Abstract
It is possible to differentiate malignant from healthy cells and to classify diseases based on identification of specific gene expression profiles. We hypothesized that gene expression profiling could also be used to identify differential activation of healthy and malignant cells, and as a model for this, we examined the molecular sequelae of CD40 activation of healthy and B-cell chronic lymphocytic leukemia (CLL) cells. Hierarchical clustering analysis of gene expression signatures grouped samples by CD40 activation status and further subclassified CD40-activated CLL cells from healthy B cells. Supervised analyses in healthy B cells compared to CLL cells identified differential regulation of genes governing cell cycle progression and apoptosis. CD40 signaling of CLL cells increases their susceptibility to immune recognition, but promotes survival and cell cycle arrest, making these cells potentially more resistant to chemotherapy. These results illustrate the utility of gene expression profiling to elucidate the molecular sequelae of signaling in healthy cells and altered signaling pathways in malignant cells. This type of approach should be useful to identify targets of therapy of malignant diseases. (Blood. 2004;104:4002-4009)
Introduction
CD40, the tumor necrosis factor receptor superfamily, member 5 (TNFRSF5) is expressed throughout B-cell development. Ligation of B-cell CD40 by its ligand CD154 (TNFSF5) expressed on activated T cells has important roles in T cell-mediated B-lymphocyte activation.1 A number of members of the family of tumor necrosis factor receptor-associated factors (TRAFs) bind to the cytoplasmic domain of CD40 and mediate activation of multiple signaling pathways that regulate B-cell survival, proliferation, differentiation, isotype switching, development of the germinal center, and the humoral memory response.2 CD40 signaling results in activation of nuclear factor κB (NF-κB),3 as well as the extracellular signal-regulated kinase (ERK), c-Jun N terminal kinase (JNK), p38 mitogen-activated protein (MAP) kinase, and phosphatidyl inositol 3-kinase (PI3K) pathways.4-6 The specific role of each of these pathways in mediating response to CD40 signaling has not been elucidated.
Ligation of CD40 also results in up-regulation of surface molecules contributing to antigen presentation on dendritic cells and monocytes as well as on healthy and malignant B cells.7 Following CD40 activation, the antigen-presenting capacity of chronic lymphocytic leukemia (CLL) cells is increased and these cells are now able to present tumor-associated antigens and induce proliferation of T cells with specificity for the leukemic cells.8,9 Autologous cytolytic T-cell (CTL) responses against CLL cells can be generated using idiotype-derived peptides as well as intracellular antigens identified by SEREX, demonstrating that tumor-associated antigens can be presented by the CLL cells, but there is increased killing of CD40-activated CLL cells.10-12 Infusion of autologous CLL cells transfected with a replication-defective adenovirus vector encoding recombinant CD40 ligand (CD154) induces antileukemic T-cell responses.13 These studies have all suggested that CD40 activation increases the antigen-presenting capacity of both healthy and malignant B cells; however, it is not clear whether other downstream signaling pathways of CD40 are intact.
Gene expression profiling has been used to compare the molecular differences and similarities between malignant and healthy cells as well as to compare the molecular signatures of subsets of malignant cells based on their biology or clinical outcome.14-20 These studies have increased our understanding of disease pathogenesis, classification, and risk stratification and identified potential new targets for treatments. CLL has a readily distinguishable gene expression pattern compared to other lymphoid malignancies and to healthy B cells, with a profile more related to resting and memory B cells, and not in keeping with the hypothesis that CLL cells are derived from CD5+ B cells.15,16
We hypothesized that gene expression profiling could also be used to identify differential molecular sequelae following activation of healthy and malignant cells. To examine this, we analyzed the gene expression profile of CLL cells compared to healthy peripheral blood B cells with or without in vitro CD40 activation. Gene expression profiling after CD40 activation of murine B cells has demonstrated that it is possible to use this approach to dissect the molecular pathways defining biologic behavior.21,22 However, such studies have not been previously performed in human cells nor used to compare the molecular consequences of signaling in healthy compared to malignant cells. We therefore performed microarray gene expression analysis on CLL cells, which had or had not been CD40 activated, from 24 previously untreated patients and compared the expression pattern with that of peripheral blood B cells, treated identically, from 11 healthy donors. The results demonstrate that microarray analysis can be used to identify different patterns of gene expression pathways following activation of malignant compared to healthy cells. This approach may therefore be used to further our understanding of the pathophysiology of disease, to study the consequences of interaction with receptors or cytokines in the tumor microenvironment, and to identify potential targets for therapy.
Patients, materials, and methods
Patient material
Peripheral blood samples were obtained from 24 patients with CLL selected to represent the wide spectrum of characteristics in this disease. All CLL patients were previously untreated. Healthy B cells were isolated from 11 healthy blood donors. All samples were obtained after receiving signed informed consent and after approval by the Institutional Review Board. Details of the CLL patients are shown in Table 1. Analyses of differences within a sample between expression levels for CD40 activated and mock activated were conducted on samples from 19 CLL patients and 6 healthy donors from whom sufficient cells were available for matched-pair analyses to be performed.
Clinical characteristics of patients
Characteristic . | Data . |
|---|---|
| Age (median), y | 38-83 (51) |
| WBC count (median), ×109/L | 13-211 (54) |
| Sex, no. | |
| Female | 12 |
| Male | 12 |
| Rai stage, no. | |
| 0 | 3 |
| I | 11 |
| II | 8 |
| IV | 2 |
| Cytogenetics, no. | |
| Normal | 7 |
| 13q del | 7 |
| 11q del | 2 |
| 17p del | 2 |
| 6p del | 1 |
| Trisomy 12 | 4 |
| Complex | 2 |
| Immunoglobulin mutation status, no. | |
| Germline | 10 |
| Mutated | 14 |
| CD38 expression, no. | |
| Less than 30% | 9 |
| More than 30% | 15 |
| CD5/CD19 lymphocytes, no. | |
| More than 95% | 19 |
| Less than 95% | 5 |
Characteristic . | Data . |
|---|---|
| Age (median), y | 38-83 (51) |
| WBC count (median), ×109/L | 13-211 (54) |
| Sex, no. | |
| Female | 12 |
| Male | 12 |
| Rai stage, no. | |
| 0 | 3 |
| I | 11 |
| II | 8 |
| IV | 2 |
| Cytogenetics, no. | |
| Normal | 7 |
| 13q del | 7 |
| 11q del | 2 |
| 17p del | 2 |
| 6p del | 1 |
| Trisomy 12 | 4 |
| Complex | 2 |
| Immunoglobulin mutation status, no. | |
| Germline | 10 |
| Mutated | 14 |
| CD38 expression, no. | |
| Less than 30% | 9 |
| More than 30% | 15 |
| CD5/CD19 lymphocytes, no. | |
| More than 95% | 19 |
| Less than 95% | 5 |
WBC indicates white blood cell.
CD40 activation
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density centrifugation from CLL blood samples or leukopaks derived from healthy donors. B cells were isolated by negative selection from the healthy PBMC samples using the B-cell isolation kit (Miltenyi Biotech, Auburn, CA), according to the manufacturer's instructions. All healthy B-cell samples were more than 95% pure. The majority of the CLL samples contained over 90% CD5+/CD19+ B cells. Therefore, PBMC samples from the patients were largely plated directly onto feeder cells. NIH3T3 cells transfected with CD40L or mock transfected were plated and adhered to 6-well plates as previously described.23 Healthy and malignant B cells were plated onto the adhered feeder cells at a density of 4 × 106/well and cultured at 37°C in 5% CO2 for 1, 2, and 3 days.
Total RNA isolation
Total RNA was extracted with Trizol (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. Following addition of chloroform, the suspension was loaded onto a Phase-Lock Gel Heavy tube (Brinkman Instruments, Westbury, NY) and centrifuged at 3000 g for 15 minutes at 4°C. The aqueous phase was removed and transferred to a fresh tube and an equal volume of ethanol was added. This mixture was then loaded onto a spin column from the SV Total RNA Isolation Kit (Promega, Madison, WI) and washed and eluted according to the manufacturer's instructions. Total RNA was analyzed for purity using RNA 6000 Nano Chips and scanned on a 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA).
Microarray expression analysis
Total RNA (15μg) was transcribed into first-strand cDNA in the presence of 100 pmol T7 (T) 24 primer (Sigma Genosys, The Woodlands, TX), 1 × first-strand buffer, 0.1 M dithiothreitol (DTT), 0.5 mM deoxynucleotide triphosphates (dNTPs), and 400 units Superscript II RNase H-Reverse Transcriptase (Invitrogen Life Technologies). Second-strand cDNA synthesis was then performed using 1 × second-strand buffer, 40 units DNA polymerase I (Invitrogen Life Technologies), 0.2 mM dNTPs, 10 U Escherichia coli DNA ligase (Invitrogen Life Technologies), and 2 U RNase H (Invitrogen Life Technologies). Double-stranded cDNA was cleaned by phenol chloroform extraction using a Phase-Lock Gel Light tube (Brinkman Instruments) and precipitated with 7.5 M NH4OAc (Sigma Genosyn), 20 mg/mL glycogen (American Bioanalytical, Natick, MA), and 100% ethanol. Cleaned cDNA was in vitro transcribed using the Ambion T7 Megascript Kit (Ambion, Austin, TX) and Bio-11-CTP and Bio-16-UTP (Sigma-Aldrich, St Louis, MO) and purified using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturers' instructions. cRNA was fragmented and hybridized to an U95Av2 chip (Affymetrix, Santa Clara, CA). Washing, staining, and scanning on a GeneArray scanner (Agilent Technologies) were performed as recommended in the Affymetrix Gene Expression Analysis Technical manual.
Validation of expression
Twelve selected genes were analyzed for expression by real-time quantitative polymerase chain reaction (PCR). RNA samples that were previously analyzed by microarray were treated with DNase using the On-Column DNase Digestion with the RNase Free DNase Set (Qiagen) to ensure that gDNA was not present. Total RNA (2 μg) was reverse transcribed into cDNA in the presence of 1 × buffer, 1 μM oligo (dT)18 primer, 2 μM dNTP mix, 40 U RNase inhibitor, and 400 U Moloney murine leukemia virus (MMLV) reverse transcriptase (Advantage RT-for-PCR kit; BD Biosciences Clontech, Palo Alto, CA) according to the manufacturer's instructions.
Primers were designed in the region of the Affymetrix probe, using Primer Express 1.0 software (Applied Biosystems, Foster City, CA). Primer concentrations were optimized using methodology described in section 4 of the SYBR Green PCR Master Mix and real time-PCR (RT-PCR) protocol (Applied Biosystems) and optimal concentrations were used in the presence of 25 μL SYBR Green PCR Master Mix (Applied Biosystems) and 20 ng cDNA. The amplification profile for all genes was 40 cycles of 50°C for 2 minutes, 95°C for 10 minutes, 95°C for 15 seconds, and 60°C for 1 minute. The specificity of PCR products was analyzed by performing a melting curve profile (1 cycle of 60°C for 15 seconds, 95°C for 20 seconds, 60°C for 15 seconds) followed by analysis on Dissociation Curve 1.0 software (Applied Biosystems). PCR products were cloned using the TOPO TA Cloning Kit (Invitrogen Life Technologies) and the cloned product diluted into normal DNA to produce standard curves to determine copy number.
Western immunoblot
Total cell protein was extracted from mock- or CD40-activated B cells from patients with CLL and healthy donors. B cells (3 × 106) were lysed with sodium dodecyl sulfate (SDS) loading buffer and the supernatant was heated 5 minutes prior to analysis by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were separated by SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane. Protein blots were performed with primary antibodies against mouse anti-human cyclin B1, rabbit anti-human Cdk1/Cdc2, rabbit anti-human FLICE/procaspase 8 inhibitory protein (FLIP), mouse anti-human clusterin-α, rabbit anti-human NF-κB/p65 (Upstate Biotechnology, Lake Placid, NY), or rabbit anti-human NF-κB/p64 RelB (Active Motif, Carlsbad CA). Proteins then were labeled with secondary antibody horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin or HRP-conjugated anti-mouse immunoglobulin and detected by Western blot chemiluminescence reagents (Perkin Elmer, Boston, MA).
Biostatistical analysis
Gene expression profiling was performed on CD40-activated and mock-activated B-CLL cells as well as on stimulated healthy B cells. We performed a supervised analysis on gene expression levels comparing CD40-activated with mock-activated healthy cells, CD40-activated with mock-activated CLL cells, and CD40-activated CLL cells with CD40-activated healthy cells. In addition, for the samples for which both CD40-activated and mock-activated expression levels were available, we performed a supervised analysis comparing the magnitude of these differences within samples between the CLL and healthy B cells. To identify the genes whose expression patterns best distinguished 2 groups in the supervised analyses, the permutation distribution of the maximum t statistic was analyzed using Permax (http://biowww.dfci.harvard.edu/~gray/permax.html). In these supervised analyses, the Permax P value was calculated by comparing observed t statistics to the permutation distribution of the largest t statistic obtained over the 12 625 genes. Permax values of P less than .01 were deemed statistically significant. DNA-Chip Analyzer (dChip) was used to normalize the Affymetrix gene array data and to obtain perfect-match only model-based expression intensities. dChip was used to perform unsupervised analyses that consisted of gene filtering (excluding genes that lacked sufficient variability across groups) and hierarchical clustering of genes and samples. The Spearman rank correlation was used to assess the correlation between the quantitative PCR analysis and Affymetrix data of selected genes.
Results
Purified B cells from healthy donors and from patients with CLL were activated by CD40-transfected or mock-transfected fibroblasts for 72 hours; total RNA was extracted and hybridized and microarray analysis was performed for each of the 4 groups. The clinical characteristics of the patients with CLL are shown in Table 1. All patients were previously untreated and were selected to represent the heterogeneity of this disease in terms of disease stage, duration of disease, cytogenetic abnormality, immunoglobulin gene mutational status, and clinical course with some patients following an indolent course and others experiencing progressive disease and subsequent requiring therapy.
Genes altered by CD40 ligation
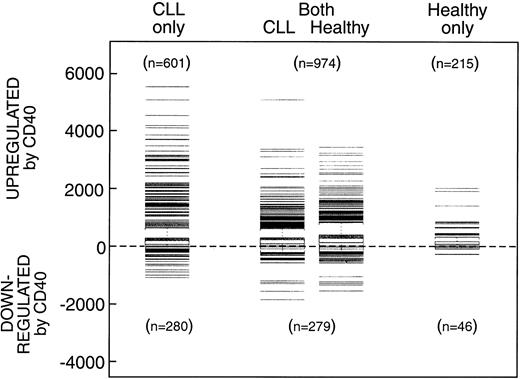
We performed a supervised analysis based on all 12 625 probe sets (genes) to determine the genes that were significantly altered (P < .01) comparing CD40-stimulated with mock-stimulated healthy cells and CLL cells following 72 hours of culture. Figure 1 shows the numbers and relative changes in gene expression in the CLL and healthy cells. A total of 1189 genes in the healthy B cells and 1575 genes in the CLL cells were significantly up-regulated after CD40 activation, 215 uniquely in healthy cells, 601 uniquely in the CLL samples, and 974 genes up-regulated in both healthy and CLL cells. Genes similarly up-regulated in both CLL and healthy B cells included major histocompatibility complex (MHC) class I and class II and the costimulatory molecules CD80 and CD86. A total of 325 genes were down-regulated in healthy B cells 46 of which were down-regulated only in healthy B cells, 279 of which were also down-regulated in both healthy B cells and CLL cells, and additional 820 genes down-regulated only in the CLL cells.
Numbers and relative changes in gene expression. Number of genes that were significantly increased or decreased (P < .01) after 3 days of culture on CD40-transfected feeder cells compared to mock-transfected cells in CLL and healthy B cells.
Numbers and relative changes in gene expression. Number of genes that were significantly increased or decreased (P < .01) after 3 days of culture on CD40-transfected feeder cells compared to mock-transfected cells in CLL and healthy B cells.
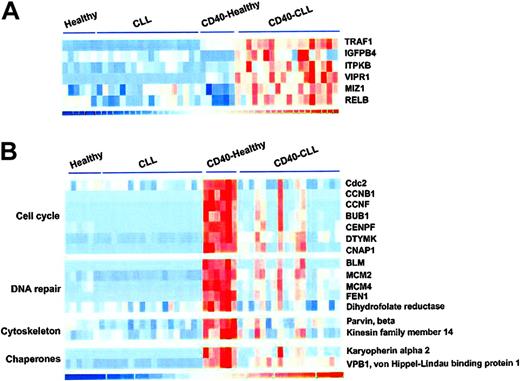
For those samples in which both CD40-stimulated and mock-stimulated expression levels were available, we performed a supervised analysis comparing the magnitude of these differences within samples between the CLL and healthy B cells. Genes whose levels of expression were differentially expressed in CD40-activated CLL samples included TRAF1, insulin-like growth factor-binding protein 4 (IGFPB4), inositol 1,4,5, triphosphate kinase B (ITPKB), vasoactive intestinal peptide receptor 1 (VIPR1), zinc finger protein 151 (ZNF151), and v-rel reticuloendotheliosis viral oncogene homolog B (RELB), as shown in Figure 2A. Larger sets of genes were differentially up-regulated after CD40 activation in healthy B cells compared to CLL cells. These included a number of genes involved in cell cycle control including cell division cycle 2, G1 to S and G2 to M (CDC2), cyclin B1 (CCNB1), cyclin F (CCNF), budding uninhibited by benzamidazole 1 homolog (BUB1), mitosin (CENPF), deoxythymidylate kinase (DTYMK), and chromosome condensation-related SMC-associated protein 1 (CNAP1); DNA replication and repair genes including Bloom syndrome protein, minichromosome maintenance deficient 2 (MCM2), MCM4, flap endonuclease 1 (FEN1), and dihydrofolate reductase; genes involved in actin biding, intracellular transport, or chaperone proteins including parvin β, kinesin family member 14 (KIF14), karyopherin α 2 (KNPA2), and von Hippel-Lindau-binding protein 1 (VPB1), shown in Figure 2B.
Representative genes differentially expressed following CD40 activation in CLL cells compared to healthy B cells based on supervised matched-pair analysis. (A) Genes whose level of expression was significantly increased in CLL cells compared to healthy B cells. (B) Genes whose level of expression was significantly increased in healthy B cells compared to CLL cells.
Representative genes differentially expressed following CD40 activation in CLL cells compared to healthy B cells based on supervised matched-pair analysis. (A) Genes whose level of expression was significantly increased in CLL cells compared to healthy B cells. (B) Genes whose level of expression was significantly increased in healthy B cells compared to CLL cells.
Hierarchical clustering analysis
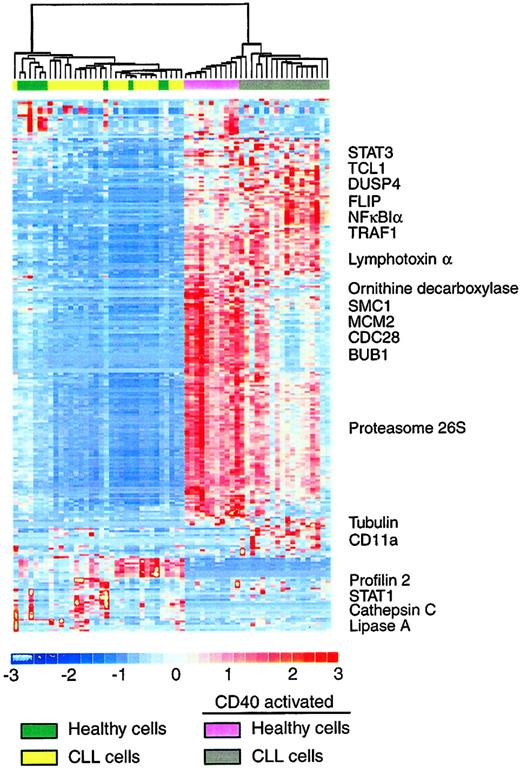
To identify the gene expression patterns across all 4 groups simultaneously, we performed an unsupervised hierarchical clustering analysis using DNA-Chip Analyzer (dChip).24 This analysis filtered all 12 625 genes to exclude genes that did not show sufficient variability across all samples and to cluster those samples that showed patterns in expression to classify the samples into distinct subgroups based on similarities in gene expression patterns. A total of 247 genes met the defined filtering criteria. Hierarchical clustering delineated 2 major clusters (Figure 3), one containing all samples that had been stimulated by CD40 and another containing the mock-stimulated samples.
Results of hierarchical clustering. Unsupervised hierarchical clustering analysis of gene expression of CLL and healthy B cells that were CD40 activated or mock activated for 3 days based on 247 genes that demonstrated sufficient variability to be included in the analysis. Representative genes are named.
Results of hierarchical clustering. Unsupervised hierarchical clustering analysis of gene expression of CLL and healthy B cells that were CD40 activated or mock activated for 3 days based on 247 genes that demonstrated sufficient variability to be included in the analysis. Representative genes are named.
This pattern of clustering demonstrates that the algorithm driving the model looking for genes that show differential patterns across all samples is indeed CD40 activation The mock-stimulated CLL cells and healthy samples were indistinguishable in the clustering analysis and did not separate into their respective groups, further supporting our hypothesis that peripheral blood B cells are appropriate controls for the CLL cells for this analysis.
Within the CD40-activated cluster there was a further division into 2 major groups, one of which contained all of the CD40-activated healthy samples plus 2 of the CD40-activated CLL samples, whereas the other branch incorporated all other CD40-activated CLL samples (Figure 3). No clinical parameter clearly differentiated these 2 patients from the others. In addition, unsupervised and supervised analyses did not identify differences between CLL cases with unmutated and mutated immunoglobulin genes, or any other clinical parameter following CD40 activation. A full listing of these 247 genes is provided in Supplemental Figure 1 (see the Supplemental Figure link at the top of the online article on the Blood website).
Differentially expressed genes within the CD40-activated samples
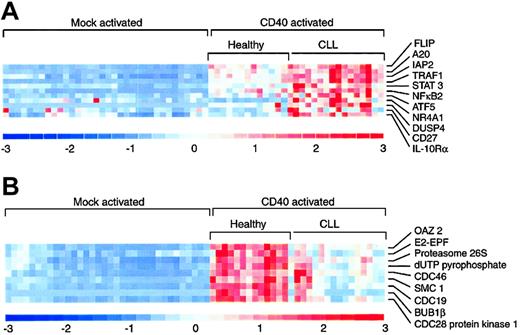
We further analyzed genes that were differentially regulated in the CD40-activated CLL samples compared to the CD40-activated healthy samples and observed a number of genes that were up-regulated in CD40-activated CLL compared to healthy cells (Figure 4A). These included many of the genes identified in the supervised analysis, including subunits of NFKB, TRAF1, and ZNF151. Additional genes identified in this analysis included several involved in the suppression of apoptosis mediated by the TNF family of receptors and NF-κB, including CASP8 and FADD-like apoptosis regulator (CFLAR; previously known as FLICE/procaspase 8 inhibitory protein or FLIP), TNF, α-induced protein 3 (TNFAIP3; also known as A20), baculoviral IAP repeat-containing 3 (BIRC3; alias cIAP2), and TRAF1. Other genes increased in CD40-activated CLL included signal transducers and activators of transcription 3 (STAT3), NFKB2, activating transcription factor 5 (ATF5), the nuclear transcription factor NRFA1, dual specificity phosphatase 4 (DUSP4), CD27, the interleukin 10 receptor (IL10R), and 3 members of the regulator of G protein-signaling family (RGS1, RGS2, and RGS10). Genes up-regulated in CLL compared to healthy B cells represent potential targets for therapy.
Representative genes from the unsupervised hierarchical clustering analysis that showed differential expression following CD40 activation. (A) Genes whose level of expression was significantly increased in CLL cells compared to healthy B cells. (B) Genes whose level of expression was significantly increased in healthy B cells compared to CLL cells.
Representative genes from the unsupervised hierarchical clustering analysis that showed differential expression following CD40 activation. (A) Genes whose level of expression was significantly increased in CLL cells compared to healthy B cells. (B) Genes whose level of expression was significantly increased in healthy B cells compared to CLL cells.
A number of genes were up-regulated in healthy B cells compared to CLL cells and relative levels of expression of representative genes including ornithine decarboxylase (OAZ2), ubiquitin carrier protein (E2EBF), 26S proteasome nonadenosine triphosphatase (ATPase) regulatory subunit 1 (PSMD1), dUTP pyrophosphatase, CDC46 homolog (MCM5), structural maintenance of chromosome 1-like 1 (SMC1L1), CDC19 (MCM2), BUB1B, and CDC28 protein kinase 1 (CKS1B) are shown in Figure 4B.
Specifically, genes in this group included those required for DNA replication and repair (dUTP pyrophosphatase, replication protein A3), polyamine biosynthesis (ornithine decarboxylase, hypoxanthine phosphoribosyltransferase 1, methylenetetrahydrofolate dehydrogenase), RNA processing and translation (eukaryotic initiation translation factor 1A, small nuclear auxiliary factor 1), and fatty acid metabolism (fatty acid desaturase) and genes involved in cell cycle progression and DNA replication including CDC19, CDC46, CDC47, and BUB1B. Because CD40 activation is known to induce B-cell proliferation, up-regulation of genes involved with regulation of cell cycle progression and metabolism in healthy B cells is not surprising. More surprising is the fact that these genes were significantly decreased in CLL cells compared to healthy cells after CD40 activation.
Validation of expression levels in gene expression profiles
Among the genes that changed expression following CD40 activation, we randomly selected 12 genes of interest to validate by quantitative real-time (RT)-PCR and correlated the results with microarray expression data. There was a high correlation between gene expression values obtained by the 2 methods (P < .0001). Quantitative RT-PCR data were expressed as mRNA copy number per microgram total RNA rather than copy number per housekeeping gene because none of the housekeeping genes investigated showed a stable level of expression after mock and CD40 activation. Glyceraldehyde phosphate dehydrogenase (GAPDH), β-actin, β2-microglobulin, and 9S all showed increased levels of expression in CD40-activated samples compared to the mock-activated samples, in keeping with previous reports of housekeeping gene variability, particularly in proliferating cells.25,26
Time-point analysis
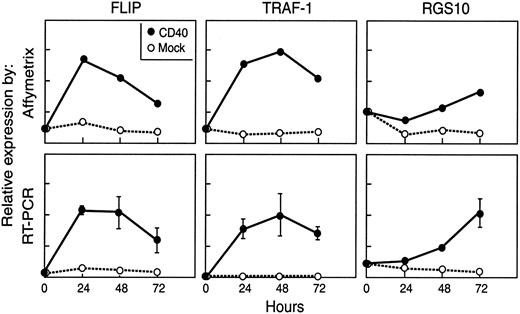
We initially chose to analyze gene expression following 3 days in culture with CD40 transfectants because our previous data had demonstrated that B cells had maximal antigen-presenting capacity at this time period.9,23 Although analysis of one time point can provide valuable data, gene regulation following any signaling event is a dynamic process. We therefore performed a time-point analysis to examine gene expression patterns in CLL cells that were unstimulated, and after 24, 48, and 72 hours on both mock or CD40 transfectants. For this type of analysis 7 microarray chips were required for each of the 2 patients studied. We further validated selected genes of interest by quantitative RT-PCR. The representative patterns of change in gene expression after CD40 activation of CLL cells from one patient are shown in Figure 5 for CFLAR (FLIP), TRAF1, and RGS10.
Gene expression levels in a patient with CLL. Gene expression levels over time after CD40 activation analysis in a patient with CLL as assessed by Affymetrix (arbitrary units) and quantitative RT-PCR (copy number/μg mRNA) for FLIP (CFLAR), TRAF1, and RGS10.
Gene expression levels in a patient with CLL. Gene expression levels over time after CD40 activation analysis in a patient with CLL as assessed by Affymetrix (arbitrary units) and quantitative RT-PCR (copy number/μg mRNA) for FLIP (CFLAR), TRAF1, and RGS10.
Expression of CFLAR and TRAF1 RNA peaked over the first 24 to 48 hours and declined thereafter. However, both genes remained increased at 72 hours in CD40-activated compared to mock-activated CLL cells. Expression of RGS10 decreased in the first 24 hours after CD40 and mock activation, followed by a steady increase over the next 48 hours in the CD40-activated cells.
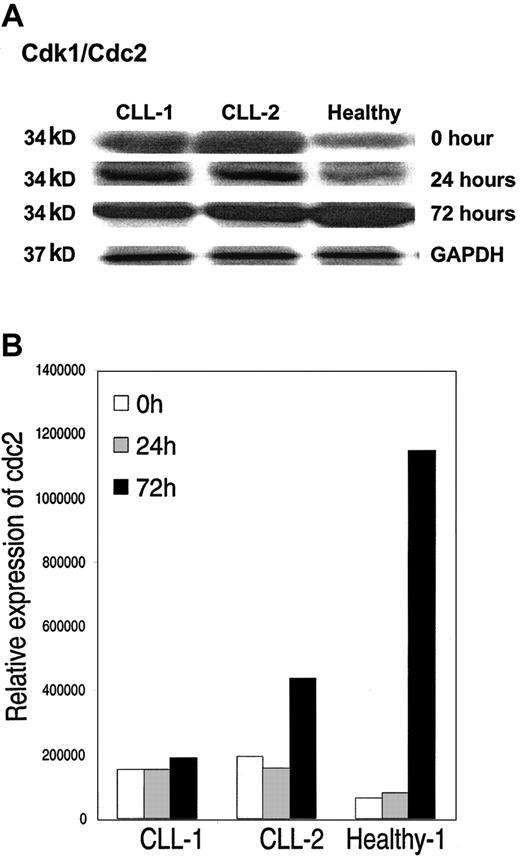
We further validated changes in protein expression for selected genes by Western immunoblot analysis. We observed concordance in the levels of expression of protein compared to that expected from the gene expression profiling. A representative example of expression by Western blot of cdk1/cdc2 34-kDa protein at 0, 24, and 72 hours in CD40-activated CLL cells compared to healthy B cells is shown in Figure 6. Similar to the pattern seen in the gene expression profiling by Affymetrix, the level of protein expression is higher in CLL cells compared to healthy B cells at time 0, but by 72 hours after CD40 activation there is increased expression in healthy B cells compared to CLL cells.
Western blot analysis. (A)Western blot analysis of cdc2 in CLL cells from 2 patients and from healthy B cells at time 0, and at 24 and 72 hours after CD40 activation. (B) Levels of expression corrected for protein loading.
Western blot analysis. (A)Western blot analysis of cdc2 in CLL cells from 2 patients and from healthy B cells at time 0, and at 24 and 72 hours after CD40 activation. (B) Levels of expression corrected for protein loading.
Discussion
Microarray technology has been widely used to profile gene expression in various diseases to improve classification, to subcharacterize diseases with heterogeneous clinical outcome, and to identify potential targets for therapy. It has been used to a lesser extent to analyze molecular events in response to physiologic activation of tumor versus healthy cell types. We sought to determine whether microarray analysis could be used as a tool to delineate similarities and differences in healthy compared to malignant cells following cell activation. We chose as our model to investigate the molecular consequences of CD40 signaling in healthy and malignant B cells because of its potential clinical utility.13 To perform this analysis, CLL cells from previously untreated patients and healthy B cells were cultured with CD154- or mock-transfected NIH3T3 feeder cells for 0, 24, 48 or 72 hours, followed by total RNA isolation and hybridization. We demonstrate that such an analysis identifies differential regulation of pathways regulated by CD40 signaling in healthy and malignant B cells suggesting that this approach can be used to identify alterations in the global signaling pathways in malignancies.
It should be stressed that gene expression analysis at the RNA level may not reflect changes in protein level because translation does not always occur. This difficulty is compounded in the present study in which we examine the consequences of intracellular signaling, often mediated by posttranscriptional modification such as phosphorylation. However, we did observe similar patterns of changes in protein expression and gene expression profiling. The consequences of CD40 activation are dependent on the time point at which the cells are studied and changes in translation likely in waves following primary and secondary events that occur after CD40 activation. Therefore, a determination has to be made at which time points analyses should be performed. It is not feasible to perform such an analysis at all time points. In 2 patients we analyzed gene expression at different time points after CD40 activation and ongoing studies will examine selected genes of particular interest based on the results obtained in this type of analysis. Based on our previous data that antigen-presenting cell (APC) activity and B-cell proliferation appears maximal at 72 hours, we chose to analyze at this time point. Results of the time-point analysis suggest that there is no ideal time point at which such a comparative study can be performed, and the timing of such an analysis should be based on other criteria related to the biology of the system being evaluated. Any comparison of malignant cells with healthy cells raises the issue of the appropriate control healthy cell population. Although CLL expresses CD5, previous microarray analyses had demonstrated that CLL cells did not appear to be the malignant counterpart of CD5+ healthy B cells, but were most closely related to resting and memory B cells.15,16 Based on these results, we compared CLL cells to healthy peripheral blood B cells rather than CD5 B cells. The fact that there was no clustering of mock-activated healthy B cells versus CLL cells lends further support that these are an appropriate control group. Finally, it should be acknowledged that genes expressed by T cells contaminating the healthy and malignant B-cell populations could influence the gene expression profile. The purity of the cell populations in all cases was high and we did not observe any differences in gene expression profiles comparing those with in whom the B-cell purity was more than 95% compared to those in which it was less than 95%, suggesting that the differences in gene expression profiling observed are not influenced by contaminating T cells.
CD40 signaling has previously been demonstrated to regulate expression of multiple genes encoding antiapoptotic proteins including bcl-xL, cIAP2, and A20.27-29 Of the genes that demonstrate increased expression in CD40-activated CLL cells compared to activated healthy cells, several are antiapoptotic, including CFLAR (FLIP), TRAF1, BIRC3 (cIAP2), and TNFAIP3 (A20). Our data demonstrated significantly higher levels of expression of these molecules following CD40 activation in CLL compared to healthy B cells and these findings were confirmed by quantitative RT-PCR validation. CFLAR (FLIP) suppresses Fas-mediated apoptosis by interfering with the activation of procaspase 8 at the level of the death-inducing signaling complex (DISC).30 FLIP expression peaked over the first 24 to 48 hours, declined thereafter, but at 72 hours remained increased in CLL cells compared to healthy B cells. TRAF1 belongs to a family of adaptor molecules that mediate the propagation of signals from TNFR superfamily receptors, including LMP1, TNFR1, TNFR2, CD30, and CD40. TRAF1 associates with other signaling molecules including TNFRSF1A-associated via death domain (TRADD), TRAF family member-associated NFKB activator (TANK), TRAF interacting protein (TRIP), receptor (TNFRSF)-interacting serine-threonine kinase 1 (RIPK1), receptor-interacting serine-threonine kinase 2 (RIPK2), TNFAIP3, BIRC2, BIRC3, and CFLAR.31 TRAF1 has a restricted tissue expression and is expressed in activated lymphocytes, dendritic cells, and certain epithelia.32 Expression is low in resting circulating peripheral blood lymphocytes but is induced on activation with CD154 and TNF-α.32,33 TRAF1 has been previously reported to be overexpressed in lymphomas and CLL cells.32,34 However, our data demonstrated that TRAF1 levels in CLL cells on day 0 were relatively low, rose rapidly after CD40 ligation, peaked after 24 to 48 hours of culture, and decreased slightly by 72 hours, at which time they were still increased compared to healthy B cells. cIAP2 interacts with TRAF1 and TRAF2 to suppress TNF-mediated apoptosis via up-regulation of NF-κB.35 Although cIAP2 expression levels have been reported to not differ between CLL and healthy B-cell samples34 or following CD40 activation,36 we observed that although CD40 ligation induced cIAP2 expression in both cell types, there were significantly higher levels of expression in CLL compared to B cells. A similar pattern was observed for A20, a zinc finger protein that interferes with recruitment of the death domain signaling adaptors TRADD and RIP to TNFR1, resulting in inhibition of TNFR1-induced apoptosis.37 Our findings are in keeping with previous reports suggesting that prevention of TNFR1-mediated apoptosis is approached at multiple sites in the signaling pathway, including disruption of events proximal to the receptor by A20 and more distal inhibition by FLIP, TRAFs, and cIAPs.35,37-39 These molecules are all induced by NF-κB activation following CD40 signaling. An additional level of complexity is added by finding that A20 is also a potent inhibitor of NF-κB,40 perhaps controlling its own expression and that of other antiapoptotic molecules in a negative feedback loop.
Among the other genes increased in CD40-activated CLL are CD27, STAT3, the IL-10 receptor, and 3 members of the regulator of G protein signaling family (RGS). CD27 is a member of the TNFR family involved with costimulatory interactions between T and B cells.41 It is synthesized as both a membrane and soluble form and increased serum levels have been detected in patients with CLL,42 which may act to impair costimulation of T cells via CD70, the CD27 ligand. Serum levels for IL-10 are also increased in B-CLL patients,43 and this cytokine has been found to serve as an autocrine growth factor in B-cell lymphoma cell lines.44 STAT3 signaling can protect cells against apoptosis and this transcription factor is constitutively active in many types of cancer, including CLL.45 The RGS family are involved with driving G proteins, which link membrane receptors with their intracellular signaling components, into their inactive GDP-bound forms and RGS2 is expressed in various hematologic malignancies.46
Genes up-regulated in CD40-activated healthy B cells and not in CLL cells were largely involved in growth, proliferation, and metabolic processes. A normal consequence of CD40 ligation on B cells is to drive growth and proliferation. Our data support this finding, but also demonstrate how refractory CLL cells appear to be to external physiologic stimuli of this kind. Of note, CD40 activation also resulted in up-regulation in CLL cells but not healthy B cells of DUSP4 and ZNF151. DUSP4 belongs to a family of phosphatases that negatively regulate members of the mitogen-activated protein (MAP) kinase superfamily (MAPK/ERK, SAPK/JNK, p38), which are associated with cellular proliferation and differentiation.47,48 ZNF151 has a potent growth arrest function that occurs via the transcriptional activation of a set of growth inhibitory genes.49,50 Taken together, it is intriguing to speculate that the down-regulation of cell cycle progression genes in CLL might be mediated by active suppression. Ongoing experiments silencing expression of these genes are being performed to address this.
We show, therefore, that short-term activation of CLL cells with CD40 ligand actually increases their potential to avoid apoptosis, without up-regulating the genes responsible for growth. The data here are consistent with the hypothesis that while CD40 signaling of CLL cells in vivo increases their susceptibility to immune recognition, at the same time it promotes survival and cell cycle arrest, making these cells potentially more resistant to chemotherapy.
Our studies further demonstrate that gene expression profiling can be applied to the analysis of differential signaling pathways in healthy and malignant cells. It may be possible therefore to use this approach to identify signal transduction pathways involved in the pathogenesis of malignancies and help to identify suitable targets for new therapeutic approaches.
Supplemental material is available online at the time of final publication only.
Prepublished online as Blood First Edition Paper, May 25, 2004; DOI 10.1182/blood-2004-02-0494.
Supported by National Institutes of Health grant CA81534 to the CLL Research Consortium and by the Eskandarian CLL Research Fund.
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Edward A. Fox and his staff of the Microarray Core facility at Dana-Farber Cancer Institute for help with the microarray analysis, Dr Sabina Chiaretti for assistance and for helpful advice, and Peter Varney for editing.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal