leukemias, the degree of marrow infiltration was higher than would be expected given the degree of splenomegaly or PB lymphocyte levels supporting preferential marrow tropism as another characteristic of this disorder. Nonetheless, cases with overlapping features between T-LGL leukemia and HSTCL or T-PLL were seen, as has been reported in case studies.29,30 Given that T-LGL leukemia is largely regarded as a low-grade tumor, diagnostic difficulties occur when the tumor presents in florid leukemic transformation.31
The general absence of defining chromosomal translocations among mature T-cell tumors, in contrast to most B-cell neoplasms, does not provide a simple molecular approach to classification and makes assignment of histogenesis more difficult.32 The categories of T-LGL leukemia and SS appear to be defined by the functional characteristics of tumor cells, with cytotoxicity and the resulting cytopenias defining T-LGL leukemia and cytokine production leading to eosinophilia and changes in vascular permeability (erythroderma) defining SS. In contrast, T-PLL appears to derive from a more quiescent T-cell population with clinical symptoms resulting from increasing tumor burden late in disease course. The occurrence of a CD4+CD8+ phenotype in a minority of T-PLL may also suggest an origin from an earlier maturation stage of T cells.
In conclusion, at the present time, besides HTLV-I/II seropositivity, there appears to be no single criterion for assignment of a newly diagnosed mature leukemic T-cell tumor to a particular WHO category. Our analysis supports the use of (1) rapidly rising PB lymphocyte counts, presence of effusions and TCL1 expression for T-PLL; (2) generalized erythroderma, PB eosinophilia, and lymphadenopathy for SS; and (3) the association of autoimmune phenomena and multiple cytopenias for T-LGL leukemias as the most useful discriminating features at presentation. However, patterns of disease presentation and progression can be overlapping in these different groups. Finally, we note that the response to different therapeutic agents is highly variable in both T-PLL and SS, suggesting a large degree of molecular heterogeneity, even within each category.
Prepublished online as Blood First Edition Paper, March 25, 2004; DOI 10.1182/blood-2004-01-0002.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Kaushali Patel, MS, for expert technical assistance, and L. Jeffrey Medeiros, MD, for critical review of the manuscript.

![Figure 3. Comparison of peak absolute lymphocyte counts among categories of leukemic mature T-cell tumors. Shown are peak levels of PB lymphocytosis that developed over the course of disease among the 5 groups of T-cell leukemia/lymphoma. The boxes around the median represent the 10th and 90th percentiles of values graphed on a logarithmic scale. The whiskers cover the range of minimum and maximum values. Patients with T-PLL developed significantly higher absolute lymphocyte counts (median, 171.3 × 109/L [171.3 × 103/μL]) compared with the other categories.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/2/10.1182_blood-2004-01-0002/6/m_zh80140463950003.jpeg?Expires=1765897511&Signature=rKVOtF8o-UyVYCfDYkwYf4b7aSU4s~~0KA6AbBVSewroYr2I5xspD7d2TVy8CC8yrEmKlUe0QYjbIfB45Nkqp5i47e47ta9cFBsf4Iu3u6e6ayud2-NZoxNU9T~aYRJXDDNEJjZNHj3SuDYhSKddnlvfkmUM48dXm8m-QBNeStaatccD4eZS7H5I0WscUa97~kx8Dux0fPfPzJNhxVuZ7graWBbqXjb05HiJpTDoGZoEYjGBO3149BDrQ7KuyKHQudQt1iRj~Mj500tcjvmI5T1eUPeBbZrldQzIMhlydmM28lZ19jMowMuH5bcgE8QUpwNKXfqkp4nbYkCo9kDPDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal