Abstract
Patients with advanced cutaneous T-cell lymphoma (CTCL) exhibit profound defects in cell-mediated immunity. Host immune functions appear to play an integral role in mediating disease-controlling responses in CTCL, therefore we investigated the effects of synthetic oligode-oxynucleotides with CpG motifs (CpG ODN), which have been recognized as immune stimulatory by virtue of activation of dendritic cells (DCs) following binding to Toll-like receptor (TLR) 9. Peripheral blood mononuclear cells (PBMCs) from patients with advanced CTCL (erythroderma with circulating malignant T cells) and healthy volunteers were cultured with either CpG-A or CpG-B ODN. Patients' PBMCs exhibited marked induction of interferon-α (IFN-α) release following culture with CpG-A. Similarly significant activation of NK cells and CD8 T cells occurred as assessed by up-modulation of CD69 expression and by natural killer lytic activity. Nevertheless, the PBMCs of patients exhibited blunted responses to CpG-A compared to healthy volunteers. In such cases, IL-15 was capable of producing levels of NK activation that were superior to CpG-A, while the combined effects of CpG-A plus IL-15 induced maximal activation of NK cells and further enhanced activation of CD8 T cells. These findings have important implications for the potential enhancement of antitumor immunity among patients with advanced CTCL.
Introduction
Cutaneous T-cell lymphoma (CTCL) is a clonally derived malignant proliferation of skin-invasive CD4 T cells. Sezary syndrome (SS), the leukemic form of CTCL, is characterized by circulating CD4, CD45RO malignant T cells, and profoundly impaired TH1 responses associated with depressed cell-mediated immunity.1-3 Consequently, decreased production of TH1-type cytokines such as interferon-γ (IFN-γ) and interleukin-2 (IL-2) and decreased NK cell activity has been observed in SS patients.4-6 Depressed TH1 responses are at least partially caused by the increased production by the malignant T cells of IL-4, IL-5, and IL-10, which are known inhibitors of TH1-type cytokine production.7-10 Moreover, decreased numbers of dendritic cells (DCs) and their products IL-12 and IFN-α, as well as decreased numbers of CD8 T cells, have also been observed in SS.6,11
Recently, there has been growing interest in biologic response modifiers that target the immune system and boost cell-mediated immunity in cancer patients, providing a potentially effective tool to control disease progression. For example, current approaches in treating CTCL include therapy with IFN-α, IFN-γ, IL-12, and IL-2, but the search for potent, effective therapeutic agents with a low occurrence of adverse effects is ongoing.12-14
Bacterial DNA, containing unmethylated nucleotides with the particular sequence motif CG, has been recognized as an immune system stimulatory molecule that triggers TH1-type responses.15 Synthetic oligodeoxynucleotides, which contain such CpG motifs (CpG ODN), resemble bacterial DNA in their ability to induce similarly effective immune responses.16 CpG motifs are recognized by TLR9 present on CD123 plasmacytoid dendritic cells (pDCs) and B cells.17-19 Binding of CpG ODN to TLR9 on pDCs triggers a cascade of signaling pathways leading ultimately to the production of significant levels of IFN-α by these cells.20-22 Additionally, stimulatory CpG ODNs support survival and maturation of pDCs and up-regulate expression of the costimulatory molecules CD80, CD86, CD40, and major histocompatibility complex (MHC) class II.23-26
The immunostimulatory potential of CpG ODN has been tested in several murine tumor models. Mice immunized with a peptide analog of melanoma antigen MART-1/Melan-A26-35 in the presence of CpG ODN generated a strong systemic CTL response against melanoma cells.27 Intratumor injections of CpG ODN lead to the generation of strong antitumor T-cell responses resulting in complete remission of established solid tumors.28
The ability of CpG ODN to generate protective antitumor responses in mice prompted similar studies in humans. A small phase 1 clinical trial of CpG-B (7909) injected intralesionally in patients with basal cell carcinoma or melanoma showed several local regressions, suggesting a potentially therapeutic effect of CpG ODN in treating human cancers.16
Synthetic oligodeoxynucleotides are divided into type A (CpG-A), B (CpG-B), and C (CpG-C), based on the biologic activity of their CpG motifs. CpG-A binds predominantly to plasmacytoid DCs and induces high levels of IFN-α; CpG-B is superior in activating B cells and induces only low levels of IFN-α from pDCs, while CpG-C combines the immune effects of A- and B-class CpG ODN, inducing significant levels of IFN-α from plasmacytoid DCs as well as B-cell stimulation.15,29 Additionally, CpG-A demonstrates the significant ability to activate NK cells and T cells via IFN-α–dependent pathways.15,30 Thus, biologic functions of CpG-A may depend solely on IFN-α, leading to activation of the same cellular compartments.
IFN-α is a cytokine with pleiotropic effects on various cell types in the immune system. It enhances the cytotoxic activity of macrophages and NK cells, facilitates proliferation of memory CD8 T cells through the induction of IL-15 in macrophages and dendritic cells, enhances T-cell survival, and acts in an autocrine manner to activate DCs.31-37 Additionally, IFN-α has the ability to induce phosphorylation of STAT4 in human activated T cells and in NK cell lines, indicating its ability to induce TH1 responses.38,39 Recently, a new function of IFN-α has been discovered that offers an explanation for its effectiveness in treating human cancers. It has been shown that IFN-α/β stimulates transcription of the gene encoding p53, resulting in an increase in cellular p53 protein, leading to apoptosis of cancer cells.40
IL-15 has many activating and homeostatic functions on lymphocytes.41 Originally, IL-15 was identified as a cytokine bearing many similarities to IL-2.42-46 Both IL-2 and IL-15 costimulate lymphocyte proliferation and activation. In addition, IL-15 potently promotes NK cell development from precursors and provides a required signal for survival of mature NK cells.41,47-49 IL-15 also stimulates proliferation and survival of naive CD8 T cells and is crucial for the survival of memory CD8 T cells.50-53
In this report we examine the stimulatory potential of CpG ODN, particularly CpG-A, and IL-15 to augment immune responses in peripheral blood mononuclear cells (PBMCs) from CTCL patients. We demonstrate the ability of CpG-A to activate patients' NK cells and CD8 T cells. Moreover, we show a superior enhancement in activation of NK cells resulting from the combined action of both CpG-A and IL-15. Our results suggest that to achieve maximal stimulation of immune responses in cancer patients, several immune compartments should be simultaneously stimulated, requiring treatments that involve a combination of different biologic response modifiers.
Patients, materials and methods
Patients
Sezary syndrome (SS) patients were diagnosed on the basis of clinical, histopathologic, and immunohistologic criteria.54 SS patients were divided into 3 groups based on tumor load in their circulation as previously reported.11 Briefly, those with 5% to 20% circulating Sezary cells were defined as low tumor burden patients; those with 21% to 49% circulating Sezary cells as medium tumor burden patients, and those with at least 50% circulating Sezary cells as high tumor burden patients. White blood cell (WBC) counts of SS patients selected for these studies were 3.5 to 5 × 106 cells/mL. All patients with SS were receiving identical treatment, consisting of the use of extracorporeal photopheresis on approximately an every 4-week schedule.55 As controls, blood samples from age-matched healthy volunteers were used. Donation of blood by patients and healthy volunteers in this study conformed to international review board–approved protocols (University of Pennsylvania and the Wistar Institute), and informed consent was obtained.
Reagents
CpG ODNs were purchased from Integrated DNA Technologies (Coralville, IO). CpG-A (ODN 2216) has a chimeric backbone in which the 5′ and 3′ ends are phosphorothioate modified, and the center portion is a phosphorodiester. CpG-B (ODN 2006) has a phosphorothioate backbone that provides a high degree of nuclease resistance.15,56 Recombinant IL-15, recombinant IFN-α, and IFN-γ were purchased from R&D Systems (Minneapolis, MN).
Preparation and culture of mononuclear cells
PBMCs from patients or healthy volunteers were collected from blood as previously described.5 Cells were cultured in RPMI 1640 (Life Technologies, Gaithersburg, MD), supplemented with 10% fetal bovine serum (FBS), penicillin/streptomycin, and L-glutamine (all reagents purchased from Gibco-BRL, Grand Island, NY). To induce immune responses in vitro, PBMCs from patients and healthy volunteers were cultured in 24-well plates at a density of 2 × 106/mL/well for 24 hours with CpG-ODN (5 μg/mL), IFNα (10 ng/mL), or IL-15 (1 ng/mL). Cells were then harvested and analyzed for surface expression of CD69 as well as for NK cytotoxic activity.
Flow cytometric analysis
To detect intracellular expression of TLR9 on CD123 subsets of peripheral blood dendritic cells, approximately 106 PBMC per sample were stained with Lin 1-FITC (lineage cocktail containing antibodies against CD3, CD14, CD16, CD19, CD20, CD56; BD Biosciences, San Jose, CA), anti–HLA-DR-APC (BD Biosciences, San Jose, CA), and anti–CD123-CyChrome (BD Biosciences, San Jose, CA) for 30 minutes on ice, followed by 15 minutes' incubation with fixation reagent A (Fix & Perm, Caltag). Cells were then washed, permeabilized with reagent B, and stained with anti–TLR9-PE antibody (eBioscience, San Diego, CA) or appropriate isotype control.
To analyze expression of CD69 on NK cells or T cells, PBMCs were stained with anti–CD3-PerCp, anti–CD56-APC, and anti–CD69-FITC or anti–CD4-APC, anti–CD8-PerCp, and anti–CD69-FITC. All antibodies were purchased from BD Biosciences, San Jose, CA.
Murine immunoglobulins of appropriate isotypes were used as a control. Cells were incubated with antibodies for 30 minutes on ice in the dark then washed twice with PBS containing 3% FBS, resuspended in 1% paraformaldehyde, and analyzed.
Cells were analyzed with the FACS Calibur (Becton Dickinson) flow cytometer using CELLQuest software (Becton Dickinson, San Jose, CA). 150 000 and 30 000 events were collected to analyze DCs or NK cells, CD4 T cells, and CD8 T cells, respectively.
Detection of cytokines
Culture supernatants were harvested and tested for the presence of cytokines, IFN-α, and IFN-γ assayed by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's recommendations, using antibody pairs from Endogen (IFN-α, Woburn, MA, sensitivity 10 pg/mL) or R&D Systems (IFN-γ, Minneapolis, MN; sensitivity, 5 pg/mL).
Natural killer cytotoxicity assay
PBMCs stimulated with cytokines or CpG ODN were the source of activated NK cells. After 24 hours of stimulation, cells were harvested, washed with PBS (Gibco-BRL, Grand Island, NY), and plated at different concentration. Human lymphoblastoma cells K562 were used as targets. A standard 4-hour Cr51-release assay was performed as previously described.5
Statistical analysis
Data are expressed as means ± SD of tested individuals. Statistical significance was determined using Student t test if applicable.
Results
The level of expression of TLR9 on plasmacytoid dendritic cells from SS patients is comparable to the level detected on cells from healthy volunteers
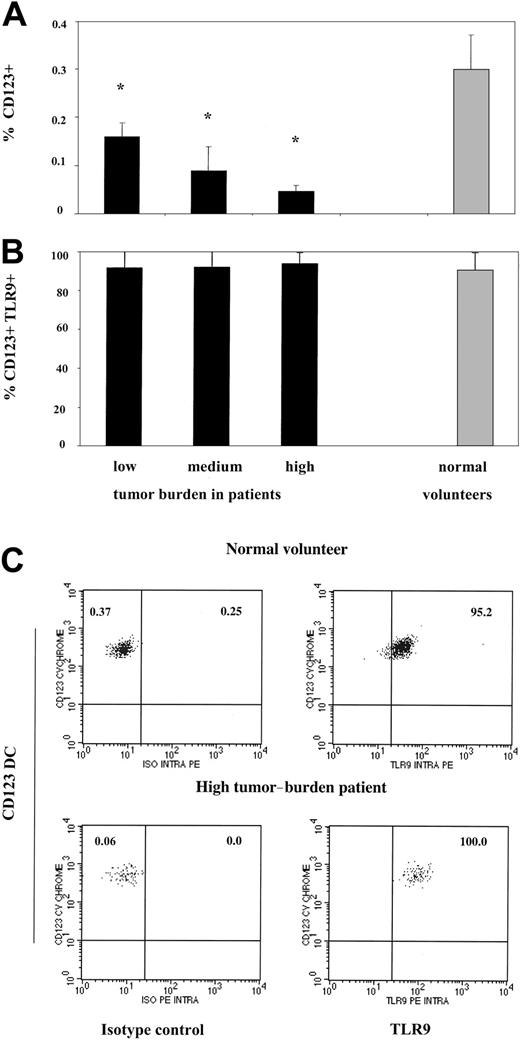
To assess the ability of CD123 pDCs from SS patients to respond to CpG, we first examined the expression of TLR9 on patients' pDCs. Consistent with our previous observations, patients with a low circulating tumor burden (< 20% Sezary cells) demonstrated a 2-fold decrease in percentage of pDCs compared to healthy volunteers (0.16% in patients versus 0.3% pDCs in healthy volunteers) (Figure 1A).11 Disease progression further diminished the pool of circulating pDCs to 0.09% in medium tumor burden patients (21%-49% Sezary cells) and to 0.05% in high tumor burden patients (50% or more tumor cells in the blood). In contrast, the expression of TLR9 did not vary between the patients with different peripheral blood tumor load. On average, 91% to 94% of the remaining dendritic cells in all 3 groups of patients expressed TLR9, similarly to dendritic cells from healthy volunteers (Figure 1B-C). Thus, pDCs in SS patients express normal levels of TLR9 and are therefore potentially able to recognize CpG motifs. Despite the normal presence of TLR9, high tumor burden patients with very few pDC remaining were unable to mount significant responses to CpG (Figure 5B), consistent with previously published reports showing severely impaired responses to influenza virus in this group.11 Therefore, the remaining data presented below focus on patients with low and medium tumor burden since they still have a significant pool of pDCs and are able to mount significant immune responses.
CD123 plasmacytoid dendritic cells from Sezary syndrome patients are decreased in numbers but express normal levels of TLR9. (A) PBMCs from SS patients with low (n = 5), medium (n = 4), and high (n = 4) peripheral blood tumor burdens and healthy volunteers (n = 6) were excluded of all lineage-positive cells (Lin 1-FITC cocktail), and lineage-negative cells were analyzed for the co-expression HLADR and CD123. Data represent means (± SD) of tested individuals and are presented as a percentage of all PBMCs gated on live cells. (B) PBMCs from SS patients and healthy volunteers were stained as described in panel A, but lineage-negative cells were analyzed for the co-expression of HLADR, CD123, and TLR9 or isotype control antibody. Control antibody stained 0.1% to 0.3% of PBMCs (data not shown). Data represent means (± SD) of tested individuals and are presented as a percentage of CD123 DCs expressing TLR9. *P < .05 compared with healthy volunteers. (C) PBMCs from a representative healthy volunteer (top row) and high tumor burden patient (bottom row) were stained as described in panels A and B. The left upper quadrant numbers of panels on the left represent the percentage of dendritic cells in the healthy volunteer (percentage of all PBMCs gated on forward and side light scatter) and the patient, whereas the right upper quadrant numbers represent the percentage of CD123 DCs stained with isotype control. The right upper quadrant numbers of the panels on the right represent percentage of CD123 DCs positive for TLR9 in the healthy volunteer (top row) and the patient (bottom row).
CD123 plasmacytoid dendritic cells from Sezary syndrome patients are decreased in numbers but express normal levels of TLR9. (A) PBMCs from SS patients with low (n = 5), medium (n = 4), and high (n = 4) peripheral blood tumor burdens and healthy volunteers (n = 6) were excluded of all lineage-positive cells (Lin 1-FITC cocktail), and lineage-negative cells were analyzed for the co-expression HLADR and CD123. Data represent means (± SD) of tested individuals and are presented as a percentage of all PBMCs gated on live cells. (B) PBMCs from SS patients and healthy volunteers were stained as described in panel A, but lineage-negative cells were analyzed for the co-expression of HLADR, CD123, and TLR9 or isotype control antibody. Control antibody stained 0.1% to 0.3% of PBMCs (data not shown). Data represent means (± SD) of tested individuals and are presented as a percentage of CD123 DCs expressing TLR9. *P < .05 compared with healthy volunteers. (C) PBMCs from a representative healthy volunteer (top row) and high tumor burden patient (bottom row) were stained as described in panels A and B. The left upper quadrant numbers of panels on the left represent the percentage of dendritic cells in the healthy volunteer (percentage of all PBMCs gated on forward and side light scatter) and the patient, whereas the right upper quadrant numbers represent the percentage of CD123 DCs stained with isotype control. The right upper quadrant numbers of the panels on the right represent percentage of CD123 DCs positive for TLR9 in the healthy volunteer (top row) and the patient (bottom row).
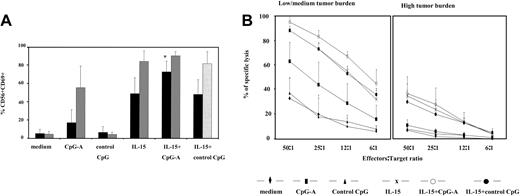
CpG-A and IL-15 in combination induce maximal activation of patients' NK cells. (A) PBMCs from SS patients (n = 11, ▪) and healthy volunteers (n = 7, ▦) were stimulated with CpG-A, control CpG ODN, IL-15 (1 ng/mL), or the combination of CpG-A and IL-15 as described in Figure 2. Cells were then harvested, stained with appropriate antibodies, and analyzed for the expression of CD69 on CD56 NK cells. Data represent means (± SD) of tested individuals and are presented as a percentage of CD56 NK cells expressing CD69. *P < .05 compared with IL-15 or CpG-A. (B) PBMCs from low to medium tumor burden patients (n = 5, left panel) and high tumor burden patients (n = 4, right panel) were stimulated with CpG-A, control CpG ODN, IL-15, or the combination of CpG-A and IL-15 as described in Figure 2, followed by a 4 hours' Cr51 release assay using K562 cells as targets. Data represent means (± SD) of tested individuals and are presented as a percentage of specific lysis.
CpG-A and IL-15 in combination induce maximal activation of patients' NK cells. (A) PBMCs from SS patients (n = 11, ▪) and healthy volunteers (n = 7, ▦) were stimulated with CpG-A, control CpG ODN, IL-15 (1 ng/mL), or the combination of CpG-A and IL-15 as described in Figure 2. Cells were then harvested, stained with appropriate antibodies, and analyzed for the expression of CD69 on CD56 NK cells. Data represent means (± SD) of tested individuals and are presented as a percentage of CD56 NK cells expressing CD69. *P < .05 compared with IL-15 or CpG-A. (B) PBMCs from low to medium tumor burden patients (n = 5, left panel) and high tumor burden patients (n = 4, right panel) were stimulated with CpG-A, control CpG ODN, IL-15, or the combination of CpG-A and IL-15 as described in Figure 2, followed by a 4 hours' Cr51 release assay using K562 cells as targets. Data represent means (± SD) of tested individuals and are presented as a percentage of specific lysis.
CpG-A induces IFN-α production, whereas CpG-B up-regulates HLA-DR on the CD123 dendritic cells of patients with Sezary syndrome
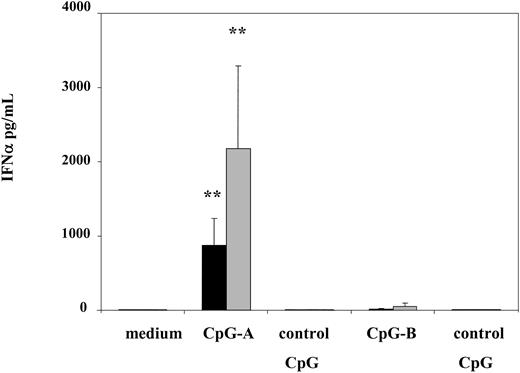
In order to define in detail the biologic potential of CD123 pDCs from patients, we analyzed 2 types of oligodeoxynucleotides with CpG motifs: CpG-A (ODN 2216), previously shown to be superior in inducing IFN-α production and NK cell activation; and CpG-B (ODN 2006), known for its ability to induce DC maturation and B-cell activation.15 Indeed, CpG-A induced significant levels of IFN-α detected in supernatants of cultured PBMCs from SS patients, reaching 869 pg/mL on average. Although induction of IFN-α from patients' cells was significant, the effects were blunted compared to healthy controls as levels of IFN-α induced by CpG-A from PBMCs of healthy volunteers were 2.5-fold higher than in patients, averaging 2179 pg/mL (Figure 2).
CpG-A stimulates production of significant levels of IFN-α from PBMCs of SS patients. PBMCs from SS patients (n = 12, ▪) and healthy volunteers (n = 6, ▦) were stimulated with 5 μg/mL of CpG-A, CpG-B, or control CpG ODN for 24 hours. Cytokine levels were measured in cell-free supernatants by ELISA. Data represent means (± SD) of tested individuals. **P < .001 compared with medium.
CpG-A stimulates production of significant levels of IFN-α from PBMCs of SS patients. PBMCs from SS patients (n = 12, ▪) and healthy volunteers (n = 6, ▦) were stimulated with 5 μg/mL of CpG-A, CpG-B, or control CpG ODN for 24 hours. Cytokine levels were measured in cell-free supernatants by ELISA. Data represent means (± SD) of tested individuals. **P < .001 compared with medium.
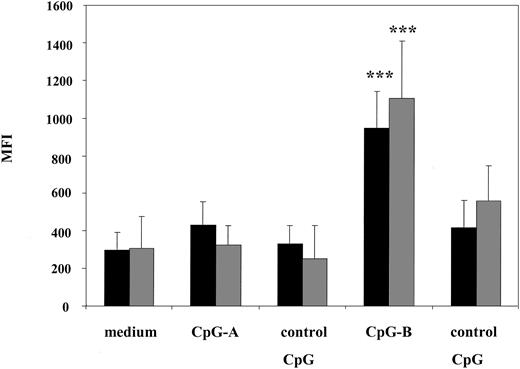
Consistent with published data, CpG-B was unable to induce equally high levels of IFN-α; an average of 21.7 pg/mL and 56.3 pg/mL of IFN-α was detected in cultured supernatants from patients and healthy volunteers, respectively. However, stimulation with CpG-B but not with CpG-A resulted in significant up-regulation of HLA-DR expression on CD123 DCs from patients as well as healthy volunteers (Figure 3). Furthermore, levels of expression of HLADR on CD123 DCs were similar among patients and healthy volunteers (MFI 947.6 in patients versus 1105.6 in healthy volunteers) suggesting that patients' pDCs maintain integrity of their signaling compartments.
CpG-B up-regulates HLADR expression on CD123 plasmacytoid dendritic cells from SS patients. PBMCs from SS patients and healthy volunteers were stimulated with CpG ODN as described in Figure 2. After 24 hours of culture, cells were harvested and PBMCs from SS patients (n = 8, ▪) and healthy volunteers (n = 5, ▦) were excluded of all lineage-positive cells (Lin 1-FITC cocktail), whereas lineage-negative cells were analyzed for the co-expression of HLADR and CD123. Data represent means (± SD) of tested individuals and presented as MFI. ***P < .0001 compared with medium or control CpG.
CpG-B up-regulates HLADR expression on CD123 plasmacytoid dendritic cells from SS patients. PBMCs from SS patients and healthy volunteers were stimulated with CpG ODN as described in Figure 2. After 24 hours of culture, cells were harvested and PBMCs from SS patients (n = 8, ▪) and healthy volunteers (n = 5, ▦) were excluded of all lineage-positive cells (Lin 1-FITC cocktail), whereas lineage-negative cells were analyzed for the co-expression of HLADR and CD123. Data represent means (± SD) of tested individuals and presented as MFI. ***P < .0001 compared with medium or control CpG.
CpG-A stimulation of PBMCs from Sezary syndrome patients results in activation of their NK cells and CD8 T cells and increased NK cytolytic activity
It has been shown that oligodeoxynucleotides containing CpG motifs produce strong activation of NK and T cells in an APC-dependent fashion.57 Furthermore, activation of NK and T cells in such settings results, at least partially, from the direct action of IFN-α produced by APC, mainly CD123 DCs, in response to CpG ODN.21,58-60 In an effort to study CpG associated activation of NK and T cells, we assessed the expression of CD69 on these cells after exposure to CpG-A. CD69 is generally absent on resting NK or T cells, but activation leads to the up-regulation of CD69 expression on their surface.57,59 Stimulation of PBMCs from SS patients with CpG-A resulted in up-regulation of CD69 on 30% of their CD56 NK cells, compared to 9% or 4% detected in medium or in control CpG ODN. However, the effect was less marked than on PBMCs from healthy volunteers, where 68% of NK cells were induced by CpG-A to express CD69 (versus 5% and 3% CD56 NK cells expressing CD69 in medium or control CpG ODN, respectively) (Figure 4A). Stimulation with recombinant IFN-α resulted in a comparable level of activation of NK cells among the PBMCs of patients (26%) and healthy volunteers (62%) (Figure 4A). In addition, CpG-A significantly activated the cytolytic machinery of NK cells in patients and healthy volunteers (P < .05; compared to medium or control CpG). Again, the effect was less marked among the PBMCs of patients (from 46%-11% of specific lysis in the range of 50:1 to 6:1 effector-to-target ratio) as compared to healthy volunteers (71%-25% in the same range of effector/target ratio) (Figure 4B).
CpG-A activates NK cells and CD8 T cells and up-regulates CD69 expression on these cells. (A) PBMCs from SS patients (n = 18, ▪) and healthy volunteers (n = 8, ▦) were stimulated with CpG-A, control CpG ODN, or IFN-α (10 ng/mL) as described in Figure 2. Cells were then harvested, stained with appropriate antibodies, and analyzed for the expression of CD69 on CD56 NK cells. Data represent means (± SD) of tested individuals and are presented as a percentage of CD56 NK cells. **P < .001 compared with medium or control CpG. (B) PBMCs from SS patients (n = 7, left panel) and healthy volunteers (n = 5, right panel) were stimulated with CpG-A, control CpG ODN, or IFN-α as described in Figure 2, followed by a 4 hours' Cr51 release assay using K562 cells as targets. Data represent means (± SD) of tested individuals and are presented as a percentage of specific lysis. (C) PBMCs from SS patients (n = 18, ▪) and healthy volunteers (n = 8, ▦) were stimulated with CpG-A, control CpG ODN, or IFN-α as described in Figure 2. Cells were then harvested, stained with appropriate antibodies, and analyzed for the expression of CD69 on CD8 T cells. Data represent means (± SD) of tested individuals and are presented as a percentage of CD8 T cells expressing CD69. *P < .05 compared with medium or control CpG.
CpG-A activates NK cells and CD8 T cells and up-regulates CD69 expression on these cells. (A) PBMCs from SS patients (n = 18, ▪) and healthy volunteers (n = 8, ▦) were stimulated with CpG-A, control CpG ODN, or IFN-α (10 ng/mL) as described in Figure 2. Cells were then harvested, stained with appropriate antibodies, and analyzed for the expression of CD69 on CD56 NK cells. Data represent means (± SD) of tested individuals and are presented as a percentage of CD56 NK cells. **P < .001 compared with medium or control CpG. (B) PBMCs from SS patients (n = 7, left panel) and healthy volunteers (n = 5, right panel) were stimulated with CpG-A, control CpG ODN, or IFN-α as described in Figure 2, followed by a 4 hours' Cr51 release assay using K562 cells as targets. Data represent means (± SD) of tested individuals and are presented as a percentage of specific lysis. (C) PBMCs from SS patients (n = 18, ▪) and healthy volunteers (n = 8, ▦) were stimulated with CpG-A, control CpG ODN, or IFN-α as described in Figure 2. Cells were then harvested, stained with appropriate antibodies, and analyzed for the expression of CD69 on CD8 T cells. Data represent means (± SD) of tested individuals and are presented as a percentage of CD8 T cells expressing CD69. *P < .05 compared with medium or control CpG.
Similarly, stimulation with CpG-A resulted in activation of CD8 T cells. CD69 was up-regulated on 13% of patients' CD8 T cells and 21% of CD8 T cells from healthy volunteers (compared to 4% and 1%, respectively, in medium). Stimulation with recombinant IFN-α has not resulted in higher expression of CD69 compared to CpG-A (Figure 4C).
CD4 T cells were not easily activated by CpG-A. Among healthy volunteers only about 6% of CD4 T cells up-regulated CD69, whereas no significant up-regulation of CD69 was detected on CD4 T cells from SS patients (1.6% in response to CpG-A versus 0.9% in medium, Figure 6B).
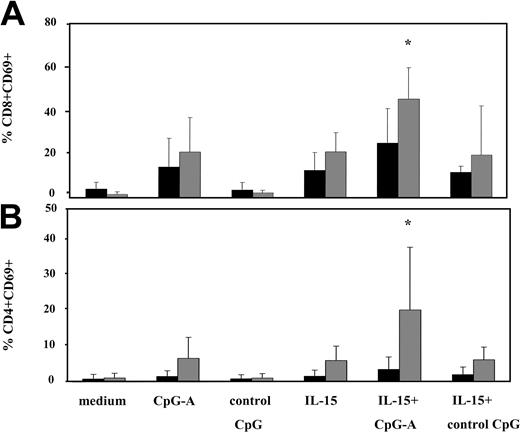
CpG-A and IL-15 in combination significantly enhances activation of T cells from healthy volunteers but not T cells of patients. PBMCs from SS patients (n = 11, ▪) and healthy volunteers (n = 7, ▦) were stimulated with CpG-A, control CpG ODN, IL-15, or the combination of CpG-A and IL-15 as described in Figure 2. Cells were then harvested, stained with appropriate antibodies, and analyzed for the expression of CD69 on CD8 (A) and CD4 (B) T cells. Data represent means (± SD) of tested individuals and are presented as a percentage of CD8 or CD4 T cells expressing CD69. *P < .05 compared with IL-15 or CpG-A.
CpG-A and IL-15 in combination significantly enhances activation of T cells from healthy volunteers but not T cells of patients. PBMCs from SS patients (n = 11, ▪) and healthy volunteers (n = 7, ▦) were stimulated with CpG-A, control CpG ODN, IL-15, or the combination of CpG-A and IL-15 as described in Figure 2. Cells were then harvested, stained with appropriate antibodies, and analyzed for the expression of CD69 on CD8 (A) and CD4 (B) T cells. Data represent means (± SD) of tested individuals and are presented as a percentage of CD8 or CD4 T cells expressing CD69. *P < .05 compared with IL-15 or CpG-A.
Enhanced activation of Sezary syndrome patients' NK cells resulting from combined stimulation with CpG-A and IL-15
We have observed a gradual decline in peripheral blood NK cell numbers in SS patients in association with an increasing burden of circulating malignant T cells (M.W., unpublished observations from our laboratory, February 2004). Thus, the full ability to activate patients' NK cells would be desirable for both cytokine production and for cytolytic activity. Since CpG-A alone produced suboptimal activation of the patients' NK and CD8 T cells, as assessed by up-regulation of CD69, we attempted to determine if the remaining NK and CD8 T cells could be activated by combining CpG-A stimulation with IL-15. The decision to use IL-15 was based on its known involvement in the development and maintenance of growth of NK cells and CD8 T cells.41
As shown in Figure 5, IL-15 activated patients' NK cells much more potently than CpG-A alone, and this also was true for NK cells from healthy volunteers. Stimulation of patients' PBMCs with IL-15 resulted in up-regulation of CD69 on 55% of CD56 NK cells compared to 30% of NK cells activated with CpG-A, whereas 86% of NK cells from healthy volunteers were activated by IL-15, compared to 68% activated by CpG-A only. Stimulation with IL-15 alone seemed to activate the majority of NK cells in the blood of healthy volunteers, while the addition of CpG-A insignificantly increased the percentage of activated NK cells to 90%. In contrast, a combination of IL-15 with CpG-A resulted in significant further activation of the patients' NK cells, up-regulating CD69 on 76% of NK cells (Figure 5A). As expected, NK cell cytolytic activity of PBMCs from low and medium tumor burden patients was increased if IL-15 was combined with CpG-A. However, IL-15 alone was quite a potent inducer of NK cytolytic activity in these patients as well as in healthy volunteers (data not shown), and the addition of CpG-A resulted in an increase in cytolytic activity that was not significant, compared to IL-15 alone (Figure 5B, left panel).
The combination of IL-15 and CpG-A has not been demonstrated to be particularly effective in activation of NK cells from high tumor burden patients. (Figure 5B, right panel). Whereas CpG-A alone failed to activate NK cytolytic activity in these patients, IL-15 induced only 35% specific lysis at the highest effector-to-target ratio of 50:1. Combining CpG-A with IL-15 did not further increase cytolytic activity of the patients' PBMCs.
CpG-A or IL-15, used as a single agent, activated a comparable percentage of CD8 T cells (14% and 13%, respectively, of patients' CD8 T cells and 21% of normal CD8 T cells). Combining both reagents resulted in additive activation of CD8 T cells (25% of patients' CD8 T cells and 45% of normal CD8 T cells up-regulated CD69); however, the increase with the combined treatment was not significant among patients when compared to CpG-A or IL-15 (Figure 6A).
Neither CpG-A nor IL-15 alone appeared to significantly enhance the expression of CD69 on the CD4 T cells from SS patients, and only 6% of CD4 T cells from healthy volunteers showed up-regulation of CD69. However, the combination of IL-15 and CpG-A did significantly enhance activation of CD4 T cells from healthy volunteers (21%) but not from patients (3%) (Figure 6B).
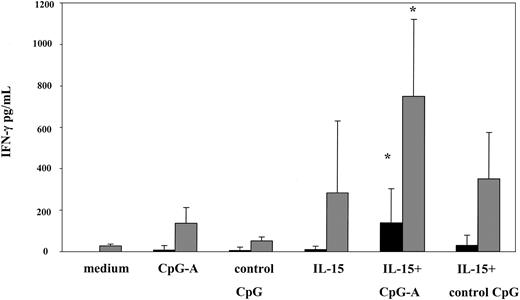
Consistent with the observed maximal activation of NK cells and CD8 T cells, the combination of CpG-A and IL-15 was superior in inducing significant levels of IFN-γ in culture supernatants from PBMCs of patients and healthy volunteers. On average, 142 pg/mL and 752.5 pg/mL of IFN-γ was detected in culture supernatants from patients and healthy volunteers, respectively. In contrast, levels of IFN-γ detected in response to CpG-A or IL-15 alone were 11- to 14-fold lower in patients (CpG-A; 9.7 pg/mL, IL-15; 12.8 pg/mL) and 2- to 5-fold lower in healthy volunteers (CpG-A; 138.2 pg/mL, IL-15; 285.5 pg/mL) (Figure 7).
CpG-A and IL-15 synergize in inducing IFN-γ production. PBMCs from SS patients (n = 7, ▪) and healthy volunteers (n = 6, ▦) were stimulated with CpG-A, control CpG ODN, IL-15 (1 ng/mL), or the combination of CpG-A and IL-15 as described in Figure 2. Cytokine levels were measured in cell-free supernatants by ELISA. Data represent means (± SD) of tested individuals. *P < .05 compared with CpG-A or IL-15 alone.
CpG-A and IL-15 synergize in inducing IFN-γ production. PBMCs from SS patients (n = 7, ▪) and healthy volunteers (n = 6, ▦) were stimulated with CpG-A, control CpG ODN, IL-15 (1 ng/mL), or the combination of CpG-A and IL-15 as described in Figure 2. Cytokine levels were measured in cell-free supernatants by ELISA. Data represent means (± SD) of tested individuals. *P < .05 compared with CpG-A or IL-15 alone.
Discussion
The decreased capacity of many types of tumor cells to induce an appropriate immunogenic signal all too often leads to an insufficient stimulation of the innate immune responses further leading to inadequate adaptive immunity and an ineffectively low frequency of tumor-specific cytotoxic T cells. The end result is a failure to eliminate or contain the cancer. The critical need to develop strategies to induce the optimal activation of the components of innate immunity, including dendritic cells and NK cells, and enhance adaptive immune responses, has been well recognized. Both CpG ODN and IL-15 play an essential role in the survival and activation of cellular components of innate and adaptive immunity.15,41
Although SS patients demonstrate the gradual loss of pDCs in the peripheral blood, remaining CD123 plasmacytoid DCs express the CpG ODN receptor, TLR9, at levels comparable to that on the cells of healthy volunteers. Quite importantly, the pDCs in the SS patients seem to maintain their functional integrity as suggested by their ability to distinguish between and to respond to 2 different types of CpG ODN: CpG-A and CpG-B. Levels of IFN-α produced by pDCs from patients in response to CpG-A were lower compared to healthy volunteers, which likely reflects the lower absolute number of IFN-α–producing cells in these patients rather than an intrinsic defect in IFN-α regulatory pathways. The level of expression of MHC class II on pDCs from patients and healthy volunteers were similar, suggesting that CpG-B-triggered signaling pathways in patients' pDCs were normal.
Our studies demonstrate that CpG-A ODNs were able to potently activate NK cells and CD8 T cells. However, our data indicate that CpG-A, used as a single agent, activates a much smaller percentage of NK cells and CD8 T cells from the patients compared to the cells from the healthy volunteers. Since activation of NK cells and CD8 T cells by CpG-A is critically dependent on IFN-α, the lower number of pDC in patients and consequently lower levels of IFN-α produced, could potentially result in lower activation of patients' NK cells and CD8 T cells. Alternatively, lower activation of NK cells and CD8 T cells may result from the intrinsic impairment in cellular signaling triggered by IFN-α. This is consistent with our observation that high doses of recombinant IFN-α were not able to induce proportionally higher levels of activation of NK cells and CD8 T cells in patients. However, it is possible that a single species of recombinant IFN-α added to the patients' cells might not fully substitute for a mixture of type I interferons released in response to CpG-A.
Presently, the basis for the less efficient activation of patients' NK cells and CD8 T cells by CpG-A in comparison to the cells of healthy volunteers is unclear. The major pathway of intracellular signaling used by IFN-α triggers the activation of signal transducer and activators of transcription such as STAT1. It remains to be established if the expression of phosphorylated STAT1 or other IFN-α–dependent signaling factors is impaired in NK cells or CD8 T cells of SS patients to the same extent as the previously demonstrated impairment in expression of STAT4 and phosphorylated STAT4, which are critical to the IL-12 signaling pathway.61
We have observed that progression of Sezary syndrome is typically associated with a decline in the number of peripheral blood CD8 T cells and NK cells. These findings imply that an expanding population of tumor cells may produce an as-yet-unidentified factor or factors that affect the homeostasis of these immune cells. The decrease in circulating NK cells and CD8 T cells and low NK activity does not seem to be a feature exclusive to Sezary syndrome. Similar observations have been made in patients with other cancers, including metastatic melanoma. Furthermore, several reports have demonstrated that NK cells from patients with cancer are prone to spontaneous apoptosis.62-65
It also is possible that the decreasing pool of NK cells and CD8 T cells in SS patients results from a depletion of “systemic” levels of IL-15, consistent with a gradual decline in the numbers of dendritic cells, which are the major IL-15 producers. Murine models have convincingly demonstrated that IL-15 deficiency can be the critical mechanism underlying low NK activity and low numbers of NK cells and CD8 T cells, which can be restored upon administration of IL-15.66-68 By comparison, IL-15 proved to be a very potent activator of NK cells from patients with SS, up-regulating CD69 expression and inducing strong cytotoxic activity. Nevertheless, IL-15 activated a higher percentage of NK cells from healthy volunteers than from patients, suggesting that IL-15 signaling pathways may not be optimal in the cells of patients. Studies of human NK cells have revealed the existence of 2 major populations that differ in the level of expression of CD56 and in their function. The CD56bright NK cell subset was found to have a high capacity for cytokine production, including IFN-γ, while the CD56dim cells failed to produce significant levels of cytokines but were more cytotoxic.69-71 Impaired IFN-γ production and overall lower responses in SS patients to IL-15, as well as to CpG-A, could thus result from partial or complete loss of one of the NK cell subpopulations, possibly CD56bright with the highest ability to up-regulate CD69 and to produce IFN-γ. Experiments are currently under way to examine in detail the populations of NK cells in SS patients.
The results of our studies clearly indicate that the combined stimulation of patients' cells with CpG-A and IL-15 produced the highest degree of activation of NK cells as assessed by CD69 up-regulation, NK cytolytic activity, and IFN-γ production.
In contrast to NK cells, similar percentages of CD8 T cells from patients and healthy volunteers up-regulated CD69 in response to either IL-15 or CpG-A. The combination of IL-15 and CpG-A produced further statistically significant enhancement in activation of normal but not patients' CD8 T cells. Thus, functional integrity of patients' CD8 T cells seems to be affected by not-yet-defined tumor-dependent factors.
Patients' CD4 T cells, unlike CD4 T cells from healthy volunteers, showed a total lack of response to CpG-A, IL-15, or a combination of both. These results seem to suggest that both the CD4 tumor cells as well as the remaining normal CD4 T cells in patients are not responsive to either stimulant, further suggesting that even a low tumor burden negatively affects their function.
In view of the typical scenario of depressed cellular immunity in association with Sezary syndrome, our data suggest that SS patients may benefit greatly from therapy combining CpG-A treatment with IL-15. In addition to the enhanced activation of NK cells and CD8 T cells achieved by a combination of both factors, their individual properties are likely to contribute to the development of effective antitumor responses. IFN-α produced in response to CpG-A may activate NK cells to kill tumor cells, leading to the release of apoptotic tumor cell bodies. The cell bodies can be captured by myeloid DCs, which then present tumor antigens to CD8 T cells in a process known as cross-priming. Recently, it has been shown that IFNα/β, induced either by virus or CpG ODN, was able to induce cross-priming through direct stimulation of DCs; however, the exact mechanisms underlying this process remain to be determined.72,73 Furthermore, it has been recently shown that stimulation with CpG-A induces secretion of the TH1-promoting chemokine, IFN-γ–inducible protein-10 (IP-10) by pDC and monocytes. The authors also found that low levels of IFN-γ induced in response to CpG ODN were sufficient to synergize with IFN-α to induce production of IP-10.74 It also has been previously demonstrated that IFN-α may have the potential to up-regulate IFN-γ expression by activating T cells and possibly NK cells, both major IFN-γ producers.75 Our data seem to agree with this notion, since CpG-A stimulation readily induced IFN-γ production by the PBMCs of healthy volunteers, although not by the PBMCs of patients. Importantly, IL-15 clearly synergized with CpG-A in inducing significantly higher levels of IFN-γ by both healthy volunteers and SS patients.
The inclusion of IL-15 in the treatment of SS patients may improve the absolute numbers of their NK cells and memory CD8 T cells. Several observations indicate that IL-15 is crucial for NK cell generation under physiologic conditions, and many studies have shown that IL-15 can potently promote NK cell development from NK cell precursors,66,68 whereas the absence of IL-15 results in a marked reduction of the numbers of NK cells.47,76 IL-15 also stimulates the proliferation and promotes survival of both naive and memory CD8 T cells.51-53
IL-15 does not seem to have a significant influence on the proliferation/survival of CD4 cells with a memory phenotype (CD44high), since these cells are present in normal numbers in IL-15-/- mice and only a low percentage of purified human CD4 T cells respond to IL-15.52,53 Nevertheless, recent studies of Geginat et all demonstrated that human effector-memory CD4 T cells divide in response to IL-15 stimulation.77
There are conflicting data concerning the role of IL-15 in supporting the growth of the malignant T-cell population in CTCL. IL-15 mRNA was found to be overexpressed in some skin biopsies from mycosis fungoides patients, in Sezary cells isolated from patients, and in some CTCL lines. The latter also had superior survival in the presence of IL-15 as compared to IL-2.78,79
A full understanding of the immunobiology of IL-15 and CpG ODN is critical if these potent immunomodulators are to be used as therapeutic agents for patients with CTCL. The results of our studies suggest that CpG ODN and IL-15 exhibit significant potential as agents with the capacity to ameliorate the profound deficiencies in cellular immunity that are observed in advanced cases of CTCL.
Prepublished online as Blood First Edition Paper, August 24, 2004; DOI 10.1182/blood-2004-03-1190.
Supported by grants CA 89442, CA81022, and CA00499 from The National Institutes of Health and by a grant from the Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal