Abstract
Previous studies have demonstrated the in vitro and in vivo activity of CC-5013 (Revlimid), an immunomodulatory analog (IMiD) of thalidomide, in multiple myeloma (MM). In the present study, we have examined the anti-MM activity of rapamycin (Rapamune), a specific mTOR inhibitor, combined with CC-5013. Based on the Chou-Talalay method, combination indices of less than 1 were obtained for all dose ranges of CC-5013 when combined with rapamycin, suggesting strong synergism. Importantly, this combination was able to overcome drug resistance when tested against MM cell lines resistant to conventional chemotherapy. Moreover, the combination, but not rapamycin alone, was able to overcome the growth advantage conferred on MM cells by interleukin-6 (IL-6), insulin-like growth factor-1 (IGF-1), or adherence to bone marrow stromal cells (BMSCs). Combining rapamycin and CC-5013 induced apoptosis of MM cells. Differential signaling cascades, including the mitogen-activated protein kinase (MAPK) and the phosphatidylinositol 3′-kinase/Akt kinase (PI3K/Akt) pathways, were targeted by these drugs individually and in combination, suggesting the molecular mechanism by which they interfere with MM growth and survival. These studies, therefore, provide the framework for clinical evaluation of mTOR inhibitors combined with IMiDs to improve patient outcome in MM.
Introduction
Interleukin-6 (IL-6) and insulin-like growth factor-1 (IGF-1) play key roles in the growth, survival, and drug resistance of multiple myeloma (MM) cells.1-9 Furthermore, their secretion in bone marrow stromal cells (BMSCs) is up-regulated by the adherence of MM cells. IL-6 and IGF-1 mediate the growth of MM cells through activation of the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3′-kinase/Akt kinase (PI3K/Akt) signaling cascades.10-16 Several studies show that PI3K/Akt signaling mediates growth, survival, migration, and cell cycle regulation in MM.14,16-18 Activated Akt, in turn, phosphorylates downstream target molecules, including forkhead transcription factor (FKHR), glycogen synthase kinase (GSK)-3β, and mammalian target of rapamycin (mTOR).15,19,20 These downstream molecules would, therefore, serve as rational targets for novel therapeutics in MM. Indeed, specific mTOR inhibitors such as rapamycin have induced cytoreduction and G1 arrest observed in MM cells.11 In vitro and in vivo studies have demonstrated marked anti-MM activity of CCI-779 (Wyeth Ayerst, St Davids, PA) an analog of rapamycin.11,12,21 CCI-779 is currently under evaluation in phase 1 and 2 studies, both in solid tumors, and mantle cell lymphoma. A phase 2 study in renal cell carcinoma has just been completed, and a phase 3 trial is currently accruing patients.22 To date, however, there are no clinical trials in MM.
Previous preclinical studies have demonstrated that thalidomide and immunomodulatory derivatives (IMiDs) such as CC-5013 (Revlimid; Celgene, Warren, NJ) and linalidomide can overcome conventional drug resistance and have, in part, delineated their molecular mechanisms of action. Furthermore, these in vitro observations have translated into clinical trials of these agents, which have resulted in significant responses even in MM patients with relapsed and refractory disease.23,24 Here we have studied the effect of combining CC-5013 with rapamycin based on rational targeting of differential signaling cascades mediating MM cell growth and survival.
Materials and methods
MM-derived cell lines
Dexamethasone (Dex)-sensitive (MM.1S) and Dex-resistant (MM.1R) human MM cell lines were kindly provided by Dr Steven Rosen (Northwestern University, Chicago, IL). Doxorubicin-resistant (Dox 40) and melphalan-resistant (LR5) RPMI 8226 human MM cells were kindly provided by Dr William Dalton (Moffitt Cancer Center, Tampa, FL). The OPM 2 cell line was obtained from Dr Lief Bergsagel (Cornell University, Ithaca, NY), and SB645 was obtained from Dr Kishimoto (Osaka University, Japan). All MM cell lines were cultured in RPMI 1640 media (Sigma Chemical, St Louis, MO) containing 10% fetal bovine serum, 2 mM L-glutamine (Gibco, Grand Island, NY), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco).
CC-5013 and rapamycin
CC-5013 was obtained from Celgene, and rapamycin was obtained from Calbiochem (San Diego, CA). The drugs were dissolved in dimethyl sulfoxide (DMSO; Sigma) at a concentration of 200 mM and were stored at -20°C until use. Drugs were diluted in culture medium (0.01-100 μM for CC-5013 and 0.01-100 nM for rapamycin) with less than 0.1% DMSO immediately before use. Diluted drugs were used within 4 hours.
Bone marrow stromal cells
Bone marrow aspirates were subjected to Ficoll Paque gradient centrifugation (Amersham Pharmacia Biotech, Piscataway, NJ), and mononuclear cells (MNCs) were separated. MNCs were placed in 25-mm2 culture flasks in RPMI 1640 media (Sigma Chemical) containing 20% fetal bovine serum, 2 mM L-glutamine (Gibco), 100 U/mL penicillin, and 100μg/mL streptomycin (Gibco). Once confluent, the cells were trypsinized and passaged as needed. For the experiments, BMSCs were incubated in 96-well culture plates (approximately 5000 to 10 000 BMSCs/well) for 24 hours. Media were washed off, and MM cells were added to the wells (2 × 104 cells/well) and incubated with media alone or with different concentrations of CC-5013, rapamycin, or a combination of the 2 drugs for 48 hours at 37°C.
Proliferation assays
DNA synthesis was measured by tritiated thymidine uptake [3H-TdR] (Perkin Elmer, Boston, MA), as previously described. Briefly, MM cells (2-3 × 104 cells/well) were incubated in 96-well culture plates (Costar, Cambridge, MA) in the presence of media or varying concentrations of CC-5013, rapamycin, or a combination of the 2 drugs for 48 hours at 37°C. For evaluation of the effect of growth factors, recombinant IL-6 (10 ng/mL) or IGF-1 (50 ng/mL) was added to the wells at the beginning of the incubation to obtain the final concentration indicated. Cells were pulsed with 3H-TdR (0.5 μCi (0.185 MBq)/well) during the last 8 hours of 48-hour culture, harvested onto glass filters with an automatic cell harvester (Cambridge Technology, Cambridge, MA), and counted by using the LKB Betaplate scintillation counter (Wallac, Gaithersburg, MD). All experiments were performed in triplicate.
Colorimetric assays were also performed to assay drug activity. Cells from 48-hour cultures were pulsed with 10 μL of 5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide (MTT; Chemicon International, Temecula, CA); the 96-well plates were incubated at 37°C for 4 hours, followed by addition of 100 μL isopropanol that contained 0.04 HCl. Absorbance readings at a wavelength of 570 nm (with correction using readings at 630 nm) were taken on a spectrophotometer (Molecular Devices, Sunnyvale, CA).
Cell cycle analysis and detection of apoptosis
MM cells (1 × 106) were cultured for 48 hours in media alone or with varying concentrations of CC-5013, rapamycin, or a combination of the 2 drugs. Cells were harvested, washed with ice-cold phosphate-buffered saline (PBS), fixed with 70% ethanol for 1 hour, and pretreated with 10 μg/mL RNase (Sigma) for 1 hour. Cells were stained with propidium iodide (PI; 5 μg/mL; Sigma), and the cell cycle profile was determined by using the RXP Cytomics software on an Epics flow cytometer (Coulter Immunology, Hialeah, FL), as in previous studies.
In addition to identifying sub-G1 cells by cell cycle analysis, as described, apoptosis was also confirmed by using annexin V/PI staining. MM cells were cultured in media or with 1 nM rapamycin, 1 μM CC-5013, or their combination at 37°C for 24, 48, or 72 hours. Cells were then washed twice with ice-cold PBS and resuspended (1 × 106 cells/mL) in binding buffer (10 mM HEPES [N-2-hydroxyethylenepiperazine-N′-2-ethanesulfonic acid], pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). MM cells (1 × 105) were incubated with annexin V-fluorescein isothiocyanate (FITC) (5 μL; MBL, Nagoya, Japan) and PI (5 μg/mL) for 15 minutes at room temperature. Annexin V+PI- apoptotic cells were enumerated by using the Epics flow cytometer.
Western blot analysis
MM cells were cultured with CC-5013 (1 μM), rapamycin (1 nM), or a combination of the 2 drugs (at same respective doses), harvested, washed, and lysed using lysis buffer (50 mM HEPES [pH 7.4], 150 mM NaCl, 1% Triton-X 100, 30 mM sodium pyrophosphate, 5 mM EDTA [ethylenediami-netetraacteic acid], 2 mM Na3VO4, 5 mM NaF, 1 mM phenylmethyl sulfonyl fluoride [PMSF], 5 μg/mL leupeptin, and 5 μg/mL aprotinin). For the detection of apoptosis-related proteins, cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membrane, and immunoblotted with antibodies against cleaved poly(ADP-ribose) polymerase (PARP) and cleaved caspase 8 (Cell Signaling Technology, Beverly, MA). The membrane was stripped and reprobed with antiactin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) to ensure equivalent protein loading.
To characterize growth signaling, immunoblotting was also performed with antiphospho-MAPK antibody (Santa Cruz Biotechnology) and with antiphospho-STAT3, antiphospho-MEK, phospho P70s kinase, phospho-Akt, and antiphospho-p65 NFκB (Cell Signaling Technology). Antigen-antibody complexes were detected by using enhanced chemiluminescence (Amersham, Arlington Heights, IL). Blots were stripped and reprobed with antiactin antibody (Santa Cruz Biotechnology) to ensure equivalent protein loading.
Detection of apoptosis in patient myeloma cells
Bone marrow aspirates were subjected to Ficoll Paque gradient centrifugation (Amersham), and mononuclear cells were separated. Mononuclear cells were suspended in RPMI 1640 media containing 20% fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were placed in a 24-well plate at a concentration of 2 × 106 cells/mL; CC-5013 and rapamycin were added to the medium to obtain the desired final concentrations. Patient cells were cultured for 48 hours and then harvested. After 2 washes with PBS, they were stained with FITC-conjugated CD38 monoclonal antibody and PE-conjugated Apo 2.7 antibody. The cells were analyzed on RXP Cytomics software on an Epics flow cytometer (Coulter Immunology). The percentage of CD38bright cells that were Apo 2.7-positive was the percentage of apoptotic myeloma cells.
Isobologram analysis
The interaction between CC-5013 and rapamycin was analyzed using the CalcuSyn software program (Biosoft, Ferguson, MO) to determine whether the combination was additive or synergistic. Data from cell viability assay (MTT) were expressed as the fraction of cells killed by the individual drugs or the combination in drug-treated compared with untreated cells. This program is based upon the Chou-Talalay method, which calculates a combination index (CI), and analysis is performed based on the following equation: CI = (D)1/(Dx)1 + (D)2/(Dx)2 + (D)1(D)2/(Dx)1(Dx)2, where (D)1 and (D)2 are the doses of drug 1 and drug 2 that have x effect when used in combination, and (Dx)1 and (Dx)2 are the doses of drug 1 and drug 2 that have the same x effect when used alone.25 When CI = 1, this equation represents the conservation isobologram and indicates additive effects. CI below 1.0 indicates synergism.
Results
Effects of CC-5013, rapamycin, and combination on DNA synthesis of MM cell lines
The effects of CC-5013 and rapamycin, alone and in combination, on DNA synthesis of MM cell lines (MM.1S, OPM2, and SB645) were determined by measuring 3H-TdR uptake during the last 8 hours of 48-hour cultures. Rapamycin (0.01-100 nM), CC-5013 (0.01-100 μM), and combinations of these agents inhibited 3H-TdR uptake of MM.1S, OPM2, and SB645 cells in a dose-dependent fashion (Figure 1A). Rapamycin (nM) and CC-5013 (μM) were used in fixed-dose combinations. Fifty percent inhibition (IC50) of proliferation in all 3 cell lines was seen at a dose between 0.1 and 1 nM rapamycin when combined with 0.1 to 1 μM CC-5013, a dose that was 10-fold lower than when either agent was used alone. The effects of these drugs on proliferation were confirmed using MTT assays (Figure 1B).
Effects of CC-5013, rapamycin, and their combination on proliferation of MM cell lines and PBMCs. (A) Time- and dose-dependent inhibition of proliferation in sensitive MM cell lines (MM1.S, OPM2, SB645), demonstrated by measuring thymidine [3H-TdR] uptake during the last 8 hours of 48-hour cultures, in the presence of CC-5013 (μM), rapamycin (nM), or both in fixed-dose treatment. (B) Time- and dose-dependent inhibition of proliferation demonstrated by MTT in MM1.S cells. (C) Inhibition of DNA synthesis as measured by 3H-TdR uptake in resistant MM cell lines treated with CC-5013 and rapamycin. (D) Lack of toxicity of combined therapy on PBMCs, demonstrated by MTT assay. Error bars indicate one standard deviation.
Effects of CC-5013, rapamycin, and their combination on proliferation of MM cell lines and PBMCs. (A) Time- and dose-dependent inhibition of proliferation in sensitive MM cell lines (MM1.S, OPM2, SB645), demonstrated by measuring thymidine [3H-TdR] uptake during the last 8 hours of 48-hour cultures, in the presence of CC-5013 (μM), rapamycin (nM), or both in fixed-dose treatment. (B) Time- and dose-dependent inhibition of proliferation demonstrated by MTT in MM1.S cells. (C) Inhibition of DNA synthesis as measured by 3H-TdR uptake in resistant MM cell lines treated with CC-5013 and rapamycin. (D) Lack of toxicity of combined therapy on PBMCs, demonstrated by MTT assay. Error bars indicate one standard deviation.
Effect of the combination of CC-5013 and rapamycin on DNA synthesis of MM cells resistant to conventional chemotherapy
To examine whether these drugs were active against cell lines resistant to conventional therapies, we similarly studied Dox40 cells, LR5 cells, and MM.1R cells. The combination of CC-5013 and rapamycin inhibited DNA synthesis of Dox40, LR5, and MM.1R cells in a dose-dependent fashion, albeit at higher concentrations than the drug-sensitive cell lines (Figure 1C). MM1.R cells were the most sensitive, suggesting a lack of cross-resistance to combined therapy compared with dexamethasone.
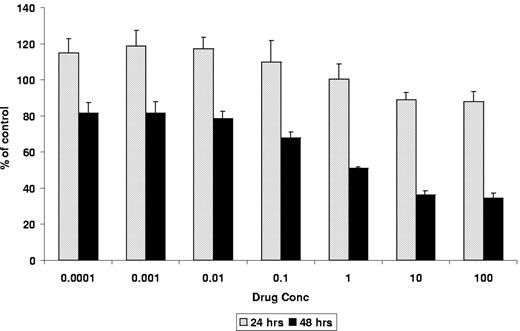
We next studied the effect of individual drugs and combinations on normal peripheral blood mononuclear cells by MTT assays. No cytotoxicity was noted at even the highest dose of rapamycin (100 nM) when combined with CC-5013 (100 μM) (Figure 1D). In time-course experiment to determine the time of maximal drug effect, cytotoxicity was minimal at 24 hours and maximal at 48 hours (Figure 2).
Time course for inhibiting the proliferation of MM1.S cells. Time course was demonstrated by 3H-TdR uptake, after fixed-dose treatment with combined CC-5013 (0.01-100 μM) and rapamycin (0.01-100 nM). Maximal dose-dependent inhibition of DNA synthesis was seen at 48 hours.
Time course for inhibiting the proliferation of MM1.S cells. Time course was demonstrated by 3H-TdR uptake, after fixed-dose treatment with combined CC-5013 (0.01-100 μM) and rapamycin (0.01-100 nM). Maximal dose-dependent inhibition of DNA synthesis was seen at 48 hours.
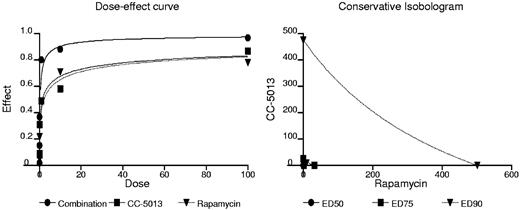
Combination CC-5013 and rapamycin is synergistic in MM cell lines
Adding increasing doses of rapamycin to CC-5013 resulted in increased cytotoxicity at every dose of CC-5013 examined. The interaction between rapamycin and CC-5013 was analyzed using the CalcuSyn software program to determine whether this combination had additive or synergistic cytotoxicity. Based on the Chou-Talalay method, to calculate CI, we generated an isobologram of varying concentrations of rapamycin with CC-5013 (Figure 3). At doses ranging from 0.1 to 100 μM CC-5013 combined with 0.1 to 100 nM rapamycin, CI ranged from 0.526 to 0.015, suggesting that this combination was highly synergistic (Table 1).
Combination CC-5013 and rapamycin is synergistic in myeloma cell lines. Demonstration of dose-effect curve and isobologram.
Combination CC-5013 and rapamycin is synergistic in myeloma cell lines. Demonstration of dose-effect curve and isobologram.
CIs generated from isobologram at increasing concentrations of rapamycin and CC-5013
CC-5013 (μM/L) . | Rapamycin (nM/L) . | Fractional activity . | CI . |
|---|---|---|---|
| 0.1 | .1 | 0.37 | 0.526 |
| 1 | 1 | 0.8 | 0.033 |
| 10 | 10 | 0.88 | 0.071 |
| 100 | 100 | 0.97 | 0.015 |
CC-5013 (μM/L) . | Rapamycin (nM/L) . | Fractional activity . | CI . |
|---|---|---|---|
| 0.1 | .1 | 0.37 | 0.526 |
| 1 | 1 | 0.8 | 0.033 |
| 10 | 10 | 0.88 | 0.071 |
| 100 | 100 | 0.97 | 0.015 |
Combination CC-5013 and rapamycin overcomes the protective effects of growth factors and BMSCs
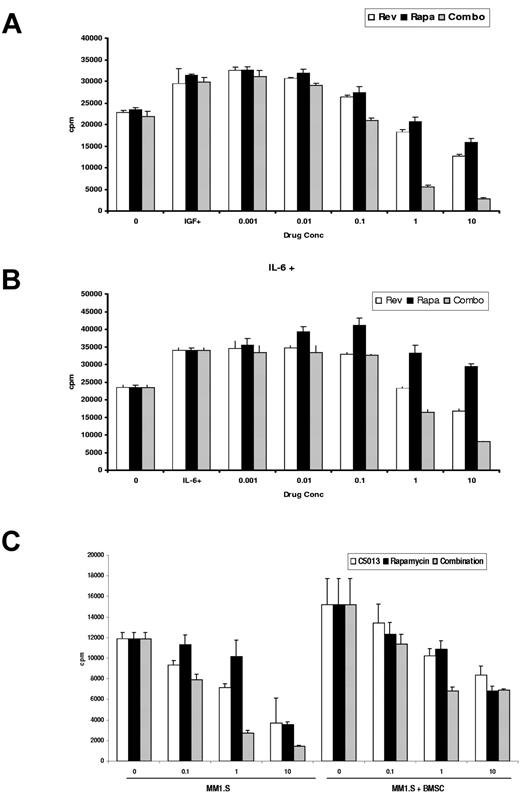
We next examined whether these drugs could overcome the MM cell growth and survival induced by IL-6 and IGF-1. MM1.S cells were cultured with increasing doses of CC-5013, rapamycin, and combinations of these drugs, in the presence of IL-6 (10 ng/mL) or IGF-1 (50 ng/mL). Rapamycin alone was unable to overcome the protective effects of these growth factors (Figure 4A-B). However, rapamycin combined with CC-5013 triggered a marked inhibition of DNA synthesis, suggesting that the combination overcomes the protective effects of these growth factors.
Combination CC-5013 and rapamycin overcomes the protective effect of IL-6, IGF-1, and adherence to the bone marrow microenvironment. Rapamycin-induced cytotoxicity is abrogated in the presence of cytokines, but the combination of CC-5013 with rapamycin overcomes the protective effects of (A) IL-6 and (B) IGF-1. (C) Combined therapy was also effective at inhibiting DNA synthesis in MM1.S cells adherent to BMSCs.
Combination CC-5013 and rapamycin overcomes the protective effect of IL-6, IGF-1, and adherence to the bone marrow microenvironment. Rapamycin-induced cytotoxicity is abrogated in the presence of cytokines, but the combination of CC-5013 with rapamycin overcomes the protective effects of (A) IL-6 and (B) IGF-1. (C) Combined therapy was also effective at inhibiting DNA synthesis in MM1.S cells adherent to BMSCs.
The effect of this drug combination on DNA synthesis of MM1.S cells in the microenvironment was similarly examined (Figure 4C). Combined CC-5013 and rapamycin inhibited 3H-TdR uptake of MM1.S cells cultured in the presence of BMSCs in a dose-dependent fashion.
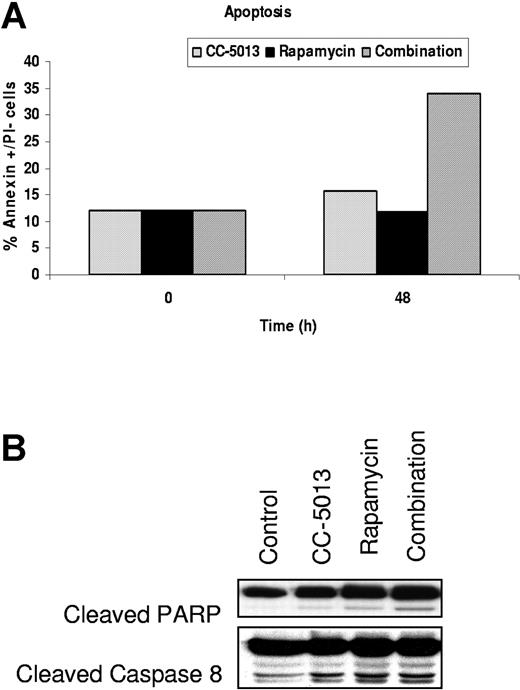
Effect of CC-5013, rapamycin, and combination therapy on cell cycle profile and apoptosis of MM cell lines
To further characterize the cytotoxic effect of these drugs on MM cell lines, we next performed cell cycle analysis on MM1.S cells cultured with media alone, CC-5013 (1 μM), rapamycin (1 nM), or a combination of these agents for 12 to 48 hours. The combination of CC-5013 and rapamycin increased the sub-G1 MM.1S cell population at 48 hours (data not shown). Annexin V/PI staining confirmed 33.9% annexin V+/PI- cells after 48 hours of exposure to combined therapy (Figure 5A). An increase in PARP and caspase 8 cleavage was induced at 24 hours by combined therapy demonstrated by Western blot analysis (Figure 5B).
Combination CC-5013 and rapamycin induces apoptosis in MM1.S cells. (A) Increase in the apoptotic cells (Annexin+ PI-) after exposure to CC-5013 (1 μM) and rapamycin (1 nM) for 48 hours, (B) associated with caspase 8 and PARP cleavage.
Combination CC-5013 and rapamycin induces apoptosis in MM1.S cells. (A) Increase in the apoptotic cells (Annexin+ PI-) after exposure to CC-5013 (1 μM) and rapamycin (1 nM) for 48 hours, (B) associated with caspase 8 and PARP cleavage.
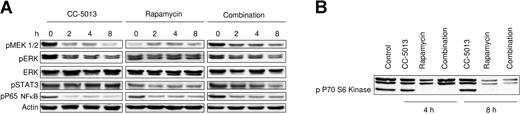
Effect of CC-5013 and rapamycin on growth signaling in MM1.S cells
To delineate the mechanisms of cytotoxicity of combined CC-5013 and rapamycin against MM cells, we incubated MM1.S cells with CC-5013 (1 μM), rapamycin (1 nM), or this drug combination for 2, 4, 6, and 8 hours; cell lysates were prepared as previously described. CC-5013 inhibited the MAPK pathway, demonstrated by the inhibition of pMEK1/2 and pERK, in a time-dependent fashion. In contrast, no inhibition of the MAPK pathway was noted with rapamycin. Conversely, at higher rapamycin doses, an upregulation of the MAPK pathway was seen, evidenced by increased phosphorylation of MEK1/2 and ERK. The combination of rapamycin and CC-5013 triggered a time-dependent inhibition of MAPK (Figure 6A). Rapamycin resulted in a time-dependent inhibition of pSTAT3, which was unaffected by CC-5013. pP65 NFκB was down-regulated by these drugs, either alone or combined. No change in pAkt was induced (data not shown) by either agent, alone or combined. p70S kinase was completely inhibited by rapamycin alone and combined with CC-5013, but CC-5013 alone had no effect (Figure 6B).
Combination CC-5013 and rapamycin affects growth and survival signaling pathways in MM1.S cells. MM1.S cells were treated with CC-5013 (1 μM), rapamycin (1 nM), or combined therapy for up to 8 hours. (A) Down-regulation of the MAPK pathway by CC-5013 and the STAT pathway by rapamycin. Rapamycin does not affect or it up-regulates MAPK pathway, evidenced by the phosphorylation of pERK and pMEK1/2. Combined CC-5013 and rapamycin inhibits both pathways, demonstrated by the down-regulation of pERK, pMEK1/2, and pSTAT. These agents, alone and in combination, inhibit pP65NFκB. (B) Complete inhibition of p70S6 kinase phosphorylation by rapamycin alone or by combination therapy, whereas CC-5013 alone has no effect.
Combination CC-5013 and rapamycin affects growth and survival signaling pathways in MM1.S cells. MM1.S cells were treated with CC-5013 (1 μM), rapamycin (1 nM), or combined therapy for up to 8 hours. (A) Down-regulation of the MAPK pathway by CC-5013 and the STAT pathway by rapamycin. Rapamycin does not affect or it up-regulates MAPK pathway, evidenced by the phosphorylation of pERK and pMEK1/2. Combined CC-5013 and rapamycin inhibits both pathways, demonstrated by the down-regulation of pERK, pMEK1/2, and pSTAT. These agents, alone and in combination, inhibit pP65NFκB. (B) Complete inhibition of p70S6 kinase phosphorylation by rapamycin alone or by combination therapy, whereas CC-5013 alone has no effect.
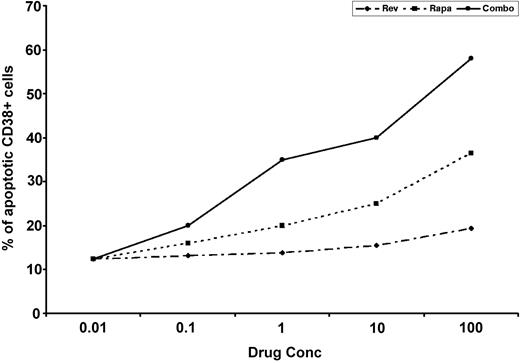
Combination CC-5013 and rapamycin induces apoptosis of patient myeloma cells
Combined CC-5013 and rapamycin therapy induced a dose-dependent increase in apoptosis of patient MM cells, evidenced by Apo 2.7 staining of CD38+ BMNCs on flow cytometry. Cells from 4 MM patients were studied, and representative data from 1 patient is shown in Figure 7.
Effect of CC-5013, rapamycin, and combination on patient myeloma cells. A dose-dependent increase in the percentage of apoptotic patient MM cells, evidenced by Apo 2.7 staining of bright CD38-positive cells, was noted after exposure to combination CC-5013 and rapamycin treatment.
Effect of CC-5013, rapamycin, and combination on patient myeloma cells. A dose-dependent increase in the percentage of apoptotic patient MM cells, evidenced by Apo 2.7 staining of bright CD38-positive cells, was noted after exposure to combination CC-5013 and rapamycin treatment.
Discussion
Our previous data suggest that targeting the PI3K/Akt pathway may have direct apoptotic and antiproliferative effects on tumor cells, in addition to overcoming cell adhesion-mediated drug resistance.14,16 In this report, we have studied the effect of combining CC-5013 with rapamycin on MM cells. Our study demonstrates a strong synergism between 2 orally bioavailable drugs that have shown in vitro activity in MM. Furthermore, rapamycin and CC-5013 can be combined at doses well below those that are pharmacologically achievable, making this an attractive combination to test in the clinic. Because a dose of 1 nM rapamycin plus 1 μM CC-5013 resulted in peak inhibition of proliferation, we used these doses for subsequent studies of cell cycle, apoptosis, and signaling. This rapamycin dose translates to 15-fold lower than that used for immunosuppression in transplantation, coupled with a 5-fold lower dose of CC-5013 than that used in the single-agent phase 2 CC-5013 trial in MM patients with relapsed or refractory disease.24
Our data also demonstrate that rapamycin alone was unable to overcome the protective effects of IL-6, IGF-1, and tumor cell adherence to BMSCs, whereas the combination of rapamycin and CC-5013 inhibited MM cell growth in the BM milieu and has potent anti-MM activity. This study demonstrates for the first time the role of mTOR inhibitors not only on tumor cells directly but also on their BM microenvironment. IGF-1-mediated protection from rapamycin-induced apoptosis has been similarly noted in rhabdomyosarcoma cells.26 Therefore, though rapamycin alone and other mTOR inhibitors show potent antitumor activity in vitro, in future clinical trials it may be necessary to combine rapamycin with agents able to overcome the protective effects of growth factors in the tumor milieu. To this end, Stromberg et al27 have recently demonstrated that rapamycin can sensitize MM cells to Dex-induced apoptosis in MM cells, suggesting that this combination was able to overcome the protective effects of IL-6 and IGF-1.
Although most studies have reported TOR inhibition with rapamycin or its analogs to result in a cytostatic response with G1 growth arrest,19 some reports demonstrate tumor cell apoptosis, as in our study.26,28 This effect may be cell-specific and may be enhanced when the 2 agents are combined. In MM, apoptosis was induced by higher doses of rapamycin alone and by combined CC-5013TM/rapamycin.
We have delineated downstream signaling cascades targeted by CC-5013 and rapamycin alone and in combination. As shown previously, CC-5013 inhibited the activation of MAPK pathway signaling,23 whereas rapamycin either did not affect, or at higher doses up-regulated, this pathway. The combination of CC-5013 and rapamycin inhibited MAPK signaling. STAT3 phosphorylation was not affected by CC-5013, but it was inhibited in a time-dependent manner by rapamycin. Combined therapy also blocked STAT3 phosphorylation in a time-dependent manner. Rapamycin-induced down-regulation of pSTAT3 has been previously demonstrated in other cell systems, including B-cell lymphomas and glial cells.29,30 In MM, STAT signaling mediates survival,10 and increasing evidence suggests cross-talk between the MAPK and the PI3K/Akt pathways mediating MM cell growth. Specific inhibition of PI3K/Akt abrogated IGF-1-induced MAPK activation and proliferation of MM cells.15 Furthermore, Qiang et al15 were able to demonstrate that the PI3K inhibitor LY294002, completely blocked protection against Dex-induced apoptosis by IGF-1. Rapamycin only partially blocked protection, whereas specific MAPK inhibitors had no effect. Taken together, these observations suggest that interrupting the PI3K pathway is a promising therapeutic strategy. Rapamycin acts downstream of the PI3K pathway, as demonstrated by the inhibition of p70S kinase and pSTAT3. CC-5013, on the other hand, inhibits the MAPK pathway. Therefore, combining an MAPK inhibitor such as CC-5013 with an mTOR inhibitor such as rapamycin may enhance antitumor response.
The role of NF-κB as a therapeutic target in MM has been previously described.31 In MM, targeting of NF-κB can overcome the growth and survival advantage conferred by MM cell-BMSC interaction and cytokines in the BM milieu. In this study, we demonstrate a time-dependent down-regulation of p65NF-κB induced by either rapamycin and CC-5013 or by combined therapy, highlighting the role this drug combination may play in overcoming drug resistance.
mTOR inhibition as an anticancer strategy has been previously tested, and ongoing clinical trials are currently evaluating CCI-779, the rapamycin ester analog.20,22,32,33 Another mTOR inhibitor undergoing in vitro and in vivo testing is RAD001.34 Although these inhibitors have shown preclinical promise, their roles as single agents in phase 2 studies have resulted in only modest responses. Our data support the notion that mTOR inhibitors will have maximum benefit when rationally combined with other agents, based on inhibiting multiple signaling cascades. We show that the combination of rapamycin and CC-5013 overcomes the protective effects of IL-6, IGF-1, and BMSCs, whereas rapamycin alone does not. The 2 drugs act by interrupting different signaling cascades, further supporting the role of combining these agents in clinical practice. We have also demonstrated their activity in MM cells resistant to conventional chemotherapy, further suggesting potential clinical testing in patients with relapsed and refractory myeloma. These preclinical studies, therefore, provide the rationale for evaluating combined rapamycin and CC-5013 therapy to overcome conventional drug resistance and to improve patient outcomes in MM.
Prepublished online as Blood First Edition Paper, August 19, 2004; DOI 10.1182/blood-2004-06-2281.
Supported by National Institutes of Health Grant Specialized Programs of Research Excellence (SPORE) IP50 CA10070-01, PO-1 78378, and RO-1 CA 50947; the Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.); the Cure for Myeloma Research Fund (K.C.A.) and PO1 HL070149-01 (J.H.A); and VA Merit Review (N.C.M.). Supported in part by a grant from Celgene Corporation.
One of the authors (D.I.S.) is employed by Celgene Corp, whose product (Revlimid) was studied in the present work.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. Effects of CC-5013, rapamycin, and their combination on proliferation of MM cell lines and PBMCs. (A) Time- and dose-dependent inhibition of proliferation in sensitive MM cell lines (MM1.S, OPM2, SB645), demonstrated by measuring thymidine [3H-TdR] uptake during the last 8 hours of 48-hour cultures, in the presence of CC-5013 (μM), rapamycin (nM), or both in fixed-dose treatment. (B) Time- and dose-dependent inhibition of proliferation demonstrated by MTT in MM1.S cells. (C) Inhibition of DNA synthesis as measured by 3H-TdR uptake in resistant MM cell lines treated with CC-5013 and rapamycin. (D) Lack of toxicity of combined therapy on PBMCs, demonstrated by MTT assay. Error bars indicate one standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-06-2281/6/m_zh80240470780001.jpeg?Expires=1769086659&Signature=4P8MFl--HcO4pxFryp2YdqAR~Wzwx2AK4KHNPqIYjeX4LQKG7mCArFx8ZlI12nyK-JcUO~UOkt3WR-rmvKGMYMKckVGIMHLMvGWGl4sQG6LDr4~fqu9dPHnj~Ivlg8aFO565hqkoIjmVrtfMpbCd7mr5K6V0JQ3bDIwLIXpneQxxJytgnPXfauRH5m8BTtV9lzDH6VKkqpmL0MfZR5sk4Sc0MU0Ms6NC88waXGorYIKDqYAHn-IWOph-GGbfGXMqy5BroyHSPiXY56P-LROpADjElS~Cyw63KBINka~ocSf-HenX6lTfkboHMKk8TulbqSh5KrgBnThYHElOqRrCaA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal