Abstract
Methotrexate (MTX) is one of the leading drugs in the treatment of leukemia, but extensive metabolism to 7-hydroxymethotrexate (7-OHMTX) can limit its therapeutic efficacy. In this study we investigated whether 7-OHMTX itself can provoke anti-folate resistance that may further disrupt MTX efficacy. For this purpose, we developed resistance to 7-OHMTX as well as MTX in 2 human leukemia cell lines (CCRF-CEM and MOLT-4) by stepwise exposure to increasing concentrations of 7-OHMTX and MTX. Consequently, both leukemia cell lines displayed marked levels of resistance to 7-OHMTX (> 10-fold) and MTX (> 75-fold). The underlying mechanism of resistance in the MTX-exposed cells was a marked decrease (> 10-fold) in reduced folate carrier (RFC)-mediated cellular uptake of MTX. This was associated with transcriptional silencing of the RFC gene in MTX-resistant CCRF-CEM cells. In contrast, the molecular basis for the resistance to 7-OHMTX was due solely to a marked decreased (> 95%) in folylpolyglutamate synthetase (FPGS) activity, which conferred more than 100-fold MTX resistance upon a short-term exposure to this drug. This is the first demonstration that 7-OHMTX can provoke distinct modalities of antifolate resistance compared with the parent drug MTX. The implications of this finding for MTX efficacy and strategies to circumvent MTX resistance are discussed.
Introduction
Methotrexate (MTX) is one of the most important drugs frequently used in the treatment of acute leukemias and solid tumors.1 It is also used in nonneoplastic disorders and as an anti-inflammatory and/or immunosuppressive drug.2 MTX has multitargeted effects at the cellular level. After cell entry, mainly via the reduced folate carrier (RFC),3 MTX undergoes polyglutamylation by an adenosine triphosphate (ATP)-dependent reaction catalyzed by folylpolyglutamate synthetase (FPGS).4 MTX is a tight-binding inhibitor of the target enzyme dihydrofolate reductase (DHFR) thereby resulting in disruption of cellular folate metabolism.5 Moreover, MTX polyglutamates (MTX-PGs) can directly inhibit thymidylate synthase (TS), as well as glycinamide ribonucleotide (GAR) transformylase and aminoimidazole carboxamide (AICAR) transformylase, enzymes involved in the de novo biosynthesis of purines and deoxythymidylate.6
MTX undergoes oxidation in the liver by aldehyde oxidase to its main metabolite 7-hydroxymethotrexate (7-OHMTX).7 The plasma concentration of 7-OHMTX can exceed that of MTX, particularly in patients treated with high-dose MTX (HDMTX), and it becomes the predominant metabolite 10 to 12 hours following MTX infusion.8 Cellular uptake of 7-OHMTX is mediated via the RFC, though with a slightly lower affinity (Michaelis-Menten constant [Km]: 9 μM) compared with MTX (Km: 5 μM).9 As such, 7-OHMTX can compete with MTX and reduced folate cofactors for cellular uptake via RFC. Intracellularly, the rate of polyglutamation of 7-OHMTX can exceed that of the parent drug MTX by a factor of 3 at equimolar extracellular drug levels in Ehrlich ascites cells.3,4 Although polyglutamated forms of 7-OHMTX are more potent inhibitors of DHFR than nonpolyglutamated compounds,10 overall 7-OHMTX has a markedly lower affinity (> 100-fold) to DHFR compared with MTX.11-13 This markedly diminished inhibitory potential of 7-OHMTX (PGs) to DHFR accounts for the loss of the growth inhibitory effect of 7-OHMTX compared with the parent drug MTX.
Nowadays combination chemotherapy including MTX can achieve 80% event-free survival in children with precursor B-cell acute lymphoblastic leukemia (ALL). Treatment is not effective in the remaining 20% of patients, possibly due to drug resistance.14,15 Drug resistance is also frequently associated with the T-lineage ALL phenotype, which is classified as a high-risk feature.14 Molecular mechanisms of MTX resistance include decreased cellular uptake via RFC, mutations in DHFR, lower affinity of MTX for DHFR, decreased FPGS, as well as alteration in TS and folylpolyglutamate hydrolase (FPGH). Recently, increased MTX efflux due to overexpression of several multidrug-resistant proteins, notably MRP1 through 4 and the breast cancer resistance protein (BCRP) was recognized as another modality of MTX resistance.16-18
In contrast to the large body of literature available on the various mechanisms of resistance to MTX, no studies are available that address the question of whether prolonged exposure to 7-OHMTX can, independently from MTX, provoke an antifolate-resistant phenotype that may disrupt MTX efficacy. To address this point, we developed here resistance to 7-OHMTX, along with resistance to MTX in 2 human leukemia cell lines using a protocol mimicking clinical exposure. This study revealed that resistance to 7-OHMTX was associated with a marked loss in FPGS activity, a resistant phenotype that was different from defective transport identified in cells selected for MTX resistance. Consequently, translating such an observation to a clinical setting, this would imply that 7-OHMTX not only induces an additional mode of resistance to MTX but may also potentially diminish the efficacy of novel polyglutamylation-dependent antifolate drugs designed to circumvent MTX resistance.
Materials and methods
Drugs and chemicals
MTX, 7-OHMTX, and MTX-polyglutamates (MTX-Glu2-7) were purchased from Schircks Laboratories (Jona, Switzerland). ZD1694 (raltitrexed (N-[5-[N-(3,4-dihydro-2-methyl-4-oxoquinazolin-6-ylmethyl)-N-methylamino]-2-thenoyl]-glutamic acid), Tomudex, (Zeneca Limited), and ZD9331 ((2S)-2-[o-fluoro-P-[N-(2,7-dimethyl-4-oxo-3,4-dihydroquinazolin-6-yl)methyl)-N-(prop-2-ynyl)amino]benzamido-4-(tetrazol-5-yl)]-butyric acid) were a gift from Dr A. L. Jackman, Institute of Cancer Research, Sutton, United Kingdom. AG2037 was a generous gift from Dr T. J. Boritzki, Agouron Pharmaceuticals, San Diego, CA. Trimetrexate (TMQ) was provided by Dr D. Fry, Warner Lambert/Park Davis (Ann Arbor, MI). [3′,5′,7-3H]-Methotrexate sodium salt (0.555 TBq/mmol [26.7 Ci/mmol]), [5-3H]-2′-deoxyuridine (407 GBq/mmol [11 Ci/mmol]), and [5-3H]-2′-deoxyuridine monophosphate (dUMP) (0.555 TBq/mmol [15 Ci/mmol]) were from Moravek Biochemicals (Brea, CA). [2,3-3H]-l-glutamic acid (22 Ci/mmol in 0.01 N HCl) was from New England Nuclear Life Science Products, Boston, MA. Acetonitrile and methanol for high-performance liquid chromatography (HPLC) were from J. T. Baker (Deventer, Holland). Tetrabutylammoniumdihydrogenphosphate, inosine, thymidine, 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyl tetrazolium bromide (MTT), l-glutamic acid, ATP, and dithiothreitol (DTT) were obtained from Sigma-Aldrich (Steinheim, Germany). Sodium dodecyl sulfate (SDS) was purchased from KEBO Lab (Stockholm, Sweden). RPMI-1640, heat inactivated fetal calf serum (FCS), l-glutamine, and penicillin-streptomycin were from Life Technologies (Paisley, United Kingdom). All reagents for polymerase chain reactions (PCRs) were obtained from Perkin-Elmer (Foster City, CA). The primers were obtained from Cyber Gene AB (Stockholm, Sweden).
Cell culture conditions and establishment of MTX- and 7-OHMTX-resistant cell lines
The Molt-4 and CCRF-CEM cell lines were originally obtained from the American Type Culture Collection (Rockville, MD). Cells were maintained in RPMI-1640 medium containing fetal calf serum (10%), penicillin (100 U/mL), streptomycin (100 μg/mL), and l-glutamine (2 mM) and were kept in humidified air, 5% CO2 at 37°C. Cells were also routinely tested and found negative for mycoplasma contamination. MTX- and 7-OHMTX-resistant subclones were selected from the original parent CCRF-CEM and Molt-4 cell lines by exposure to increasing concentrations of MTX or 7-OHMTX starting from 1 and 50 nM, until final concentrations of 0.3 μM and 30 μM, respectively. Single clones were isolated using the limiting dilution method. The cells were subcultured twice weekly, and MTX and 7-OHMTX resistance was maintained by adding relevant concentrations of drugs. Cells were cultured in drug-free medium for at least 3 passages before use in experiments. Cultured cells during logarithmic phase (approximately 0.8-1.5 × 106 cells/mL) were used for each experiment. A Coulter Multisizer (Coulter Electronics, Luton, United Kingdom) was used to determine cell numbers.
Cytotoxicity assay
Cytotoxicity was assessed using the MTT assay as described by Mosmann.19 Briefly, Molt-4 and CCRF-CEM cells (2 × 105 cells/mL) were plated in 100 μL in each well of 96-well round-bottomed microtiter plates. Drugs were added at the desired concentrations and the plates were incubated at 37°C for 72 hours in a 5% CO2 humidified atmosphere. At the end of the incubation period, 10 μL of a stock solution of 5 mg/mL tetrazolium salt (MTT), were added to each well, and the plates were incubated for another 4 hours at 37°C. Formazan salt crystals were dissolved with 100 μL of 10% SDS in 10 mM HCl solution overnight in 37°C. Absorbance was measured using an enzyme-linked immunosorbent assay (ELISA) plate reader (Labsystems Multiscan RC, Helsinki, Finland) at a wavelength of 540 nm with reference at 650 nm. Growth inhibition was measured by dividing the mean absorbance of treated wells per mean absorbance of control wells (drug-free wells) and is expressed as a percentage. IC50 values were defined as the drug concentrations at which cell growth was inhibited by 50% compared with drug-free controls. In some experiments, cells were incubated for a short time period of 4 hours with a concentration range of MTX (0-500 μM). Cells were then washed 3 times in prewarmed medium (37°C) to allow efflux of unpolyglutamated MTX, after which cells were resuspended in fresh drug-free medium and incubated for another 68 hours.
Transport of MTX
Logarithmically growing cells were centrifuged and rinsed twice in a HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)-buffered saline solution (HBSS) containing 107 mM NaCl, 20 mM HEPES, 26.2 mM NaHCO3, 7 mM d-glucose, 5.3 mM KCl, 1.9 mM CaCl2, and 1 mM MgCl2, adjusted to pH 7.4 with NaOH as described previously.20 Cells were then resuspended in the same buffer at a density of 106 cells/mL and incubated at a final concentration of 1 μM[3H]MTX (specific activity: 26.7 Ci/mmol) in a HEPES-buffered medium at 37°C. Accumulation of MTX was terminated at 3, 10, 20, 30, and 45 minutes by addition of 10 volumes of ice-cold phosphate-buffered saline (PBS). Cells were then centrifuged (600g, 5 minutes, 4°C) and rinsed twice with 10 mL ice-cold PBS. Cell pellets were finally dissolved in water and boiled for 10 minutes. Radioactivity was determined by liquid scintillation counting.
Determination of cellular MTX polyglutamates by HPLC
A total of 5 million leukemic cells from the logarithmic phase of growth were centrifuged (600g, 5 minutes), the supernatant was removed, and cell pellets were resuspended in 10 mL fresh medium containing 10 μM inosine and 5 μM thymidine. After 15 minutes, radiolabeled [3H]MTX (final concentration: 1 μM; specific activity: 2250 disintegrations per minute [dpm]/pmol) was added and cells were incubated for 24 hours at 37°C. Cells were harvested, centrifuged (600g, 4°C, 5 minutes), and washed 3 times with 10 mL ice-cold PBS. For cell lysis, 2 mL of 60% methanol was added, mixed, and incubated at -20°C overnight. After centrifugation at 13 500g for 15 minutes, the supernatant was transferred to a glass tube and evaporated under vacuum. The dry residue was dissolved in 200 μL distilled water and 75 μL supernatant was injected into an HPLC system with gradient elution as previously described,20 with minor modifications. Briefly, the separation of metabolites was done on column μBoundpack phenyl 3.9 × 150 mm (Waters). Mobile phases consisted of 5 mM tetrahydrobutyldihydrogenphosphate in water-acetonitrile (80:20) for solution A and 20:80 for solution B. The column was eluted isocratically from 0 to 10 minutes with 100% of mobile phase A and 0% of B, then increasing up to 50% of B during 25 minutes. The HPLC system consisted of a CM4000 pump (Milton Roy, LDC Division, Riviera Beach, FL), a CMA-240 autosampler (Carneige Medicine, Stockholm, Sweden) equipped with a flow scintillation analyzer 500TR (Packard, Downers Grove, IL). The amount of polyglutamates was calculated as pmol/107 cells.
Folylpolyglutamate synthetase (FPGS) activity
The FPGS activity assay was carried out as described previously.21 FPGS substrates MTX and [3H]-l-glutamic acid (12 dpm/pmol) were used at final concentrations of 250 μM and 4 mM, respectively.
Folylpolyglutamate hydrolase (FPGH) activity
FPGH activity was measured as described earlier.22 Briefly, 107 cells were sonicated in 100 μL of 0.1-M Tris-HCl, pH 6.9 (4 × 5 seconds with 10-second intervals, on ice). The reaction mixture contained 100 μM MTX-diglutamate in 0.1 M Tris-HCl, pH 6.9. After the addition of 25 μg protein from the crude cell extract, the reaction mixture was incubated for 1 hour in a 37°C water bath. The reaction was stopped by boiling the samples for 5 minutes. Samples were centrifuged (5 minutes, 13 500 g at 4°C) and the supernatants were collected. MTX-diglutamate was separated from the product MTX by HPLC as described.
Thymidylate synthase (TS) activity
In order to measure the TS activity, we used a method previously described by Van der Wilt et al.23 Briefly, a cell pellet of 2 × 107 cells was suspended in 1 mL assay buffer (0.2 M Tris, 100 mM NaF, 15 mM cytidine-5′-monophosphate, pH 7.5) and lysed by sonication using 3 × 5 seconds with 10-second intervals. The reaction mixture consisted of 25 μL cell extract, 5 μL of 6.5-mM 5,10-methylenetetrahydrofolate (CH2-THF), and 10 μL assay buffer, pH 7.4. The reaction was initiated by the addition of 1 μL [5-3H]-dUMP (1-μM final concentration) with or without MTX-Glu4 (1 μM). After 30 minutes of incubation at 37°C, the reaction was terminated by addition of an ice-cold charcoal mixture (17.6 g charcoal in 100 mL of 4% trichloroacetic acid in methanol). After centrifugation, 50 μL of the supernatant containing tritiated water was counted by liquid scintillation.
SDS-polyacrylamide gel electrophoresis (PAGE) and Western analysis
Cells were centrifuged and rinsed twice with PBS. Pellets were resuspended in lysis buffer (10 mM NaCl, 1.5 mM MgCl2, and 10 mM Tris-HCl, pH 7.4), containing a mixture of protease inhibitors (Complete mini tablets; Boehringer-Mannheim, Indianapolis, IN), and sonicated for 3 × 5 seconds with 10-second intervals. To determine multidrug-resistance associated proteins (MRP1 through MRP4), membrane protein extracts were prepared as previously described.24 Proteins were resolved in 7.5% SDS-polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA) run at 120 V and electrophoretically transferred to membranes for 1 hour at 60 V. Polyvinylidene difluoride (PVDF) was used as a membrane for MRPs. The membranes were soaked overnight in a suspension of 5% nonfat dry milk in TBST buffer (25 mM Tris [pH 8.0], 150 mM NaCl, and 0.5% Tween 20). The nylon membranes were then incubated for 1 to 2 hours with primary antibodies for MRP1-4 (diluted 1:100 in TBST; Santa Cruz Biotechnology, Santa Cruz, CA). After 3 washes with TBST, immunodetection was carried out by incubation with anti-goat secondary antibody for MRPs and horseradish peroxidase-linked antibody (dilution 1000; Amersham International, United Kingdom) for 1 hour at room temperature. Immunoreactive bands were visualized using the enhanced chemiluminescence immunodetection system as described by the suppliers (Amersham International) after exposure to x-ray film.
Semiquantitative RT-PCR analysis of RFC gene expression. Cells (1 × 107) from the mid-log phase of growth were harvested by centrifugation, washed with PBS, and total RNA was isolated using the Tri-Reagent kit according to the instructions of the manufacturer (Sigma). A portion of total RNA (20 μg in a total volume of 20 μL) was reverse transcribed using Moloney murine leukemia virus (M-MLV, 180 units; Promega, Madison, WI) in a reaction buffer containing random hexamer primers, deoxynucleoside triphosphates (dNTPs), and a ribonuclease inhibitor Rnasin (Promega). Portions of cDNA (∼ 50 ng) synthesized from parental cells and their antifolate-resistant sublines were amplified using 10 pmols of each primer in 2xReddyMix PCR master mix reaction buffer according to the instructions of the manufacturer (ABgene, Surrey, United Kingdom). The PCR reaction was performed as follows: initial melting at 95°C for 5 minutes, followed by 30 cycles each of 1 minute at 95°C, annealing at 60°C for 45 seconds, elongation at 72°C for 1 minute, followed by 10 minutes extension at 72°C. Then, the PCR products were resolved on 2% agarose gels containing ethidium bromide. The RFC and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers used were as previously described.25
Electrophoretic mobility shift assays. Nuclear extracts were prepared from exponentially growing cells (2 × 107 cells) as previously described.25 DNA-protein complexes were formed by incubating nuclear extract proteins (6 μg) with [α-32P] deoxycytidine triphosphate (dCTP) or [aα-32P] deoxy-ATP (dATP) end-labeled CRE, GC-box, AP-1, AP-2, Mzf1, and E-box double-stranded oligonucleotides as detailed elsewhere.25,26 A 2-fold or more decrease in the binding intensity as revealed by scanning densitometry relative to parental cells was scored as altered binding. Furthermore, decreased binding of a single band upon electrophoretic mobility shift assay was sufficient for scoring it as altered binding to a given consensus site.
RNA extraction and reverse transcription-PCR analysis
For each cell line, 2.5 × 107 cells were grown and centrifuged to harvest the cells. RNA extraction was performed according to the manufacturer's instructions (RNeasy Midi Handbook; Qiagen, KEBO Lab, Spånga, Sweden). RNA concentrations were assessed using an RNA/DNA calculator (GeneQuant; Pharmacia Biotech, Cambridge, United Kingdom). cDNA was synthesized from 5 μg total RNA in a 100-μL reaction mixture according to the manufacturer's instructions using an RNA PCR kit (GeneAmp; Perkin-Elmer) with random hexamers. To amplify the cDNA, about 50 ng of the reversed transcribed cDNA from cell lines was subjected to 30 cycles of PCR in 25 μL of 1x PCR buffer; 2 mM MgCl2; 0.2 mM each of dATP, dCTP, deoxyguanosine triphosphate (dGTP), and deoxythymidine triphosphate (dTTP); 1 μM primers; and 1.2 milliunits of Taq DNA polymerase. The thermal profiles and primers for MRPs have been described by Decleves et al.27 The PCR procedure was performed for at least 3 RNA isolations for each sample.
FPGS mRNA analysis was performed as described previously.28
Statistical evaluation
Statistical analysis was performed using GraphPad Prism version 3.0a for Macintosh (GraphPad Software, San Diego, CA). A P value of less than .05 was regarded as statistically significant.
Results
Development of MTX- and 7-OHMTX-resistant cell lines and cross-resistance pattern to various cytotoxic agents
Resistant cell lines were developed by stepwise exposure of parental CCRF-CEM and MOLT-4 for 12 to 18 cycles (96 hours) of increasing concentrations of MTX (from 1 nM to 300 nM) or 7-OHMTX (from 50 nM to 30 μM). The antifolate-resistant cell lines were comparable with the parental cells with regard to doubling time and cell cycle parameters, as well as tritiated leucine, uridine, and thymidine incorporation rates (data not included).
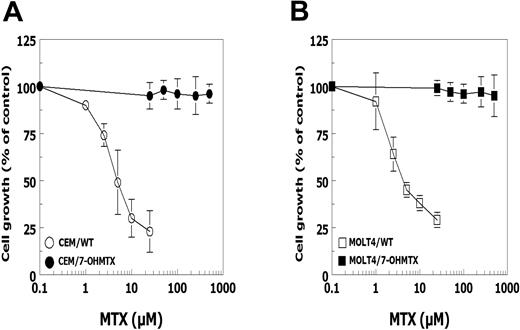
Characterization of the drug-resistant cell lines revealed that (for a 72-hour drug exposure) the cell lines selected for MTX resistance (CCRF-CEM/MTX and MOLT-4/MTX) acquired more than 50-fold resistance to MTX and 14-fold cross-resistance to 7-OHMTX, relative to parental cells (Table 1). The cell lines selected for 7-OHMTX resistance (CCRF-CEM/7-OHMTX and MOLT-4/7-OHMTX) became more than 15-fold resistant to 7-OHMTX compared with wild-type cells, but retained sensitivity to MTX. However, when exposure to MTX was limited to 4 hours rather than 72 hours, CCRF-CEM/7-OHMTX cells and MOLT-4/7-OHMTX cells were more than 100-fold resistant to MTX (IC50: > 500 μM) compared with parental CEM cells (IC50: 5.7 ± 2.4 μM) and parental MOLT-4 cells (IC50: 4.3 ± 0.6 μM) (Figure 1A-B).
Growth inhibitory effects of selected antifolates against human CCRF-CEM and MOLT-4 leukemia cells with acquired resistance to MTX and 7-OHMTX
Cell line . | MTX, nM*†¶ . | 7-OHMTX, μM . | TMQ, nM† . | ZD1694, mM*‡¶ . | ZD93331, nM*‡ . | AG2037, nM*§¶ . |
|---|---|---|---|---|---|---|
| CCRF-CEM/WT | 12.6 ± 0.2 | 17.7 ± 5.5 | 1 ± 4.6 | 3.9 ± 0.4 | 10.4 ± 2.6 | 13.1 ± 1.6 |
| CCRF-CEM/MTX | 1175 ± 36 | > 250 (> 14) | 9.1 ± 1.0 (0.4) | 176 ± 5 (45) | 601 ± 195 (58) | 83.5 ± 24.2 (6.3) |
| CCRF-CEM/7-OHMTX | 15.9 ± 2.1 | > 250 (> 14) | 6.5 ± 2.1 (0.3) | 106 ± 64 (27) | 11.5 ± 2.9 (1.1) | 37.3 ± 9.2 (2.8) |
| Molt-4/WT | 13.5 ± 2.1 | 3.5 ± 1.4 | 19.1 ± 2.1 | 4.5 ± 0.3 | 15.4 ± 1.6 | 41.6 ± 2.6 |
| Molt-4/MTX | 962 ± 42 | > 250 (> 71) | 3.9 ± 0.7 (0.2) | 667 ± 38 (148) | 1304 ± 182 (85) | 842 ± 204 (20) |
| Molt-4/7-OHMTX | 15.6 ± 3.6 | > 250 (> 71) | 8.3 ± 2.9 (0.4) | 111 ± 56 (25) | 10.7 ± 2.3 (0.7) | 36.1 ± 1.5 (0.9) |
Cell line . | MTX, nM*†¶ . | 7-OHMTX, μM . | TMQ, nM† . | ZD1694, mM*‡¶ . | ZD93331, nM*‡ . | AG2037, nM*§¶ . |
|---|---|---|---|---|---|---|
| CCRF-CEM/WT | 12.6 ± 0.2 | 17.7 ± 5.5 | 1 ± 4.6 | 3.9 ± 0.4 | 10.4 ± 2.6 | 13.1 ± 1.6 |
| CCRF-CEM/MTX | 1175 ± 36 | > 250 (> 14) | 9.1 ± 1.0 (0.4) | 176 ± 5 (45) | 601 ± 195 (58) | 83.5 ± 24.2 (6.3) |
| CCRF-CEM/7-OHMTX | 15.9 ± 2.1 | > 250 (> 14) | 6.5 ± 2.1 (0.3) | 106 ± 64 (27) | 11.5 ± 2.9 (1.1) | 37.3 ± 9.2 (2.8) |
| Molt-4/WT | 13.5 ± 2.1 | 3.5 ± 1.4 | 19.1 ± 2.1 | 4.5 ± 0.3 | 15.4 ± 1.6 | 41.6 ± 2.6 |
| Molt-4/MTX | 962 ± 42 | > 250 (> 71) | 3.9 ± 0.7 (0.2) | 667 ± 38 (148) | 1304 ± 182 (85) | 842 ± 204 (20) |
| Molt-4/7-OHMTX | 15.6 ± 3.6 | > 250 (> 71) | 8.3 ± 2.9 (0.4) | 111 ± 56 (25) | 10.7 ± 2.3 (0.7) | 36.1 ± 1.5 (0.9) |
Growth inhibitory effects were analyzed after 72 hours of drug exposure and expressed as IC50 concentration, drug concentration required to inhibit cell growth by 50%. Results are presented as the mean ± SD of 3 to 5 separate experiments. Values in parentheses depict resistance factor; ratio IC50 value resistant cell line versus parental cell line.
RFC-dependent antifolate
Primary target, DHFR
Primary target, TS
Primary target, GARFT
Polyglutamylation-dependent antifolate
Growth-inhibiting effects of MTX against parental and 7-OHMTX-resistant sublines of CEM and MOLT-4 after short-term drug exposure. (A) Parental CCRF-CEM cells (○) and CEM/7-OHMTX cells (•) and (B) parental MOLT-4 cells (□) and MOLT-4/7-OHMTX cells (▪) were incubated with a range of MTX concentrations for 4 hours, after which cells were washed and resuspended in drug-free medium for another 68 hours and monitored for inhibition of cell growth. Results are presented as the mean of 3 independent experiments ± SD.
Growth-inhibiting effects of MTX against parental and 7-OHMTX-resistant sublines of CEM and MOLT-4 after short-term drug exposure. (A) Parental CCRF-CEM cells (○) and CEM/7-OHMTX cells (•) and (B) parental MOLT-4 cells (□) and MOLT-4/7-OHMTX cells (▪) were incubated with a range of MTX concentrations for 4 hours, after which cells were washed and resuspended in drug-free medium for another 68 hours and monitored for inhibition of cell growth. Results are presented as the mean of 3 independent experiments ± SD.
In order to identify the mechanistic basis for the MTX- and 7-OHMTX-resistant phenotypes, resistant cells were analyzed for cross-resistance to other antifolates that differentially use RFC or FPGS, or inhibit target enzymes other than DHFR (eg, TS and glycinamide ribonucleotide formyltransferase [GARFT]). All resistant cell lines displayed 3.5- to 4.9-fold hypersensitivity to trimetrexate (TMQ), a lipophilic antifolate that achieves its cytotoxic effect without relying on RFC transport or polyglutamylation. Of note, MTX-resistant CCRF-CEM and MOLT-4 cells displayed cross-resistance to other antifolates that depend on RFC transport for their growth inhibitory effect; these include, for example, ZD1694 (45- and 148-fold), ZD9331 (58- and 85-fold), and AG2037 (6- and 20-fold). In contrast, 7-OHMTX-resistant CCRF-CEM and MOLT-4 cells were 25-fold cross-resistant to the polyglutamylation-dependent antifolate ZD1694, but retained sensitivity to ZD9331 and AG2037. The cytotoxicity of nonantifolate drugs such as cytosine arabinoside, cladribine, etoposide, daunorubicin, vincristine, hydroxyurea, and melphalan did not differ between the parental and MTX- or 7-OHMTX-resistant cell lines (data not shown).
MTX transport
Since the antifolate cross-resistance pattern for MTX-exposed CCRF-CEM and MOLT-4 cells as well as hypersensitivity to TMQ were indicative of altered cellular uptake via RFC, we determined the influx and accumulation of radiolabeled MTX in these cells, compared with parental and 7-OHMTX-resistant cells. Accumulation of [3H]MTX over a period of 60 minutes was profoundly impaired in CCRF-CEM/MTX cells (Figure 2A) and MOLT-4/MTX cells (Figure 2B), compared with parental and 7-OHMTX-resistant cells. Consistently, the initial rates of [3H]MTX transport in CCRF-CEM/MTX cells and MOLT-4/MTX cells were 13-fold and 11-fold lower than in parental cells, respectively (Figures 2A and 2B). 7-OHMTX-resistant CCRF-CEM cells and MOLT-4 cells exhibited only a minor decrease in [3H]MTX influx, 1.4-fold and 1.2-fold, respectively, compared with parental cells.
Influx of MTX in wild-type and resistant cells. Time course of [3H]MTX accumulation in CCRF-CEM (A) and MOLT-4 (B) parental cells (▴) and their MTX-(•) and 7-OHMTX-(○) resistant sublines. Extracellular concentration of [3H]MTX was 1 μM. Bottom panels: Relative linear uptake rates of [3H]MTX determined over 0- to 3-minute incubation time. Unidirectional influx rates of [3H]MTX for each parental cell line are set at 100%. Results are presented as the mean of 3 independent experiments in which SD was less than 17%. Further experimental details are described in “Materials and methods.”
Influx of MTX in wild-type and resistant cells. Time course of [3H]MTX accumulation in CCRF-CEM (A) and MOLT-4 (B) parental cells (▴) and their MTX-(•) and 7-OHMTX-(○) resistant sublines. Extracellular concentration of [3H]MTX was 1 μM. Bottom panels: Relative linear uptake rates of [3H]MTX determined over 0- to 3-minute incubation time. Unidirectional influx rates of [3H]MTX for each parental cell line are set at 100%. Results are presented as the mean of 3 independent experiments in which SD was less than 17%. Further experimental details are described in “Materials and methods.”
Transcriptional silencing of the RFC gene
In order to identify the molecular basis for the severe transport defect in MTX-resistant CCRF-CEM and MOLT-4 cells we first determined RFC gene expression. Semiquantitative RT-PCR analysis revealed markedly decreased RFC mRNA levels in CCRF-CEM/MTX but not in MOLT-4/MTX cells; the identical GAPDH levels in the various cell lines confirmed that equal amounts of cDNA were being analyzed (Figure 3A). Detailed genomic PCR single-strand analysis of the entire RFC gene29 failed to identify any RFC mutations. Indeed, DNA sequencing confirmed that no RFC mutations were present that could explain the antifolate transport defect in either MTX-resistant cell line. We further explored the possibility of whether the markedly decreased RFC mRNA expression in CCRF-CEM/MTX cells was associated with transcriptional silencing of the RFC promoter, which was recently identified as a novel mode of RFC transport-related antifolate resistance.25,30 To this end, we used electrophoretic mobility shift assays with consensus [32P]-labeled oligonucleotides that are present in the human RFC promoter. Indeed, decreased binding (or a complete loss) of various transcription factors to their cis-acting elements, including CRE, E-box, AP1, Mzf-1, and GC-box, was observed in CCRF-CEM/MTX cells when compared with their parental cells (Figure 3B). In contrast, a completely normal AP2 binding was retained in CCRF-CEM/MTX cells; this was in accord with our previous results.25,26
RFC gene expression and electrophoretic mobility shift assay in parental cells and their MTX-resistant sublines. Semiquantitative RT-PCR analysis of the RFC gene (A) along with a GAPDH control was performed as previously described.25,26,29 Electrophoretic mobility shift assay (B) was performed by the binding of nuclear proteins to their [32P]-labeled consensus double-stranded oligonucleotides followed by visualization with a Phosphorimager as detailed elsewhere.25,26
RFC gene expression and electrophoretic mobility shift assay in parental cells and their MTX-resistant sublines. Semiquantitative RT-PCR analysis of the RFC gene (A) along with a GAPDH control was performed as previously described.25,26,29 Electrophoretic mobility shift assay (B) was performed by the binding of nuclear proteins to their [32P]-labeled consensus double-stranded oligonucleotides followed by visualization with a Phosphorimager as detailed elsewhere.25,26
MTX polyglutamylation
Rather than a transport defect, the antifolate cross-resistance profile of CCRF-CEM/7-OHMTX and MOLT-4/7-OHMTX cells was consistent with an impaired antifolate polyglutamylation. For this purpose, we measured FPGS activity in the 7-OHMTX selected cell lines compared with the MTX-resistant counterparts. FPGS activity was found to be severely decreased, 56-fold in CCRF-CEM/7-OHMTX (Figure 4A) and 92-fold in MOLT-4/7-OHMTX (Figure 4C) cells, relative to parental cells. In contrast, FPGS activity was slightly elevated in CCRF-CEM/MTX cells (1.3-fold) and MOLT-4/MTX cells (1.5-fold) compared with parental cells.
FPGS activity and polyglutamylation formations in wild-type and MTX- and 7-OHMTX-resistant cells. (A,C) FPGS activity in parental CCRF-CEM (A) and MOLT-4 (C) cells and their MTX- or 7-OHMTX-resistant sublines. Enzyme activity ± SD is depicted relative to parental cells (100%). Control values for FPGS activity in parental CCRF-CEM cells and MOLT-4 cells were 958 ± 478 and 739 ± 171 pmol [3H]-glutamate incorporated/mg protein/hour, respectively. (B,D) [3H]MTX-polyglutamate formation in parental CCRF-CEM (B) and MOLT-4 (D) cells and their MTX- and 7-OHMTX sublines after 24 hours of exposure to 1 μM[3H]-MTX. Formation of individual polyglutamate conjugates is depicted as percentage of total [3H]MTX accumulation. Total [3H]MTX accumulation (expressed as pmol [3H]-MTX/107 cells) after 24 hours for the individual cell lines were as follows: CCRF-CEM, 63.3 ± 1.8; CCRF-CEM/MTX, 26.5 ± 2.4; CCRF-CEM/7-OHMTX, 32.9 ± 1.3; MOLT-4, 70.5 ± 1.2; MOLT-4/MTX, 18.2 ± 4.1; and MOLT-4/7-OHMTX, 22.1 ± 0.7 pmol/107 cells. Results are presented as means ± SD of 3 separate experiments.
FPGS activity and polyglutamylation formations in wild-type and MTX- and 7-OHMTX-resistant cells. (A,C) FPGS activity in parental CCRF-CEM (A) and MOLT-4 (C) cells and their MTX- or 7-OHMTX-resistant sublines. Enzyme activity ± SD is depicted relative to parental cells (100%). Control values for FPGS activity in parental CCRF-CEM cells and MOLT-4 cells were 958 ± 478 and 739 ± 171 pmol [3H]-glutamate incorporated/mg protein/hour, respectively. (B,D) [3H]MTX-polyglutamate formation in parental CCRF-CEM (B) and MOLT-4 (D) cells and their MTX- and 7-OHMTX sublines after 24 hours of exposure to 1 μM[3H]-MTX. Formation of individual polyglutamate conjugates is depicted as percentage of total [3H]MTX accumulation. Total [3H]MTX accumulation (expressed as pmol [3H]-MTX/107 cells) after 24 hours for the individual cell lines were as follows: CCRF-CEM, 63.3 ± 1.8; CCRF-CEM/MTX, 26.5 ± 2.4; CCRF-CEM/7-OHMTX, 32.9 ± 1.3; MOLT-4, 70.5 ± 1.2; MOLT-4/MTX, 18.2 ± 4.1; and MOLT-4/7-OHMTX, 22.1 ± 0.7 pmol/107 cells. Results are presented as means ± SD of 3 separate experiments.
The marked fall in FPGS activity was not associated with a concomitant decrease in FPGS mRNA. Relative to parental cells, FPGS mRNA level in CCRF-CEM/7-OHMTX was 0.54 and in MOLT-4/7-OHMTX cells, 1.24. For comparison, CCRF-CEM/MTX and MOLT-4/MTX cells had FPGS mRNA levels of 0.85 and 1.70, respectively, relative to parental cells.
Consistent with the markedly decreased FPGS activity, CCRF-CEM/7-OHMTX (Figure 4B) and MOLT-4/7-OHMTX (Figure 4D) cells were essentially devoid of the ability to convert MTX to long-chain polyglutamate derivatives, following 24 hours of incubation with 1 μM [3H]MTX. In both cell lines, more than 96% of the total accumulated [3H]MTX was present in its monoglutamate (ie, original) form. In contrast, in parental CCRF-CEM and MOLT-4 cells, more than 70% of [3H]MTX was converted to its long-chain polyglutamate forms (up to hexaglutamate). Lack of conversion to long chain [3H]MTX polyglutamates (< 5% of parental cells) was also identified in CCRF-CEM/MTX and MOLT-4/MTX; this could be accounted for by the poor cellular accumulation of [3H]MTX in these cells.
Other antifolate resistance parameters
Although RFC transport defects in MTX-resistant cells and polyglutamylation defects in 7-OHMTX-resistant cells were identified as major contributors to the resistant phenotypes, we further examined the potential contribution of alternative (minor) mechanisms of antifolate resistance (summarized in Table 2). Elevated DHFR levels as a resistance factor was ruled out due to the lack of cross-resistance to the lipophilic DHFR inhibitor TMQ (Table 1). Increased breakdown of MTX-polyglutamates associated with an increased activity of folylpolyglutamate hydrolase (FPGH) can confer antifolate resistance.26,31 Except for a minor increase (1.7-fold) in FPGH activity in CCRF-CEM/7-OHMTX cells, no alterations in FPGH activity were noted in CCRF-CEM/MTX, MOLT-4/MTX, and MOLT-4/7-OHMTX cells, relative to parental cells (Table 2). A modest elevation in TS activity was observed in CCRF-CEM/MTX cells (1.7-fold) and MOLT-4/MTX cells (2.4-fold), but not in the 7-OHMTX-resistant cell lines. Kinetic properties of TS, reflected by potency of inhibition by MTX-polyglutamates, were not altered in the resistant cell lines (data not shown). Enhanced cellular efflux of antifolates by members of the multidrug resistance protein family, notably MRP1 through MRP4,16,18 does not seem to play a role in the MTX- and 7-OHMTX-resistant cells, as no changes in MRP1 through MRP4 expression were observed, either at the mRNA or the protein levels (Table 2).
Summary of antifolate resistance parameters in 7-OHMTX–resistant and MTX-resistant CCRF-CEM and MOLT-4 cells compared with their parental cells
Cell line . | RFC-mediated transport*(fold change) . | FPGS activity†(fold change) . | FPGH activity‡(fold change) . | TS activity§(fold change) . |
|---|---|---|---|---|
| CCRF-CEM/MTX | ↓ ↓ (13) | ↑ (1.3) | No change | ↑ (1.7) |
| CCRF-CEM/7-OHMTX | ↓ (1.4) | ↓ ↓ (56) | ↑ (1.7) | No change |
| MOLT-4/MTX | ↓ ↓ (11) | ↑ (1.5) | No change | ↑ (2.4) |
| MOLT-4/7-OHMTX | No change | ↓ ↓ (92) | No change | No change |
Cell line . | RFC-mediated transport*(fold change) . | FPGS activity†(fold change) . | FPGH activity‡(fold change) . | TS activity§(fold change) . |
|---|---|---|---|---|
| CCRF-CEM/MTX | ↓ ↓ (13) | ↑ (1.3) | No change | ↑ (1.7) |
| CCRF-CEM/7-OHMTX | ↓ (1.4) | ↓ ↓ (56) | ↑ (1.7) | No change |
| MOLT-4/MTX | ↓ ↓ (11) | ↑ (1.5) | No change | ↑ (2.4) |
| MOLT-4/7-OHMTX | No change | ↓ ↓ (92) | No change | No change |
MRP1 through MRP4 mRNA and protein levels were assayed by RT-PCR and Western blotting; no change was observed in any cell line.
RFC transport is depicted as [3H]MTX influx rates at an extracellular concentration of 1 μM [3H]MTX. Control values for parental CCRF-CEM and MOLT-4 cells were 0.87 ± 0.13 pmol [3H]-MTX/min/107 cells and 0.34 ± 0.05 [3H]-MTX/min/107 cells, respectively
FPGS activity was determined at substrate concentrations of 250 μM MTX and 4 mM [3H]-L-glutamic acid. Control values for parental CCRF-CEM and Molt-4 cells were 958 ± 478 and 739 ± 171 pmol [3H]-glutamate incorporated/h/mg protein, respectively
FPGH activity was determined at substrate concentration of 100 μM MTX-Glu2. Control values for parental CCRF-CEM and MOLT-4 cells were 8460 ± 374 and 2658 ± 42 pmol MTX-Glu2 breakdown/h/mg protein, respectively
TS activity was measured either in cell extracts or in intact cells in situ. Control values for parental CCRF-CEM and Molt-4 cells were 4.8 ± 0.23 and 2.7 ± 0.24 nmol/h/mg protein, respectively
Discussion
7-OHMTX is well recognized as the major metabolite of MTX that can hamper the therapeutic effect of MTX due to its slow elimination and, at the cellular level, by competition with MTX for cellular uptake and intracellular polyglutamylation. In this report, we describe for the first time an additional adverse effect of exposure to 7-OHMTX, that is, the induction of a mechanism of antifolate resistance that is associated with defective polyglutamylation that concomitantly confers resistance to MTX. In contrast, exposure to MTX itself provoked an impaired RFC transport-based resistance phenotype. These observations were made independently for 2 human leukemia T-cell lines, CCRF-CEM and MOLT-4.
The plasma concentration of 7-OHMTX may exceed that of MTX 3 to 10 hours following infusion of high-dose MTX; when the plasma concentration of MTX is in the range of 0.1 μM, the level of 7-OHMTX is 17- to 140-fold higher.8 The concentration of 7-OHMTX was also 2- to 5-fold higher than that of MTX in the bone marrow of children 24 hours after an oral dose of 30 mg/m2 MTX.32 Although 7-OHMTX and its metabolites have a weak inhibitory effect on cell growth in the low-dose MTX treatment, it was postulated that a pharmacologic activity of 7-OHMTX might be more pronounced in high-dose MTX protocols.33 In such a clinical setting, 7-OHMTX (polyglutamates) may accumulate to appreciable levels in tumor cells, as the efficiencies of RFC-mediated transport and FPGS-dependent polyglutamylation are similar to those of the parent drug MTX. 7-OHMTX polyglutamates can exert an inhibitory effect on DHFR and TS,10-12 however their potency is approximately 2 orders of magnitude lower than that for MTX-polyglutamates.
In order to establish whether long-term exposure to 7-OHMTX could ultimately affect the efficacy of MTX, we set out to expose human CCRF-CEM and MOLT-4 leukemia cells to gradually increasing concentrations of 7-OHMTX, and, in parallel, to MTX. Strikingly, both selection schedules resulted in completely different mechanisms of drug resistance. In leukemia cells selected for resistance to MTX, defective cellular uptake via RFC could account for the resistance phenotype, whereas in cells selected for 7-OHMTX resistance, defective polyglutamylation associated with a marked reduction in FPGS activity was the predominant mode of resistance. Other potential mechanisms of antifolate resistance were also evaluated (Table 2), for example, increased activity of TS34 or FPGH, or increased cellular efflux via MRP1 through MRP5, but changes in one or more of these parameters appeared rather small and may contribute to the resistance phenotype only to a minor extent.
Defective transport via RFC is an established mechanism of antifolate resistance.21,35 Consistent with studies by others,21,29,36,37 we observed here that selection of CCRF-CEM and MOLT-4 cells with low doses of MTX resulted in impaired antifolate transport. Interestingly, the molecular basis for the transport defect in MTX-resistant CCRF-CEM cells appeared to be associated with the simultaneous loss of binding of multiple transcription factors to cis-acting elements present in the RFC promoter. Consequently, markedly decreased RFC mRNA levels and RFC protein expression resulted in impaired antifolate transport even in the absence of mutations in the RFC gene.21,30 This novel mechanism of antifolate resistance was recently described by Rothem et al.25,30 However, the molecular mechanism underlying the MTX transport defect in MOLT-4/MTX was associated neither with inactivating mutations in the RFC gene nor with changes in RFC mRNA levels. Hence, it remains to be determined whether poor translatability of RFC transcript(s) and/or posttranslational mechanisms contributed to the loss of RFC-dependent transport activity.
Rather than a transport defect, resistance to 7-OHMTX appeared to be accompanied by a polyglutamylation defect. Defective polyglutamylation associated with a marked decrease in FPGS activity is a common mechanism of resistance to antifolates that heavily rely on polyglutamylation for their inhibitory effect on folate-dependent enzymes.36,38,39 Decreased FPGS activity as a mode of antifolate resistance is frequently observed following short-term exposure with relatively high doses (micromolar range) of antifolates.36,38-41 In this respect, it is of interest to note that long-term exposure of the leukemia cells to 7-OHMTX also elicits a marked decrease in FPGS activity, and concomitantly, a high level of resistance to MTX upon short-term exposure to this antifolate (Figure 1A-B). Thus, it seems apparent that sustained intracellular concentrations of 7-OHMTX can exert a selective pressure on FPGS expression that is comparable with short-term/high-dose protocol of other polyglutamylation-dependent antifolates. In line with other studies,38,39,41 we observed no major reduction in FPGS mRNA in CCRF-CEM/7-OHMTX and MOLT- 4/7-OHMTX cells, suggesting that the decrease in FPGS activity is due to posttranscriptional or posttranslational alterations probably involving protein instability.41
Methotrexate is the anchor drug in the treatment of childhood acute lymphoblastic leukemia.1,14 B-cell ALL appears to be better responsive to MTX than T-cell ALL, part of which is explained by more efficient MTX polyglutamylation due to a higher activity of FPGS in B-cell ALL than T-cell ALL.42,43 However, analysis of leukemia samples revealed large interpatient variations in both RFC transport capacities and FPGS mRNA/enzyme activities.15,28,43-45 The data from the present study may provide a new dimension into the molecular basis of variability of FPGS activity in leukemia cells, that is, decrease in FPGS activity as an important mode of resistance may not necessarily be enforced by MTX itself but rather by its metabolite 7-OHMTX. The finding that MTX and its metabolite 7-OHMTX provoke disparate mechanisms of antifolate resistance, transport impairment versus polyglutamylation defect, respectively, can have important implications for designing strategies aimed at circumvention of MTX resistance. Results presented in Table 1 show that new-generation antifolates including ZD9331 and AG2037 retain activity against FPGS-deficient/7-OHMTX-resistant CCRF-CEM and MOLT-4 cells. In contrast, MTX-resistant CCRF-CEM and MOLT-4 cells with impaired drug transport displayed cross-resistance to these novel antifolates. The lipophilic compound TMQ was the only antifolate that retained activity against MTX- and 7-OHMTX-resistant leukemia cells. In fact, TMQ activity was even greater than for parental cells, which is explained by the fact that RFC and FPGS deficiency leads to a depletion of intracellular folate pools that then diminishes competition for interactions with DHFR.21,36,38
In summary, this study provides the first evidence that MTX as well as its metabolite 7-OHMTX can independently provoke distinct modes of antifolate resistance, that is, transport impairment and polyglutamylation defect. It is anticipated that this phenomenon may be particularly prevalent after repeated cycles of HD-MTX. Consequently, in a clinical setting, this can impair the efficacy of MTX or various antifolates that depend on transport and/or polyglutamylation for their cytotoxic activity.
Prepublished online as Blood First Edition Paper, August 12, 2004; DOI 10.1182/blood-2004-04-1493.
Supported by grants from the Swedish Children Cancer Foundation and the Swedish Cancer Foundation (grant to F.A.), the Dutch Cancer Society (grant NKB-VU 2000-2237 to G.J.P. and G.J.), as well as the Israel Cancer Association and the Star Foundation (Y.G.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 2. Influx of MTX in wild-type and resistant cells. Time course of [3H]MTX accumulation in CCRF-CEM (A) and MOLT-4 (B) parental cells (▴) and their MTX-(•) and 7-OHMTX-(○) resistant sublines. Extracellular concentration of [3H]MTX was 1 μM. Bottom panels: Relative linear uptake rates of [3H]MTX determined over 0- to 3-minute incubation time. Unidirectional influx rates of [3H]MTX for each parental cell line are set at 100%. Results are presented as the mean of 3 independent experiments in which SD was less than 17%. Further experimental details are described in “Materials and methods.”](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-04-1493/6/m_zh80240471130002.jpeg?Expires=1769104103&Signature=tgUV2RrbPJ5fYOhAgUkPFmgjgY00Mpm0NjtbSBiyT6mgue7Hyu57RYbzL2Wf7CbS6jO6Ks6lc~Cclg3p-kg~chjp6TfzKi27z4Lgvucpyai9M6nvkPkUlTCZZX1bIVpZvuQKXX32zDckVWKKV06CVPJUbDgrvdOT8vK0yEj3hX6yMsprrUUYU5WilW2bxy4pihOIiJpZXd1ZJVeO1nwd57uMN9p3i88vCnWVY~PBbGSIdHqVzOOIvM~WIpJMlQde-SRIl1uVKh4P0JtBZ7OyU-M5XiU2W-s6dSJP5o~LWxfQPFIRSoxlEXjRs8NgQ6p7B1eqDJSLOe8ZfcjoLOQUAA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. RFC gene expression and electrophoretic mobility shift assay in parental cells and their MTX-resistant sublines. Semiquantitative RT-PCR analysis of the RFC gene (A) along with a GAPDH control was performed as previously described.25,26,29 Electrophoretic mobility shift assay (B) was performed by the binding of nuclear proteins to their [32P]-labeled consensus double-stranded oligonucleotides followed by visualization with a Phosphorimager as detailed elsewhere.25,26](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-04-1493/6/m_zh80240471130003.jpeg?Expires=1769104103&Signature=FKGQ~IDJRmVTRvq5ktSp1hv~WRBu3jAZPjqn2-SY6HIvnZ~egdMifWtyHdxbnd0SVkCbnD4kaX1kma~egIVse-DW5Acrp0aQ-4GVyd0XvcRlOT217KuujQLskMOnSMvZv6iixkERektxNR~uQX1Lrub5SKvfLv43BxPc8H~jk19e~ZA4SzO9XuS0YLyna5N1DMVFBrBenjKh8MWX3UYwRlaqREYsRqLdnlIpykpyuBS7lfBOIumXqrS8i1MqZZDJWm78TIRSpTQ9YaywOekosUO0Huero42H5B79VC4OJZl8aaqQAo8iBzsef4HNeU9Sl7VqrV0wtoDZNr78qwmtpw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. FPGS activity and polyglutamylation formations in wild-type and MTX- and 7-OHMTX-resistant cells. (A,C) FPGS activity in parental CCRF-CEM (A) and MOLT-4 (C) cells and their MTX- or 7-OHMTX-resistant sublines. Enzyme activity ± SD is depicted relative to parental cells (100%). Control values for FPGS activity in parental CCRF-CEM cells and MOLT-4 cells were 958 ± 478 and 739 ± 171 pmol [3H]-glutamate incorporated/mg protein/hour, respectively. (B,D) [3H]MTX-polyglutamate formation in parental CCRF-CEM (B) and MOLT-4 (D) cells and their MTX- and 7-OHMTX sublines after 24 hours of exposure to 1 μM[3H]-MTX. Formation of individual polyglutamate conjugates is depicted as percentage of total [3H]MTX accumulation. Total [3H]MTX accumulation (expressed as pmol [3H]-MTX/107 cells) after 24 hours for the individual cell lines were as follows: CCRF-CEM, 63.3 ± 1.8; CCRF-CEM/MTX, 26.5 ± 2.4; CCRF-CEM/7-OHMTX, 32.9 ± 1.3; MOLT-4, 70.5 ± 1.2; MOLT-4/MTX, 18.2 ± 4.1; and MOLT-4/7-OHMTX, 22.1 ± 0.7 pmol/107 cells. Results are presented as means ± SD of 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-04-1493/6/m_zh80240471130004.jpeg?Expires=1769104103&Signature=oRdPlemON16SJaXZRH2ImSoYp7RTQ6NbxwmjSaw7pESepD~8olZJwA5xeqiULI55I8J6lUv-ueutY5gMdxT-ZCwRFizjmoqkmMjsVSzrMXDUrQxFcJXnlY9363Y4fHCJgNxUyZBni-gdyXx1wAXovQckFXdJSIIttIadX5syFkt2ayfX-VJ0ji9u~ZVym2CMKtoHWcxDhL1~u8pm9H6SR8Jdfwihjm4j1yuqHU0jVBg7X0ZQMri9byOoSTaVMqk3kuYRNnM8iL49tXdVEr1fMKu8lSPvoaXfw5dpNhZUvB~1q8ebR1zSYwvFM1Ho9bKCcqbStQOZsHPtWbefRE-YzA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal