Abstract
Interleukin-7 receptor (IL-7R) levels are tightly controlled during ontogeny: high on double-negative (DN) cells, absent on double-positive (DP) cells, and high once again on thymocytes undergoing positive selection. To determine if loss of IL-7–mediated survival signals in DP cells is necessary for normal antigen-specific selection, we created T-lineage–specific IL-7R α chain (IL-7Rα) transgenic (Tg) mice in which IL-7R is expressed throughout ontogeny. There was no effect of the IL-7Rα Tg on negative selection. Surprisingly, however, although the thymi of IL-7Rα Tg mice were comparable at birth, there was a decrease in thymocyte number as the mice aged. This was found to be due to competition between DN and IL-7R–expressing DP cells for endogenous IL-7, which resulted in decreased levels of Bcl-2 in DN cells, increased DN apoptosis, and decreased DN cell number. Therefore, the down-regulation of IL-7R on DP cells is an “altruistic” act required for maintaining an adequate supply of local IL-7 for DN cells.
Introduction
Interleukin-7 (IL-7) is a nonredundant trophic factor for immune cell development that acts primarily by promoting cell survival.1,2 In the thymus, IL-7 is produced by stromal cells (primarily, major histocompatibility complex [MHC] class II–positive epithelial cells) and effective concentrations are achieved only locally, perhaps by retention in extracellular matrix glycosaminoglycans.3
The receptor for IL-7 is a heterodimer of an α subunit (IL-7Rα or CD127) and the cytokine receptor common γ chain (γc).4,5 The latter is shared by other cytokine receptors, including those for IL-2, IL-4, IL-9, IL-15, and IL-21.6,7 Heterodimerization of IL-7Rα and γc upon IL-7 binding exposes tyrosines in the intracellular region of IL-7Rα to the γc-associated Janus kinase 3 (Jak3), permitting their phosphorylation, recruitment of Jak1, and subsequent activation of the Jak/STAT signaling pathway. It has been reported that phosphatidylinositol-3 kinase also is recruited to IL-7Rα and that Src kinases are activated, which presumably also contribute to survival, proliferation, and/or differentiation.8
Reduction of physiologic IL-7 signaling has severe repercussions in immune system development. IL-7 knockout mice have a 20-fold decrease in thymic size and a vastly constricted peripheral T-cell pool,1 similar to γc-deficient animals.9 Unlike humans, IL-7 deficiency in mice is accompanied by B-cell developmental arrest.1 Compared to IL-7 and γc knockouts, IL-7Rα–deficient mice have an even more striking paucity of T and B cells, arguing for a role for a IL-7R ligand other than IL-7, which has been attributed to thymic stromal lymphopoietin (TSLP).10-12 In these mice, γδ T cells are completely absent due to the absence of TCRγ rearrangement.13 Thus far, 5 IL-7 Tg mice have been described.14-18 When placed under the control of an MHC-II promoter, IL-7 overexpression resulted in a 30-fold increase in peripheral T and B cells without affecting thymic cellularity or CD4/CD8 ratios.14 On the other hand, when driven by the IgH promoter and enhancer, IL-7-overproducing animals had a small thymus (5- to 6-fold decrease) with an almost completely absent DP population and a lymphoproliferative disorder in the periphery with T- and B-cell lymphomas.15
IL-7Rα is expressed on T-, B-, and natural killer (NK)–lineage cells. It can first be detected on common lymphoid progenitor (CLP) cells in bone marrow (BM), and its levels are tightly regulated during lymphocyte development, with alterations depending on cell maturity and activation status.19 Recently, it has been suggested that early thymic progenitors that give rise to T-lineage cells do not express measurable levels of IL-7R and are not IL-7 responsive, and thus may be derived independently from CLPs or have down-regulated the receptor.20 Once committed to the T lineage, IL-7R levels are tightly regulated: high levels of expression are found on CD4-CD8-(DN) cells, while CD4+CD8+ (DP) thymocytes do not express IL-7R at detectable levels. IL-7R levels increase once again on thymocytes undergoing positive selection and mature single-positive (SP) cells and remain high on peripheral T cells3,21 unless activated.22,23 IL-7 production also is tightly regulated during ontogeny, with peak production in fetal development accompanied with massive thymic expansion and a substantial decrease in the aging thymus.24
The tight coordination of IL-7R levels with state of T-cell maturation strongly suggests that the precise regulation of IL-7R signaling is necessary for normal T-cell development. Given that IL-7 has prosurvival functions, one obvious and intriguing possibility is that loss of IL-7R on DP thymocytes is required to allow antigen-specific negative selection to occur.25 To test this, we created transgenic animals in which IL-7R levels would be maintained in all thymocyte subsets by putting IL7Rα under the control of the CD2 promoter. Although we found antigen-specific selection to be unaltered, thymocyte numbers were profoundly reduced in an age-dependent manner. The following analysis indicates that this is due to competition between the expanding numbers of IL-7R+ DP thymocytes and their DN precursors for limiting amounts of endogenous IL-7, and therefore that normal IL-7R down-regulation is necessary to maintain a functional balance between IL-7 production and IL-7 consumption.
Materials and methods
Reagents and antibodies
For flow cytometry, directly conjugated or biotinylated monoclonal antibodies for CD4 (clones RM4.5 and RM4.4), CD8a (53-6.7), CD8b.2 (53-5.8), TCRβ (H57-597), IL-7R (SB/14 and B12-1), CD25 (7D4 and PC61), CD44 (IM7), Thy1.2 (53-2.1), CD3 (145-2C11), Mac-1 (M1/70), B220 (RA3-6B2) TCRγδ (GL-3), NK-1.1 (NKR-P1C), CD45.1 (A20), CD45.2 (104), Thy1.1 (OX-7), Bcl-2, dUTP, FcγIII/II receptor (2.4G2), IgG isotype control as well as fluorescein isothiocyanate (FITC), CyChrome, or phycoerythrin (PE)–labeled streptavidin were obtained from BD Pharmingen (San Diego, CA). Recombinant mouse IL-7 (rmIL-7) was purchased from R&D Systems (Minneapolis, MN), and human IL-7 was a gift from Terry Fry and Crystal Mackall. Moth cytochrome c (MCC) peptide (88-103) was purchased from Macromolecular Resources (Fort Collins, CO). Saponin and sodium azide (NaN3) were purchased from Sigma (St Louis, MO), bovine serum albumin (BSA) was obtained from ICN Biomedicals (Aurora, OH). For fetal thymic organ cultures (FTOCs), filters were obtained from Whatman (Clifton, NJ), and Nuclepore Track-Etch membrane and Gelfoam sponges were purchased from Pharmacia & Upjohn (Toronto, ON, Canada).
Mice
C57BL/6 (Thy1.2/Ly5.2) mice were obtained from Frederick Cancer Research Facility (Frederick, MD). Congenic B6.PL-Thy-1.1 (Thy1.1/Ly5.2) and C57BL/6-SJL (Thy1.2/Ly5.1) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). To create IL-7Rα Tg mice, murine IL-7Rα cDNA (kindly provided by Scott Durum, NCI-Frederick, MD) was ligated into the human CD2 promoter and enhancer expression cassette p29Δ2(EcoR I-Sal 1-) (generously supplied by Paul Love, National Institutes of Health, Bethesda, MD). The internal EcoRI site of cDNA of IL-7Rα in pLXIN vector was eliminated using Quick Change Site–directed Mutagenesis Kit (Stratagene, La Jolla, CA). The mutation introduced was silent as it preserved amino acid sequence (GAA to GAG in codon 98). The primers used were as follows; forward: 5′-TTTTATAAAGACATCAGAGTTCTTACTGATTGGTAG-3′ and reverse: 5′-GCTACCAATCAGTAAGAACTCTGCTGTCTTTATAAAA3′ (the nucleotide change is underlined). The newly made pLXIN IL-7Rα was used as the template for polymerase chain reaction (PCR) reaction to introduce EcoRI and SalI ligation sites. Primers for the PCR reaction were: forward 5′-CCGGAATTCATGATGGCTCTGGGTAGAGCTTTCG3′ and reverse: 5′-GGA CGCGTCGACTCATTTGTTTTGGTAAAAACTAGACATGG3′ (EcoRI and SalI sites are underlined, respectively). The amplified product was ligated into p29Δ2 and injected into C57BL/6 oocytes at the National Cancer Institute (NCI) transgenic core facility in Frederick, MD. The IL-7Rα Tg mice were bred in NCI animal facility to C57BL/6 mice and carried as heterozygotes. TCR-Cyt-5CC7-I/Rag2 (5CC7) mice were obtained from Taconic (Germantown, NY) and bred to IL-7Rα Tg mice. IL-7 Tg mice (designated IL-7-TgA) on the C57BL/6 background were generated as described18 and crossed to IL-7Rα Tg mice in our animal facility.
Fetal thymic organ culture and deletion assays
Fetal thymi from C57BL/6 × IL-7Rα Tg and 5CC7 × IL-7Rα Tg mating were recovered from embryos at gestational days 15-16. Thymic lobes were placed on Millipore filters floating on a Gelfoam sponge in RPMI 1640 (Biofluids; Gaithersburg, MD) supplemented with 10% heat-inactivated fetal calf serum, 100 U/mL penicillin, 4 mM glutamine, 10 mM sodium pyruvate, nonessential amino acids, and 5 μM 2-mercaptoethanol (complete medium). For deletion assays thymic lobes were cultured for 3 days, and then 10 μg/mL of anti-TCRβ (H57-597) or 0.1 to 1 μM MCC peptide was added to lobes originating from C57BL/6 × IL-7Rα Tg and 5CC7 × IL-7Rα matings, respectively. Thymic lobes were harvested 24 hours after deletion initiation. To some cultures, recombinant mouse or human IL-7 was added at a final concentration of 10 ng/mL.
Cell staining and flow cytometry
Thymocytes were stained for surface markers for 45 minutes on ice in the presence of FcγIII/II receptor blocking antibodies and analyzed either on FACS Scan or FACSCalibur (Becton Dickinson, San Jose, CA). In some experiments, analysis of DN cells was performed by excluding cells expressing CD4, CD8, TCRγδ, TCRβ, Mac-1, NK1.1 and B220. All data were analyzed with FlowJo software (Tree Star, San Carlos, CA). Intracellular staining for Bcl-2 was done as described previously.26,27 Briefly, 2 × 106 cells were harvested after 18 to 20 hours in vitro culture with or without supplementing IL-7 (3-10 ng/mL). Upon staining for surface markers, cells were washed with FACS buffer (Hanks balanced salt solution with 2% BSA and 0.01% sodium azide) and fixed with 2% formaldehyde in phosphate-buffered saline (PBS) for 15 minutes on ice. Subsequently, cells were permeabilized in saponin buffer (0.03% saponin, 2% BSA, 0.1% NaN3) and stained with antibodies against Bcl-2 for 30 minutes.
TUNEL assay
Terminal deoxytransferase nick end labeling (TUNEL) was performed on thymocytes isolated from 6- to 10-week-old C57BL/6 and IL-7Rα Tg mice and cultured in complete medium for 12 to 24 hours with or without recombinant mouse IL-7 (3-10 ng/mL). Upon washing, cells were stained with surface markers, and TUNEL assay was performed with Pharmingen Apo-Direct kit (San Diego, CA) by manufacturer's instructions.
Preparation of bone marrow chimeras
Bone marrow (BM) donors were IL-7Rα Tg and congenic C57BL/6.Ly5.1 mice. For most of the experiments BM was T-cell depleted by negative selection on MACS columns according to manufacturer's protocol. Prior to column separation, BM cells were incubated with rat monoclonal anti–Thy1 antibody (G7) followed by incubation with a mixture of MACS magnetic beads: anti-CD4, anti-CD8, and anti–rat IgG. T-cell–depleted or unfractionated BM cells were transplanted into lethally irradiated (1000 rad) C57BL/6 or B6-Thy1.1 mice by tail vein injections. Seven to 10 × 106 cells were transferred per animal. BM chimeras were reconstituted with just IL-7Rα Tg or C57BL/6-Ly5.1 cells. For competition experiments BM chimeras were made with mixture of equal numbers of IL-7Rα Tg and wild-type cells. Analysis of the chimeras was performed 4 to 7 weeks after BM reconstitution.
Results
Expression of a functional transgenic IL-7Rα
A cDNA expression vector was made in which murine IL-7Rα was placed under the control of the human CD2 promoter to ensure expression of the transgene solely in the T-cell lineage. This construct was injected into C57BL/6 oocytes, generating 7 mice that were Southern blot positive for the transgene. Three of these animals were selected as founders for further study: 2 had a high level of transgene incorporation (C10 and B1) and one had less (D4) (data not shown). IL-7Rα surface expression on thymocytes from representative mice from each founder is shown (Figure 1A). The ratios of the major thymocyte subpopulations (DN, DP, and SP) in the mice at 10 days of age were similar in all of the mice, indicating that the transgene did not have a major effect on early T-cell development (Figure 1B). The relative levels of IL-7R on the surface of different thymocyte subpopulations were assessed (Figure 1C). As previously shown, IL-7R is highly expressed on DN cells in wild-type mice, absent on DP cells, and present once again on SP cells.3 C10 (Figure 1C) and B1 (data not shown) founders expressed IL-7Rα levels that were approximately 5 times higher than physiologic levels on DN and SP cells, while the D4 founder had approximately 3 times the normal level. Importantly, and as expected because of the CD2 promoter, transgenic IL-7R was expressed on DP cells. Similar relative expression levels were observed throughout development.
IL-7Rα expression in different thymic subsets of 3 founder mice. (A) Unfractionated thymocytes from wild-type and the indicated IL-7Rα Tg mice (D4, B1, and C10) were stained for IL-7Rα expression. (B) Thymocytes from 10-day-old mice were stained for CD4 and CD8. Representative profiles from wild-type, Tg low-expressor (D4), and Tg high-expressor (C10) lines are shown. (C) IL-7Rα expression is shown in thymocyte subpopulations (DN, DP, and SP) in wild-type (thin line), D4 (dashed line), and C10 mice (thick line); for clarity, the negative staining control is shown only in the first histogram (dotted line).
IL-7Rα expression in different thymic subsets of 3 founder mice. (A) Unfractionated thymocytes from wild-type and the indicated IL-7Rα Tg mice (D4, B1, and C10) were stained for IL-7Rα expression. (B) Thymocytes from 10-day-old mice were stained for CD4 and CD8. Representative profiles from wild-type, Tg low-expressor (D4), and Tg high-expressor (C10) lines are shown. (C) IL-7Rα expression is shown in thymocyte subpopulations (DN, DP, and SP) in wild-type (thin line), D4 (dashed line), and C10 mice (thick line); for clarity, the negative staining control is shown only in the first histogram (dotted line).
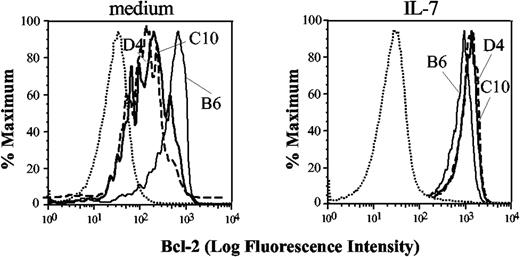
Although Tg IL-7Rα was expressed on DP cells, it is possible that it might not function when expressed in this thymocyte subset. This could occur, for example, if the common γ chain was limiting in DP cells. To determine if the IL-7R was functional on DP cells, we measured IL-7–induced up-regulation of Bcl-2, which is thought to be one of the major mechanisms by which IL-7 exerts its prosurvival activity. Intracellular staining with antibodies against Bcl-2 and quantitation by flow cytometry facilitates detection of IL-7 responsiveness in distinct thymocyte subsets.26,27 As previously shown,26,28 gated wild-type DP cells (Figure 2) had almost unmeasurable basal levels of Bcl-2 (left panel), and there was little if any change after the addition of recombinant mouse IL-7 (right panel). Bcl-2 in cultured DP cells from IL-7Rα Tg mice also was largely undetectable. Importantly, a large number of IL-7Rα Tg DP thymocytes responded to IL-7 stimulation by up-regulating Bcl-2 (right panel). Thus, the transgenic IL-7R is functional in DP cells.
IL-7 responsiveness of Tg mice. Staining for Bcl-2 is shown in wild-type (dashed line) and Tg thymocytes (filled line) upon 18 hours in vitro treatment with 3 ng/mL of recombinant murine IL-7 (right panels) or vehicle control (dimethyl sulfoxide [DMSO]; left panels) in gated DP cells. One representative experiment out of 4 is shown; all mice were young adults, age matched per individual experiment. Control antibody staining is shown only in the first histogram (dotted line).
IL-7 responsiveness of Tg mice. Staining for Bcl-2 is shown in wild-type (dashed line) and Tg thymocytes (filled line) upon 18 hours in vitro treatment with 3 ng/mL of recombinant murine IL-7 (right panels) or vehicle control (dimethyl sulfoxide [DMSO]; left panels) in gated DP cells. One representative experiment out of 4 is shown; all mice were young adults, age matched per individual experiment. Control antibody staining is shown only in the first histogram (dotted line).
Negative selection is not perturbed in IL-7Rα Tg mice
To determine the possible impact of enforced IL-7R expression on DP cells in development and negative selection, we quantitated the cellularity of FTOCs.29 Thymi of IL-7Rα Tg mice taken on fetal day 15-16 and cultured for 4 days were similar to or slightly smaller than wild-type thymi (Figure 3A-B). Treatment of FTOCs with antibodies against TCRβ results in deletion of cells that successfully rearranged TCRβ genes and is a commonly used model for negative selection.30,31 As expected, stimulation of wild-type thymi with anti-TCRβ resulted in deletion. Notably, there was no difference between the deletion observed with wild-type and with IL-7Rα Tg thymi (Figure 3A). Another more physiologic model of negative selection is to provide specific antigen to thymocytes expressing a complementary TCR. To this end, IL-7Rα Tg animals were bred to 5CC7 mice, which express a transgenic αβ TCR that recognizes a cytochrome c peptide presented by the MHC class II molecule I-Ek.32 In this instance, deletion can be induced by adding moth cytochrome c peptide 88-103 to the FTOC. Thymocyte numbers were similar in 5CC7 and 5CC7/IL-7R Tg mice after 4 days of culture (Figure 3B). Moreover, antigen-induced deletion was identical between the 2 groups. To address the possibility that IL-7 was a limiting factor in these FTOCs, exogenous IL-7 was added to FTOCs of IL-7R Tg thymi with or without anti-TCRβ (Figure 3C). The supplemental IL-7 did not influence cell number over the course of the 4-day culture. Moreover, it did not prevent stimulation with anti-TCRβ from causing deletion, as the recovery in the presence of IL-7 was equivalent to nonsupplemented cultures. Together, these data argue strongly that the presence of IL-7R on DP cells does not have a marked effect on early thymocyte development or negative selection.
Negative selection is not perturbed in IL-7Rα Tg mice. (A) Thymocytes were counted after 4 days of in vitro FTOCs from wild-type (bars on left) and B1 Tg thymi (bars on right) that were taken at embryonic days 15 to 16. Where indicated, deletion was initiated with 10 mg/mL of anti-TCRβ antibody (H57-597) 24 hours before harvesting the cultures. Number of thymi in the study is shown on the top of each column. The bars indicate standard errors. (B) FTOC cellularity after 4 days in vitro culture of 15-day-old fetal thymi from 5CC7 (bars on left) or 5CC7 × B1 mice (bars on right) is shown. Where indicated, 0.1 μM MCC (88-103) peptide was added for the last 24 hours. The bars indicate standard errors. (C) Thymi from B1 thymi taken at day 15 were incubated in vitro for 4 days with or without 10 ng/mL of rhIL-7. Deletion was induced as described in panel A. The bars indicate standard deviations.
Negative selection is not perturbed in IL-7Rα Tg mice. (A) Thymocytes were counted after 4 days of in vitro FTOCs from wild-type (bars on left) and B1 Tg thymi (bars on right) that were taken at embryonic days 15 to 16. Where indicated, deletion was initiated with 10 mg/mL of anti-TCRβ antibody (H57-597) 24 hours before harvesting the cultures. Number of thymi in the study is shown on the top of each column. The bars indicate standard errors. (B) FTOC cellularity after 4 days in vitro culture of 15-day-old fetal thymi from 5CC7 (bars on left) or 5CC7 × B1 mice (bars on right) is shown. Where indicated, 0.1 μM MCC (88-103) peptide was added for the last 24 hours. The bars indicate standard errors. (C) Thymi from B1 thymi taken at day 15 were incubated in vitro for 4 days with or without 10 ng/mL of rhIL-7. Deletion was induced as described in panel A. The bars indicate standard deviations.
Age-dependent thymic hypoplasia in IL-7Rα Tg mice
The analysis of fetal thymi revealed no obvious phenotypic differences between wild-type and IL-7Rα Tg mice. However, we were surprised to observe that Tg thymocyte numbers decreased compared to wild type as the mice grew older. Thymocyte numbers in fetuses and young animals up to 1 week of age were similar between IL-7R Tg and wild-type mice (Figure 4A). However, by 10 to 14 days of age, concomitant with a period of extensive thymocyte accumulation, there was a striking difference among the groups. In the period from 7 to 10 to 14 days of age, wild-type thymi more than tripled in cell number, with an average of more than 150 × 106 cells per thymus. Thymi from all 3 founder IL-7R Tg mice high were small, with levels approximately half (D4) or one third (B1 and C10) as large as wild type. By adulthood (5 to 12 weeks of age), thymocyte numbers averaged approximately 175 × 106 per animal in wild-type mice, but remained at approximately 50 × 106 per animal in all IL-7R Tg mice (Figure 4A), and this dichotomy remained as mice aged (data not shown). The CD4/CD8 profiles were largely similar in adult mice (Figure 4B), although we consistently observed a slightly lower percentage of cells in the DN gate. To further explore differences in immature thymocytes, we narrowed the focus to αβ T-cell precursors by excluding myeloid, B-lineage, NK and NKT, and TCRγδ-positive cells.33,34 Such an analysis revealed a large decrease in DN absolute numbers in the IL-7Rα Tg thymi (Figure 4C). The fraction of DN cells at each of the 4 stages of maturity was assessed by measuring the expression of CD44 and CD25 (DN1 through DN4: CD44+CD25- → CD44+CD25+ → CD44-CD25+ → CD44-CD25-)34,35 (Figure 4D). Compared to wild-type cells, IL-7R Tg DN thymocytes accumulated in the DN2 and DN3 subsets, with a corresponding decrease in the most mature (DN4) subset. The averaged results from 3 independent experiments are shown in Figure 4E. This phenotype is consistent with a partial block in progression at the hyperproliferative phase of DN cell development that occurs coincident with expression of the pre-TCR. The exact stage of DN progression at which the Tg IL-7R was expressed was determined (Figure 4F). There were not enough cells in the DN1 gate to analyze. However, gating on the other DN subsets revealed that the Tg IL-7Rα receptor is expressed at least as early as the DN2 stage and persists at the same level throughout DN cell progression (Figure 4D). Therefore, the block in the DN2/DN3 → DN4 progression cannot be explained by the timing of expression of the Tg receptor.
Age-dependent changes IL-7Rα Tg thymus. (A) Thymic cellularity was enumerated at the indicated ages; the bars represent the SEM for each point. Data from B1 and C10 mice, which have similar Tg IL-7R levels and behaved similarly, were pooled. Two to 12 mice are shown per experimental time point. (B) Thymocyte CD4/CD8 profiles are shown for representative mice at 8 weeks of age. (C) The absolute number of DN thymocytes from 3 wild-type and 4 B1 or C10 mice of 8 to 10 weeks of age is shown. (D) DN thymocytes from the indicated strains were examined for expression of CD44 and CD25. (E) The averaged absolute numbers of wild-type (▪) or B1 and C10 (□) cells present in the different DN subpopulation from 3 independent experiments are shown. (F) Surface expression of IL-7Rα is shown on the indicated DN subpopulation, as gated in panel D: DN2 (CD44+CD25+), DN3 (CD44-CD25+), and DN4 (CD44-CD25). The curves represent cells from wild-type (thin line), D4 (dashed line), and C10 (thick line) thymi. Because of the different numbers of cells recovered between the strains, the results are normalized to the number of cells in the largest peak. Control staining is shown only in the first histogram. The bars represent the SEM.
Age-dependent changes IL-7Rα Tg thymus. (A) Thymic cellularity was enumerated at the indicated ages; the bars represent the SEM for each point. Data from B1 and C10 mice, which have similar Tg IL-7R levels and behaved similarly, were pooled. Two to 12 mice are shown per experimental time point. (B) Thymocyte CD4/CD8 profiles are shown for representative mice at 8 weeks of age. (C) The absolute number of DN thymocytes from 3 wild-type and 4 B1 or C10 mice of 8 to 10 weeks of age is shown. (D) DN thymocytes from the indicated strains were examined for expression of CD44 and CD25. (E) The averaged absolute numbers of wild-type (▪) or B1 and C10 (□) cells present in the different DN subpopulation from 3 independent experiments are shown. (F) Surface expression of IL-7Rα is shown on the indicated DN subpopulation, as gated in panel D: DN2 (CD44+CD25+), DN3 (CD44-CD25+), and DN4 (CD44-CD25). The curves represent cells from wild-type (thin line), D4 (dashed line), and C10 (thick line) thymi. Because of the different numbers of cells recovered between the strains, the results are normalized to the number of cells in the largest peak. Control staining is shown only in the first histogram. The bars represent the SEM.
DN cells in Tg thymus show evidence of IL-7 starvation
Given that IL-7 signaling results in up-regulation of the antiapoptotic molecule Bcl-2 and that addition of IL-7 to FTOC had no deleterious effect on fetal thymocyte development, the observation that enforced expression of IL-7R on DP cells resulted in thymic hypoplasia was unexpected. Furthermore, the finding that DN cells were partially blocked at the CD25+ stage resembles the phenotype observed in IL-7R–deficient animals.36,37 This raised the possibility that the reduced cell numbers in adult IL-7Rα Tg thymi could be due to competition for endogenously produced IL-7 by the increased numbers of IL-7R–bearing DP cells. If so, the cause of thymic hypoplasia could be lack of proliferation and/or enhanced apoptosis of DN thymocytes. Because enhanced survival via IL-7 signaling is mediated, at least in part, by up-regulation of Bcl-2,27,38 we measured Bcl-2 levels in DN cells that were treated or not with exogenous IL-7. Thymocytes were maintained in suspension culture for 18 hours in the absence or presence of recombinant mouse IL-7 (Figure 5). Strikingly, basal Bcl-2 levels in Tg DN cells were much lower than their wild-type counterparts (Figure 5, left panel). This was completely rectified by the addition of IL-7 to the culture, which resulted in up-regulation of Bcl-2 in IL-7Rα Tg DN cells to levels similar to wild-type cells (Figure 5, right panel). These data suggest that DN cells in the IL-7Rα Tg mice are “starved” for IL-7.
Bcl-2 response DN thymocyte subpopulation upon in vitro treatment with IL-7. Thymocytes from young adult mice as described for Figure 2 were treated with vehicle control (DMSO; left panel) or 3 ng/mL IL-7 (right panel) and stained for Bcl-2. Wild type (thin line), D4 Tg cells (dashed line), and C10 Tg cells (thick line) are shown with DP cells serving as a control (dotted line). Similar results were obtained from 4 independent experiments, and at doses of IL-7 from 3 to 10 ng/mL, equal effect was observed.
Bcl-2 response DN thymocyte subpopulation upon in vitro treatment with IL-7. Thymocytes from young adult mice as described for Figure 2 were treated with vehicle control (DMSO; left panel) or 3 ng/mL IL-7 (right panel) and stained for Bcl-2. Wild type (thin line), D4 Tg cells (dashed line), and C10 Tg cells (thick line) are shown with DP cells serving as a control (dotted line). Similar results were obtained from 4 independent experiments, and at doses of IL-7 from 3 to 10 ng/mL, equal effect was observed.
IL-7Rα Tg DN cells are prone to spontaneous apoptosis
Because Bcl-2 is a potent antiapoptotic effector, we quantitated DN cell apoptosis by TUNEL, an assay that measures DNA nicking and fragmentation.39,40 Thymocytes were placed in suspension culture with or without IL-7 for 22 hours (Figure 6). A little more than 40% of DN cells from wild-type mice were TUNEL-positive at this time (Figure 6A, upper left panel). Corresponding with reduced Bcl-2 expression, increased numbers of DN cells from IL-7Rα Tg mice became apoptotic during this period (Figure 6A, lower left panel). Furthermore, exogenous IL-7 resulted in extensive protection from apoptosis, which was even more apparent in IL-7R Tg than wild-type cells (Figure 6A, right panels). Thus, it is likely that DN cells from IL-7Rα Tg mice are functionally deprived of IL-7.
Apoptotic susceptibility of IL-7Rα Tg thymocytes. TUNEL assay was performed on thymocytes that were cultured in vitro for 20 hours with or without 3 ng/mL rmIL-7. To measure DNA fragmentation, staining for dUTP (expressed as TUNEL-positive) is shown in gated DN cells (A) and DP cells (B) of wild-type (upper row) or B1 mice (lower row). Percentages of TUNEL-positive and TUNEL-negative cells are indicated. One representative experiment of 3 is shown.
Apoptotic susceptibility of IL-7Rα Tg thymocytes. TUNEL assay was performed on thymocytes that were cultured in vitro for 20 hours with or without 3 ng/mL rmIL-7. To measure DNA fragmentation, staining for dUTP (expressed as TUNEL-positive) is shown in gated DN cells (A) and DP cells (B) of wild-type (upper row) or B1 mice (lower row). Percentages of TUNEL-positive and TUNEL-negative cells are indicated. One representative experiment of 3 is shown.
DP thymocytes also die in prolonged suspension culture: in the representative experiment shown in Figure 6B approximately half of both wild-type and IL-7Rα Tg DP cells became TUNEL positive after 22 hours. Although the addition of IL-7 did not change the percent of wild-type DP cells that underwent apoptosis, it conferred a modest amount of protection to IL-7R Tg-bearing DP cells, consistent with the induction of Bcl-2 in this population. There was a comparable survival advantage for CD4+ and CD8+ SP thymocytes in cultures containing IL-7 treatment for both wild-type and IL-7R Tg cells (data not shown).
The effect of IL-7R expression on DP cells is not cell autonomous and is mitigated by additional IL-7
If the thymus in IL-7Rα Tg mice is small because of competition between DN and DP cells for limiting amounts of IL-7, one would expect that the effect would not be cell autonomous (ie, in a mixed environment both wild-type and IL-7Rα Tg thymocytes would be affected). If, on the other hand, the enforced expression of the IL-7R has cell intrinsic untoward effects on thymocyte survival or proliferation, in such an environment, wild-type cells should develop normally while transgenic cells should not. To test this, we established irradiated mixed bone marrow (BM) chimeric mice and examined them for competition among cells with wild-type or transgenic IL-7R expression (Figure 7). BM chimeras were made by lethal irradiation of wild-type mice and reconstitution with a 1:1 ratio of BM cells from wild-type and either D4 or B1 IL-7Rα Tg animals. To distinguish between donor cells, C57BL/6 BM was obtained from Ly5.1 (CD45.1) and IL-7Rα Tg BM from Ly5.2 (CD45.2) congenic mice. Control animals were generated by reconstituting recipients with wild-type or IL-7Rα Tg BM only. The reconstituted animals were analyzed 4 to 7 weeks after BM transplant, a time at which almost 100% of thymocytes originate from donor BM cells.41 Thymi from mice reconstituted with just wild-type BM were the largest, having an average of 130 × 106 cells at 6 weeks. As expected, the thymi of mice reconstituted with B1 BM cells were much smaller, having on average approximately 15 × 106 thymocytes. D4-resconstituted thymi were on average somewhat larger than those reconstituted with B1 BM (Figure 7A). Importantly, in mixed chimeras reconstituted with equal number of wild-type and IL-7R Tg BM cells the average thymic size was substantially smaller than thymi reconstituted with wild-type BM. D4–to–wild-type mixed chimeras were at approximately one third the size of wild-type only chimeras, an effect that was even more pronounced in B1–to–wild-type chimeras, consistent with the higher levels of IL-7R on B1 versus D4 cells.
Mixed bone marrow chimeras show dominant effect of the IL-7Rα Tg cells on the size of the reconstituted thymus. (A) Thymic cell counts are shown for BM chimeras reconstituted with 100% wild-type, 100% D4, 100% B1, or a 50:50 mixture of wild-type and Tg BM 6 weeks after reconstitution. Error bars represent SEM. (B) The pooled results of multiple experiments in which reconstitution was examined in mixed chimeric animals from 4 to 7 weeks after BM transfer. The black columns represent the percent reconstitution by wild-type thymocytes, and the white columns represent percent reconstitution by IL-7Rα Tg thymocytes. Error represent SEM. (C) B1 IL-7Rα Tg mice were crossed to IL-7 Tg mice, and representative F1 animals from that breeding are shown. Error bars represent SEM.
Mixed bone marrow chimeras show dominant effect of the IL-7Rα Tg cells on the size of the reconstituted thymus. (A) Thymic cell counts are shown for BM chimeras reconstituted with 100% wild-type, 100% D4, 100% B1, or a 50:50 mixture of wild-type and Tg BM 6 weeks after reconstitution. Error bars represent SEM. (B) The pooled results of multiple experiments in which reconstitution was examined in mixed chimeric animals from 4 to 7 weeks after BM transfer. The black columns represent the percent reconstitution by wild-type thymocytes, and the white columns represent percent reconstitution by IL-7Rα Tg thymocytes. Error represent SEM. (C) B1 IL-7Rα Tg mice were crossed to IL-7 Tg mice, and representative F1 animals from that breeding are shown. Error bars represent SEM.
The reconstituted thymus of each chimeric animal was analyzed for the relative ratios of Tg+ to wild-type cells. Although there was some variability between individual mice, something that is commonly seen in mixed BM chimeras,42 the average ratio of wild-type to IL-7Rα Tg thymocytes was approximately 1:1 (wild-type–to–B1) or slightly in favor of Tg cells (wild-type–to–D4) (Figure 7B). There was no difference between the various thymocyte subpopulations between wild-type and IL-7Rα Tg cells in the mixed chimeras (data not shown). These results demonstrate that the developmental effect of the IL-7Rα transgene is not cell autonomous, but rather expansion of both transgene-expressing and wild-type cells is altered, consistent with the establishment of a thymic microenvironment in which IL-7 is limiting.
To confirm that IL-7 is limiting in the IL-7R Tg mice in vivo, the IL-7Rα Tg mice were crossed to mice transgenically expressing IL-7 under the control of the proximal lck promoter.18 At 3 weeks of age, IL-7 Tg mice had a slightly larger thymus than the control animals, and the B1 IL-7R Tg thymi were approximately 25% as large (Figure 7C). Notably, B1 × IL-7-TgA mice had thymi that were approximately 2.5-fold larger than B1. These numbers approached but did not quite reach the levels seen in wild-type thymi, suggesting that IL-7 is still limiting, although to a much smaller degree.
Discussion
Thymic IL-7 production and T-lineage IL-7R expression are tightly regulated throughout ontogeny. In the developing thymus, the earliest IL-7 production can be observed on embryonic day 12, peaks on day 15, and subsequently decreases over the following 5 days.43,44 At the other extreme, the loss of function in an aging thymus correlates with a decrease of IL-7 secretion by epithelial cells.45 Although epithelial cell numbers do not appear to change with age, IL-7 expression significantly declines. Antibody-blocking experiments and IL-7 or IL-7Rα knockout mice have defined an early developmental checkpoint at which IL-7 signaling is critically required and where most thymocyte division occurs: DN2 and late DN3 (ie, prior to or just following TCRβ or TCRγ rearrangement).27,46 The profound block in thymocyte progression that occurs in IL-7R–deficient mice can be partially bypassed by the expression of a transgenic αβTCR,46,47 Bcl-2 overexpression,38,48 or Bax deficiency.49
The massive expansion of late DN and early DP cells is accompanied by the complete loss of IL-7R on the cell surface,50 due to transcriptional down-regulation of IL-7Rα expression, which persists throughout the DP cell stage.51 IL-7R levels begin to rise again in the subset of DP cells that is undergoing positive selection, and IL-7 has been implicated in supporting the survival of cells committed to the CD8 lineage prior to their re-expression of CD8 and maturation.52 Furthermore, IL-7 is essential for the post-selection expansion of positively selected CD4 and CD8 thymocytes in the neonatal thymus.53 IL-7R provides survival signals to peripheral T cells, especially those destined to be memory cells.21,54
These observations provide multiple rationales for the up-regulation and maintenance of high levels of IL-7R on antigen-selected thymocytes and peripheral T cells. But one is left with the intriguing question of why there is a need for carefully coordinated and massive down-regulation of IL-7R when cells pass from the DN to the DP stage. One possibility is that the down-regulation follows efficient TCRβ rearrangement and pre–TCR-dependent expression and that once pre-TCR is able to signal IL-7 becomes dispensable for survival. Down-regulation of IL-7R in this case may be a mechanism to limit the continued expansion of DN/early DP clones, which if unchecked might have a deleterious effect on repertoire diversity because a relatively few clones would expand to fill the DP compartment. Another possibility is that for efficient negative selection to occur, DP cells must lose the IL-7R and, thus, the antiapoptotic signals it provides.25 To investigate these and other possible reasons for IL-7R down-regulation in thymocyte development, we generated IL-7Rα Tg mice in which expression was not subject to the normal pattern of developmental regulation. Perhaps surprisingly, studies with these animals do not support either of the possibilities mentioned above. There was no expansion of the DN or DP compartment, even in fetal thymi. Furthermore, there was no disturbance of development in different models of antigen-specific negative selection. Therefore, we conclude that IL-7R down-regulation in DP cells is not vital for preventing DN cell expansion or for antigen-specific selection.
Despite normal early development, absolute thymocyte numbers in the IL-7Rα Tg mice decreased as a function of age. The most marked differences between wild-type and IL-7Rα Tg mice occurred at a time corresponding to the rapid expansion of the thymus. Moreover, thymocytes showed evidence of IL-7 starvation, with diminished Bcl-2 levels in DN cells and an increase in DN cell apoptosis upon culture. We did not observe an increase in TUNEL positivity or a decrease in Bcl-2 in DN cells examined immediately ex vivo (data not shown). It is likely that this is because DN cells that begin to lose Bcl-2 rapidly undergo apoptosis and are quickly cleared by resident phagocytotic cells.55,56 Consistent with this is the fact that DN cell numbers were reduced in IL-7R Tg mice and were restored by the introduction of IL-7 both in vitro and in vivo. It is also possible, however, that a reduction in available intrathymic IL-7 might affect the expression and/or function of prosurvival mediators other than Bcl-2. Studies with irradiated mixed BM chimeras confirmed that the effect of the IL-7R up-regulation was not cell autonomous but also affected wild-type bystander cells, consistent with depletion of IL-7 from the thymic microenvironment. Importantly, these results are not simply a reflection of IL-7R overexpression. Although D4 mice have a modest increase in DN thymocyte IL-7R levels compared to wild-type animals, they were still able to profoundly affect the expansion of wild-type cells even when they constitute only half the cells in chimeric animals. Moreover, an IL-7Rα transgene under the control of the proximal lck promoter was previously introduced into IL-7R-/- mice.36 The DP cells expressed IL-7R levels similar to, or slightly less than, normal DN cell levels, and the thymus size was decreased by 25% to 30%. Together, these data support the notion that expression of IL-7R on DP cells, even at relatively low levels, is sufficient to compete for limiting amounts of endogenous IL-7. Although TSLP also is a ligand for the IL-7R, it is unlikely that depletion of this cytokine is responsible for the thymic phenotype, because TSLP has only minor effects on thymic development57 and, more important, thymic hypoplasia in our IL-7R Tg mice was reversed by introducing the IL-7 transgene.
The present findings with IL-7Rα also are noteworthy because of previous observations in AKR/J mice, which spontaneously develop thymomas. In these mice the cell population that proliferates and is tumorigenic has high levels of IL-7Rα.41 This led to the conclusion that simple overexpression of IL-7R confers a survival advantage to early thymocytes in the normal thymic environment. Our results with mixed bone marrow chimeras containing both wild-type and IL-7Rα Tg cells argues that this is in fact not the case. We did not observe enhanced proliferation of thymocytes from IL-7Rα Tg mice nor did they outcompete the wild-type cells in mixed BM chimeras. To the contrary, while the average ratio of Tg to wild cells was around 1:1, we observed a substantial decrease in thymic size that correlated with the level of Tg expression (ie, Tg high-expressor thymocytes caused a larger decrease low-expressor thymocytes). It is possible, therefore, that the proliferative/survival advantage of AKR/J thymocytes in mixed chimeras reflects differences in strain background genes rather than IL-7R levels.
Prepublished online as Blood First Edition Paper, August 24, 2004; DOI 10.1182/blood-2004-06-2484.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Lionel Feigenbaum for help in making transgenic mice; Scott Durum, Paul Love, Terry Fry, and Crystal Mackall for reagents; Peter Mercado for expert assistance with animal experiments; and Rémy Bosselut for insightful discussions and critical review of the manuscript.


![Figure 2. IL-7 responsiveness of Tg mice. Staining for Bcl-2 is shown in wild-type (dashed line) and Tg thymocytes (filled line) upon 18 hours in vitro treatment with 3 ng/mL of recombinant murine IL-7 (right panels) or vehicle control (dimethyl sulfoxide [DMSO]; left panels) in gated DP cells. One representative experiment out of 4 is shown; all mice were young adults, age matched per individual experiment. Control antibody staining is shown only in the first histogram (dotted line).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-06-2484/6/m_zh80240470920002.jpeg?Expires=1765956291&Signature=4OjDHIE9O8tSZIQ6-16hGXWLNt70sBckAtxmXK9e9VIpT4E2lrX0Pbs1eY0tfZGPEg5fHOHfHG1EzGU7JZ6vVMsu0hF9rwjUQR4ZFvTHbAGHp60VY9w-FqYTMevIhQoS2fPPnO5VC0qWf62hbSpTXD1pAOECyI97DBXI6lVvIDvziI3jKzCBYdObMAlCsgzUrSXfbsBMPYWT3iO484aRVE4Vh4a-BDColit2BA-kAg2KD1Ha5BGS8qXTUorEGEGzTkp4PJ~ypYFaf1fh1dQ~6aThybRutc~NVAXm4TujX8yPLXSLptGCl1-exEk2pmTLWWlaFAEXB9SCZq1LIVMhqg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal