Abstract
Although the Stroke Prevention Trial in Sickle Cell Anemia (STOP) demonstrated the efficacy of blood transfusions for primary stroke prevention in high-risk children with sickle cell disease (SCD) in 1998, the impact of this trial on public health has not been studied. Our objective was to determine whether stroke rates in Californian children with SCD have declined since 1998. Using a California-wide hospital discharge database, we identified all first admissions for stroke in children with SCD from 1991 through 2000. Annual stroke incidence rates were calculated as the number of admissions divided by the estimated population of Californian children with SCD in that year. For 1991-2000, 93 children with SCD were admitted to Californian hospitals with a first stroke during 12 030 person-years of follow-up; 92.5% were ischemic and 7.5% hemorrhagic. Overall, the rate of first stroke was 0.77/100 person-years. For the study years 1991-1998, the rate for first stroke was 0.88/100 person-years compared to 0.50 in 1999 and 0.17 in 2000 (P < .005 for trend). Since the publication of the STOP study in 1998, annual rates of admissions for first stroke for Californian children with SCD have declined. (Blood. 2004;104:336-339)
Although stroke in childhood is generally rare, occurring with an annual incidence of approximately 2.5/100 000 person-years,1-3 approximately 1 in 10 children with sickle cell disease (SCD) suffer a stroke by the age of 20 years.4-6 Chronic blood transfusion therapy has been considered an effective method of secondary stroke prevention (ie, prevention of recurrent stroke) in children with SCD since the 1970s.7 In 1992, transcranial Doppler (TCD) ultrasonography was shown to identify those children with SCD who were at highest risk for stroke.8 This tool for identifying high-risk patients made “the primary prevention of stroke an achievable goal,”8 by allowing targeting of costly transfusion therapy. The Stroke Prevention Trial in Sickle Cell Anemia (STOP) randomized high-risk, but as yet stroke-free, children to transfusions or standard care, and showed that transfusion therapy resulted in a 92% relative decrease in the rate of first stroke, resulting in early termination of the trial.9 The National Heart, Lung, and Blood Institute issued a Clinical Alert announcing the results of the trial on September 18, 1997, and the STOP study was formally published on July 2, 1998.
Although more than 6 years have elapsed since the trial results were announced, the effect of the STOP study on public health has not been established. Rates of utilization of transfusion therapy in children with SCD and its impact on stroke rates have not been assessed. We sought to determine temporal trends in stroke incidence rates among children with SCD in California and test the hypothesis that rates have declined since the publication of the STOP study in 1998.
Patients and methods
We searched the databases of the Office of Statewide Health Planning and Development (OSHPD) of California to identify all childhood admissions for SCD in California from 1991 through 2000. These databases contain information on all admissions to nonfederal hospitals in California, including discharge diagnoses (up to 25 diagnoses per admission), age, sex, ethnicity, first 3 digits of zip code of residence, dates of admission and discharge, source of admission, source of payment for the admission, length of stay, and patient disposition. Admissions for SCD were identified using the International Classification of Disease, 9th revision (ICD-9) code for SCD (282.6) in any discharge code position.10,11
Inclusion/exclusion criteria
We included all childhood admissions for SCD to hospitals in California during the study period. The code for SCD (282.6) includes hemoglobin-SS, -SC, -SD, and -SE disease. Admissions for sickle cell trait (282.5) were excluded. Childhood was defined as younger than 20 years of age. Patients residing outside of California were excluded. Ethnicity, self-reported by the patient/guardian, was not used as an inclusion criterion because a study of SCD prevalence in California identified cases of SCD among neonates identified by their parents as belonging to a race other than black.12
First admissions for stroke
Among the childhood admissions for SCD, we identified all first admissions for stroke. Stroke admissions were identified by the following ICD-9 codes in any diagnostic position: for hemorrhagic stroke, 431 (intracerebral hemorrhage [ICH]) and 430 (subarachnoid hemorrhage [SAH]); for ischemic stroke, 433 through 437.9, excluding 435 (transient ischemic attack). We did not include admissions for subdural or epidural hemorrhage (432).
Within the OSHPD databases, a discharge diagnosis of stroke could reflect either a new stroke or a remote stroke. Because only new strokes were of interest in our study, we included only first stroke admissions during the study period; a unique patient identifier was used to exclude any subsequent stroke admissions from the stroke analysis. However, particularly in the early years of the study period, it is possible for a “first” stroke admission to actually reflect a remote stroke that occurred prior to the study period. The unintentional inclusion of remote strokes in the early years of the study would lead to an ascertainment bias (a bias resulting from the manner in which the cases were identified). Specifically, it could artificially inflate the number of stroke cases identified in the early years of the study. To reduce this potential ascertainment bias, we excluded first stroke admissions that were also that patient's first admission overall, unless that admission had a diagnostic code that was previously shown to be highly associated with an acute stroke (430, 431, 434, 436)13-15 and was in the primary diagnostic position.
Study base
We defined a secondary study base of at-risk children with SCD in California during the study years (1991-2000) based on state-wide population data from the US Census Bureau16 and the published prevalence of SCD in Californian newborns from 1990-1994.12 SCD prevalence rates were stratified by ethnicity in that report (86% of cases were in black children; 7% Hispanic; 1% white; 6% “other” ethnicity). To account for temporal changes in ethnic diversity in the Californian population, we used these race-stratified prevalence rates to calculate the number of Californian children with SCD within each different ethnic group reported in the US Census for each year of the study. We assumed that the prevalence of SCD within each ethnic group remained stable throughout the study period. These estimated annual totals of Californian children with SCD were considered to be the population “at risk” for SCD-related admissions to Californian hospitals.
Incidence/case fatality rates
Annual stroke incidence rates were estimated as the number of first admissions with a diagnosis of stroke divided by the population of Californian children with SCD for that year. Person-years were defined as the average size of the population of interest over the period of the study times the number of years of study.17 Case fatality rates were based on in-hospital deaths. We also evaluated the total length of the hospital stay and the total charge for the admission.
Other SCD admissions
For comparison, we calculated annual rates of all SCD admissions as the total number of childhood SCD admissions in 1 year divided by the population of Californian children with SCD for that year. In addition, we identified all hospital admissions for sickle cell crisis using the ICD-9 code 282.62; annual rates of sickle cell crisis admissions were similarly calculated.
Coexisting stroke risk factors
Coexisting conditions that may predispose to stroke in a child with SCD were ascertained from secondary discharge diagnoses. These conditions included dehydration, meningitis/encephalitis, endocarditis, sepsis/bacteremia, head trauma, and osteomyelitis. Because there is no code for acute chest syndrome, this diagnosis could not be evaluated.
Statistical analysis
We used the Royston nonparametric χ2 test for trend to compare the rate of strokes in children with SSD in the years prior to 1999 (combined data for 1991-1998) to the rates of strokes in the years 1999 and 2000.18 To test for trends in length of stay and total charge, we used the Cuzick nonparametric test for trend, an extension of the Wilcoxon rank-sum test, to compare admissions in the years prior to 1999 to admissions in 1999 and 2000.19 We used Stata (version 8.0; Stat Corp, College Station, TX) for statistical calculations.
Results
The estimated study base included a total of 12 030 person-years of Californian children with SCD (an average of 1203 children per year of study). During the study period, we identified a total of 24 576 hospital admissions of Californian children with SCD (an average of 2.04 admissions per person-year at risk). Among these admissions, there were 93 first admissions for stroke, yielding an overall annual rate for first stroke of 0.77/100 person-years. The vast majority (92.5%) of these strokes was ischemic, whereas the remaining 7.5% were hemorrhagic (a total of 3 admissions for SAH and 4 admissions for ICH). Fifty-three percent of admissions for first stroke were girls. There was only one death during the 93 first admissions for stroke, yielding a case fatality rate of 1.1%.
Age at first stroke
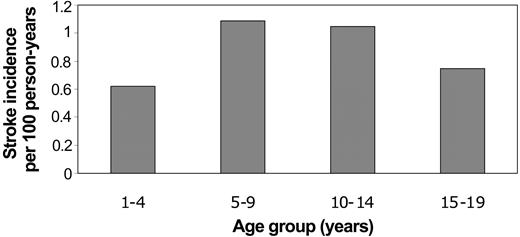
The mean age at the time of first stroke was 9.5 years (SD 4.9). There were no strokes related to SCD during infancy. The majority of first strokes occurred during mid childhood (34% from ages 5-9 years; 28% from ages 10-14 years), although 19% occurred from age 1 to 4 years. The remainder (18%) occurred from ages 15 to 19 years. Incidence rates for first stroke were similarly highest during mid childhood (Figure 1).
Incidence rates of first stroke in Californian children with SCD by age at time of first stroke.
Incidence rates of first stroke in Californian children with SCD by age at time of first stroke.
Temporal changes in stroke rates
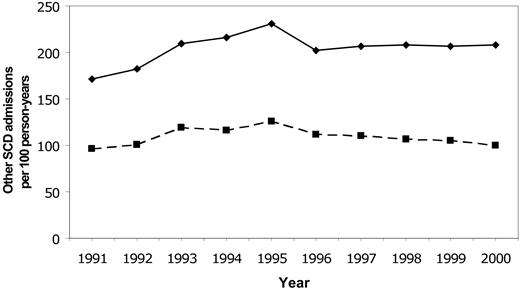
Rates for first stroke in Californian children with SCD were stable between 1991 and 1998, with an overall incidence of 0.88/100 person-years (Figure 2). The incidence declined to 0.50 in 1999 and 0.17 in 2000 (P = .0047 for trend). Rates of all SCD-related admissions and admissions for “sickle cell crisis” were stable throughout the same time period (Figure 3).
Temporal trends in admissions for first stroke for SCD in Californian children, 1991-2000.
Temporal trends in admissions for first stroke for SCD in Californian children, 1991-2000.
Temporal trends in SCD-related hospitalizations. Temporal trends in rates of all hospitalizations for SCD (solid line) and hospitalizations for sickle cell crisis (dashed line) in Californian children from 1991-2000.
Temporal trends in SCD-related hospitalizations. Temporal trends in rates of all hospitalizations for SCD (solid line) and hospitalizations for sickle cell crisis (dashed line) in Californian children from 1991-2000.
Comorbid diagnoses
Thirty-four (37%) of the cases of first stroke also had a diagnosis of “sickle cell crisis” for the same admission. Three (3.2%) had a comorbid diagnosis of dehydration. Approximately 1% had a coexisting diagnosis of sepsis/bacteremia, meningitis/encephalitis, or head trauma. Thirty-one patients (33%) received some sort of blood transfusion during the hospital admission.
Admission charge/length of stay
The median total charge for an admission for first stroke was $14 785 (mean, $32 317; range, $2056-$215 058) compared with a median total charge of $7146 (mean, $12 107; range, $244-$761 688) for an SCD admission not related to stroke. The median length of stay for an admission for first stroke was 6 days (mean, 9.6; range, 1-59 days) compared with 3 days (mean, 4.8; range, 0-134 days) for a nonstroke SCD admission. There was no change in length of stay or total charge for admissions for first stroke prior to 1999 compared to those in 1999 or 2000 (test for trend P = .51 for length of stay; P = .87 for total charge).
Discussion
Among Californian children with SCD, stroke rates were relatively stable in the 8 years leading up to the STOP study and then declined significantly (by > 80%) over the subsequent 2 years. Overall rates of SCD admissions (of which stroke admissions account for < 5%) did not show a similar decline. Although causality cannot be established with a retrospective observational study such as this, the temporal trend in stroke rates that we observed is consistent with the hypothesis that the results of the STOP study have had an impact on stroke rates in Californian children with SCD.
It is conceivable that the implementation of blood transfusion therapy could have had this effect by 1999. Although the STOP study was not formally published until 1998,9 its results were made public as a Clinical Alert by the National Heart, Lung, and Blood Institute in 1997. In addition, the effect of transfusion therapy appears to be rapid. In the STOP study, the stroke-free survival curves of the transfusion versus standard-care groups diverged within 6 months of treatment.9
It is also plausible that the degree of change that we observed is related to the implementation of chronic transfusion therapy. In the STOP study, the absolute risk of stroke was approximately 10% in the control group versus less than 1% in the treatment group, yielding a number needed to treat of approximately 10 high-risk children to prevent one stroke.9 There are approximately 120 high-risk children with SCD in California at any time (based on the average number of Californian children with SCD of approximately 1200/y, and a 10% rate of meeting the TCD ultrasound criteria for high risk for stroke).8 Therefore, to get the effect size that we observed (an absolute reduction in admissions for first stroke from an average of 10.6/y for 1991-1998, to 2 in 2000), approximately two thirds of the high-risk children in California would have had to be identified and treated with blood transfusion therapy. Given that the medical care of children with SCD tends to be highly centralized into sickle cell clinics, and the major SCD clinic in Northern California participated in the STOP study, this observed effect size is plausible.
The importance of a method of primary stroke prevention in this population is also demonstrated by our study. The rate of stroke in children with SCD is extraordinarily high; we observed an overall first stroke incidence rate of 773/100 000 person-years, which is more than 300 times that in Californian children as a whole (2.3/100 000 person-years).3 The high stroke incidence among children with SCD has been previously reported; a cohort of patients from Southern California in the 1970s had a rate for first stroke of 760/100 000 person-years in the first 2 decades of life.6 A US-wide multicenter prospective cohort enrolled between 1978 and 1988 (the Cooperative Study of Sickle Cell Disease) had a rate for first stroke of 610/100 000 person-years for sickle cell patients of all ages, including adults. The stroke incidence was higher in young children (1020/100 000 person-years for ages 2-5 years; 790 for ages 6-9) compared to young adults (520 for ages 20-29).4 Thus, the slightly lower overall incidence rate observed in this cohort is likely due to the broader age range.
The importance of primary stroke prevention in children with SCD is also reflected in the socioeconomic burden of stroke admissions, both in terms of length of stay and total admission costs. The median total length of stay for stroke-related admissions was twice that for nonstroke SCD admissions; long hospital stays translate into greater numbers of work days lost for the parents of these children. There was a similar 2-fold difference in the median total cost of the admissions. Given that strokes tend to recur in children with SCD, the total socioeconomic burden for any one individual child is even greater and would also include any disability produced, which could affect the child and the family for many years.
We observed a high proportion of ischemic (93%), as opposed to hemorrhagic, stroke in our study. Prior studies of stroke in children with SCD have similarly demonstrated proportions of ischemic stroke ranging from 75% to 95%.4,5,20 This contrasts to childhood stroke in general, approximately half of which are ischemic,2,3,21 and is likely related to the intracranial vasculopathy that underlies the pathophysiology of stroke in children with SCD.22,23
Our study is limited in that we used an administrative database and were therefore unable to validate stroke cases or confirm complete case ascertainment. In addition, our method of estimating the number of at risk children with SCD in California using race-stratified census population data and race-stratified prevalence rates of SCD in Californian newborns is imperfect. However, this methodology accounted for temporal changes in the racial demographics of the Californian population, and it is unlikely that the prevalence of SCD within an ethnic group changed significantly during our study period. Therefore, even if our definition of the study base is biased, such bias should not affect the temporal trends that we observed.
In summary, we observed declining stroke rates in Californian children with SCD, coincident with the publication of the STOP study demonstrating the efficacy of blood transfusions in primary stroke prevention. Further studies are needed to demonstrate the causality of this association and to explore in greater detail both the implementation and benefit of transfusion therapy. The public health implications of this issue are great, both because of the substantial costs of blood transfusion therapy24 and the substantial potential benefit.
Prepublished online as Blood First Edition Paper, March 30, 2004; DOI 10.1182/blood-2004-02-0636.
Supported by a Neurological Sciences Academic Development Award from the National Institutes of Health (K12 NS01692-05). The authors have no conflicts of interest to disclose. Presented in abstract form at the American Heart Association, International Stroke Conference, San Diego, CA, February 6, 2004.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to acknowledge Drs Elliot Vichinsky, Jacob S. Elkins, and Yvonne W. Wu for helpful discussions regarding this project.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal