Abstract
Heparin cofactor II (HCII) is a plasma protein that inhibits thrombin rapidly in the presence of dermatan sulfate or heparin. We previously reported that the time to thrombotic occlusion of the carotid artery after photochemical injury was shorter in HCII-deficient mice than in wild-type control animals. In this paper, we describe the antithrombotic activity of dermatan sulfate in wild-type and HCII-deficient mice. Intravenous administration of porcine skin dermatan sulfate induced a dose-dependent prolongation of the carotid artery occlusion time in HCII+/+ mice that was not observed in HCII-/- animals. Pharmacokinetic studies suggested that porcine skin dermatan sulfate expresses antithrombotic activity after being transferred from the plasma to sites in the vessel wall. Using invertebrate dermatan sulfate preparations, we showed that N-acetylgalactosamine-4-O-sulfate residues are required for the HCII-dependent antithrombotic effect. Furthermore, the invertebrate dermatan sulfates, which have higher charge densities than mammalian dermatan sulfate, slightly prolonged the thrombotic occlusion time of HCII-/- mice. These results indicate that HCII mediates the antithrombotic effect of porcine skin dermatan sulfate after injury to the carotid arterial endothelium in mice, whereas more highly charged dermatan sulfates possess weak antithrombotic activity independent of HCII. (Blood. 2004;104:3965-3970)
Introduction
Heparin cofactor II (HCII) inhibits thrombin in a glycosaminoglycan-dependent manner. Both heparin and dermatan sulfate bind to HCII and increase the rate of thrombin inhibition approximately 10 000-fold.1 Following the report that dermatan sulfate prolongs the activated partial thromboplastin time (aPTT) of plasma in vitro,2 Buchanan et al found that intravenous infusion of porcine skin dermatan sulfate into rabbits inhibits thrombosis in a venous stasis model.3 A number of groups subsequently showed that dermatan sulfates isolated from porcine or bovine intestinal mucosa4 or from marine invertebrates5 not only stimulate HCII activity in vitro but also have antithrombotic activity in experimental models of venous and arterial thrombosis (reviewed in Linhardt and Hileman6 ). Furthermore, human trials demonstrated the efficacy of dermatan sulfate for prevention of thrombosis after hip surgery or during hemodialysis, and dermatan sulfate has been used successfully to anticoagulate patients with heparin-induced thrombocytopenia.7
The mechanism of the antithrombotic effect of dermatan sulfate remains poorly understood. By immunodepletion of HCII from human plasma, Sié et al demonstrated that the ability of dermatan sulfate to prolong the aPTT in vitro depends on the presence of HCII.8 Prolongation of the aPTT, however, does not accurately predict the antithrombotic activities of various glycosaminoglycans.9 Furthermore, the antithrombotic activities of certain chemically modified forms of dermatan sulfate do not correspond with their abilities to activate HCII in vitro, suggesting that dermatan sulfate may alter some aspect of platelet function or coagulation independent of HCII.6 For example, dermatan sulfate has been reported to promote fibrinolysis in vivo, perhaps by causing release of tissue plasminogen activitor.10,11 Therefore, despite the fact that dermatan sulfate activates HCII in vitro, there is no direct evidence that HCII mediates the antithrombotic effects of dermatan sulfate observed in experimental animals or humans.
The availability of HCII-deficient mice has allowed us to investigate the function of HCII in vivo. We previously reported that formation of an occlusive thrombus in the carotid artery after injury to the endothelium occurs more rapidly in HCII-deficient mice than in wild-type mice, demonstrating for the first time that HCII has antithrombotic activity in vivo.12 In the current study, we show that porcine skin dermatan sulfate inhibits thrombosis following carotid injury in wild-type mice but has no effect in HCII-deficient mice. In contrast, the antithrombotic activity of heparin does not depend on the presence of HCII, which is consistent with the belief that antithrombin is the major mediator of heparin's effect. Furthermore, we demonstrate that the antithrombotic activity of dermatan sulfate correlates with the presence of N-acetylgalactosamine-4-O-sulfate residues, which are required for binding of HCII to dermatan sulfate,13,14 and that highly charged invertebrate dermatan sulfates have weak antithrombotic activity that is HCII independent. Our results provide direct evidence that HCII mediates the antithrombotic effect of mammalian dermatan sulfate and raise the possibility that dermatan sulfate molecules present in the vessel wall stimulate HCII activity after vascular injury.
Materials and methods
Mice
HCII-deficient mice were generated by homologous recombination in 129/SvJ-derived embryonic stem cells.12 The resulting chimeric animals were backcrossed with inbred C57BL/6 mice purchased from Taconic (Germantown, NY). All experimental protocols were approved by the Washington University Animal Studies Committee.
Glycosaminoglycans
Dermatan sulfate from the ascidians Styela plicata and Ascidia nigra was prepared as previously described.14 Porcine skin dermatan sulfate, porcine intestinal heparin (183 U/mg), and chondroitin 4-sulfate were purchased from Sigma (St Louis, MO). Dermatan sulfate was treated with nitrous acid to remove contaminating heparin or heparan sulfate prior to use.2
Proteins
Arterial thrombosis model
Carotid artery thrombosis was induced as described previously.12 Briefly, adult male and female mice (∼ 25 g body weight) were anesthetized by intraperitoneal injection of sodium pentobarbital (80 mg/kg), secured in the supine position, and placed under a dissecting microscope. The right common carotid artery was isolated through a midline cervical incision, and an ultrasonic flow probe (model 0.5 VB; Transonic Systems, Ithaca, NY) was applied. A 1.5-mW, 540-nm laser beam (Melles Griot, Carlsbad, CA) was applied to the artery from a distance of 6 cm. Injury was initiated by injection into the lateral tail vein of rose bengal (50 mg/kg body weight; Fisher Scientific, Fair Lawn, NJ) dissolved in phosphate-buffered saline (PBS), and flow was monitored until complete and stable (> 5 minutes) occlusion occurred.
Antithrombotic activity of glycosaminoglycans
Glycosaminoglycans dissolved in 0.15 M NaCl were injected intravenously into the lateral tail vein at various intervals before injection of rose bengal, and the occlusion time was determined as described in the preceding paragraph. The total volume of material injected intravenously did not exceed 10% of the estimated blood volume of the mouse.
Pharmacokinetics of 3H-labeled dermatan sulfate
Porcine skin dermatan sulfate was radiolabeled and purified according to Höök et al.17 In this procedure, dermatan sulfate was partially N-deacetylated with hydrazine in the presence of hydrazine sulfate and then was reacetylated with [3H]acetic anhydride to regenerate the native polymer. 3H-labeled dermatan sulfate (320 000 dpm, ∼ 35 μg) was injected into the lateral tail vein together with 10 mg/kg unlabeled dermatan sulfate. The animal was killed after 5 to 60 minutes by exsanguination, blood was collected by cardiac puncture into one-tenth volume of 0.105 M sodium citrate, and urine was aspirated from the bladder. Platelet-poor plasma was obtained by centrifugation of the blood for 5 minutes at 16 000g. The animal was then perfused through the left ventricle of the heart with 50 mL PBS at a pressure of 100 mmHg, and various organs were harvested. Glycosaminoglycans were extracted from the organs by papain digestion and partially purified by cetylpyridinium and ethanol precipitation according to Pavão et al14 after addition of 0.4 mg unlabeled dermatan sulfate to each sample. The radioactivity present in the organs, plasma, and urine was determined by liquid scintillation counting. Each sample was recounted after addition of a tritium standard to correct for quenching.
Formation of 125I-thrombin-HCII complex ex vivo
Human thrombin was labeled with 125I as described previously.16 Mice were killed at various times after being injected with 10 mg/kg porcine skin dermatan sulfate, and plasma was prepared as described in the preceding paragraph. Plasma (4 μL) was incubated with 125I-thrombin (0.3 nM, ∼ 150 000 cpm) for 2 minutes at room temperature in 10 μL 0.15 M NaCl, 0.02 M Tris (tris(hydroxymethyl)aminomethane)-HCl, and 1 mg/mL polyethylene glycol, pH 7.4. The sample was then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 7.5% polyacrylamide gel under reducing conditions, and radioactive bands were detected by autoradiography.16 The 125I-thrombin-HCII band was cut from the gel and counted in a gamma counter.
aPTT assay
Citrate-anticoagulated mouse plasma (90 μL) was mixed with 10 μL of a glycosaminoglycan solution and 100 μL Alexin HS reagent (Sigma). After a 2-minute preincubation at 37°C, 100 μL 0.25 M CaCl2 was added and the clotting time was determined.
Thrombin inhibition assay
Glycosaminoglycans were incubated at room temperature with mouse thrombin (16 nM) and mouse antithrombin (50 nM) in 100 μL 50 mM Tris-HCl, 150 mM NaCl, 1 mg/mL poly(ethylene glycol), pH 7.4. Thrombin was added last to initiate the reaction. After 60 seconds, 500 μL 100 μM tosyl-Gly-Pro-Arg-p-nitroanilide (Roche Molecular Biochemicals, Indianapolis, IN) was added, and the absorbance at 405 nm was determined continuously for 100 seconds. The rate of change of absorbance was proportional to the residual thrombin activity.
Statistical analysis
Statistical significance was determined with the Student 2-tailed t test for independent samples. P < .05 was considered significant.
Results
Antithrombotic activity of porcine skin dermatan sulfate in HCII+/+ mice
The interval between endothelial injury and thrombotic occlusion of the carotid artery in HCII+/+ animals not treated with dermatan sulfate varied with their genetic background (Table 1). As previously noted,12,18 the presence of 129/SvJ alleles was associated with shorter occlusion times. Therefore, all of the experiments in this study were performed with commercial inbred C57BL/6 mice or HCII knock-out mice backcrossed for at least 10 generations into the C57BL/6 background.
Thrombotic occlusion times of untreated HCII+/+ mice
Generation . | Heterozygous Ioci, % . | Occlusion times, s, mean ± SD . |
|---|---|---|
| F1 agouti | 100 | 24 ± 5 (n = 5)* |
| N6F1 | 3 | 39 ± 10 (n = 18)* |
| N10 or greater | 0.2 or lower | 59 ± 5 (n = 6) |
| Inbred C57BL/6 | 0 | 56 ± 7 (n = 9) |
Generation . | Heterozygous Ioci, % . | Occlusion times, s, mean ± SD . |
|---|---|---|
| F1 agouti | 100 | 24 ± 5 (n = 5)* |
| N6F1 | 3 | 39 ± 10 (n = 18)* |
| N10 or greater | 0.2 or lower | 59 ± 5 (n = 6) |
| Inbred C57BL/6 | 0 | 56 ± 7 (n = 9) |
F1 agouti mice were generated by mating C57BL/6 females with chimeric males derived from 129/SvJ embryonic stem cells.12 The F1 agouti mice were then backcrossed with inbred C57BL/6 mice. The approximate percentage of loci containing an allele from both strains (heterozygous loci) is indicated.
Data are from He et al.12
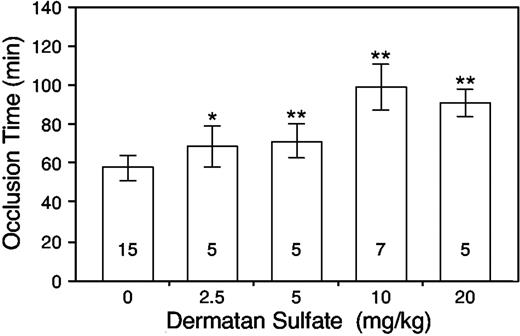
Initially, we injected HCII+/+ mice intravenously with 2.5 to 20 mg porcine skin dermatan sulfate per kilogram body weight 15 minutes prior to the injection of rose bengal. The occlusion time increased in a dose-dependent manner, reaching a maximum of approximately twice the baseline value at a dose of 10 mg/kg (Figure 1). No further increase was observed at a dose of 20 mg/kg.
Porcine skin dermatan sulfate prolongs the carotid artery occlusion time of HCII+/+ mice. Dermatan sulfate was administered intravenously at the doses indicated. Then, 15 minutes later, vascular injury was initiated by injection of rose bengal and the time to thrombotic occlusion was determined. Error bars represent the mean ± SD. The number within each column indicates the number of mice studied. *P = .012 versus control (no dermatan sulfate); **P ≤ .001 versus control.
Porcine skin dermatan sulfate prolongs the carotid artery occlusion time of HCII+/+ mice. Dermatan sulfate was administered intravenously at the doses indicated. Then, 15 minutes later, vascular injury was initiated by injection of rose bengal and the time to thrombotic occlusion was determined. Error bars represent the mean ± SD. The number within each column indicates the number of mice studied. *P = .012 versus control (no dermatan sulfate); **P ≤ .001 versus control.
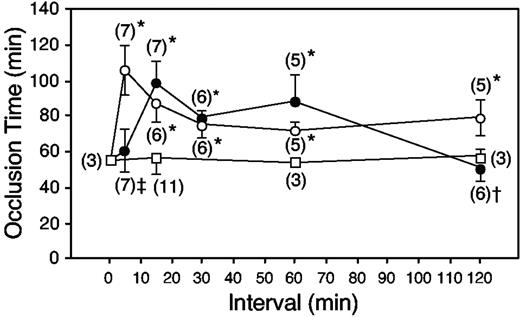
To investigate the time of onset and the duration of antithrombotic activity, we injected porcine skin dermatan sulfate 5 to 120 minutes prior to the injection of rose bengal. At a dermatan sulfate dose of 10 mg/kg, no prolongation of the occlusion time was observed when the interval between injections was only 5 minutes (Figure 2, solid circles). The occlusion time was prolonged when the interval between injections was 15 to 60 minutes but returned to baseline when injury was induced 120 minutes after injection of 10 mg/kg dermatan sulfate. In contrast, at a dose of 20 mg/kg, antithrombotic activity was observed at the 5-minute time point and persisted for the duration of the experiment (Figure 2, open circles). The occlusion time was not prolonged in control animals injected with 0.15 M NaCl 15 to 120 minutes prior to rose bengal (Figure 2, open squares).
Onset and duration of antithrombotic activity. HCII+/+ mice were given porcine skin dermatan sulfate at a dose of 10 mg/kg (•) or 20 mg/kg (○), or an equal volume of 0.15 M NaCl (□), by intravenous injection at time = 0 minute. At specified intervals thereafter, vascular injury was initiated by injection of rose bengal, and the time to thrombotic occlusion was determined. Error bars represent the mean ± SD. Numbers in parentheses indicate the number of mice studied. P values indicate statistical significance relative to mice that did not receive dermatan sulfate: *P < .0001; †P = .068; and ‡P = .28.
Onset and duration of antithrombotic activity. HCII+/+ mice were given porcine skin dermatan sulfate at a dose of 10 mg/kg (•) or 20 mg/kg (○), or an equal volume of 0.15 M NaCl (□), by intravenous injection at time = 0 minute. At specified intervals thereafter, vascular injury was initiated by injection of rose bengal, and the time to thrombotic occlusion was determined. Error bars represent the mean ± SD. Numbers in parentheses indicate the number of mice studied. P values indicate statistical significance relative to mice that did not receive dermatan sulfate: *P < .0001; †P = .068; and ‡P = .28.
Pharmacokinetics of porcine skin dermatan sulfate in HCII+/+ mice
We considered several possible explanations for the delayed onset of antithrombotic activity observed at the lower dose (ie, 10 mg/kg) of dermatan sulfate: (1) dermatan sulfate injected into the tail vein could be sequestered temporarily by saturable binding sites in the pulmonary vascular bed; (2) it could be metabolized to a more active form; or (3) a portion of the dermatan sulfate could transfer from the plasma into the arterial wall in a time- and concentration-dependent manner. To investigate these possibilities, we determined the kinetics of clearance of 3H-labeled dermatan sulfate.
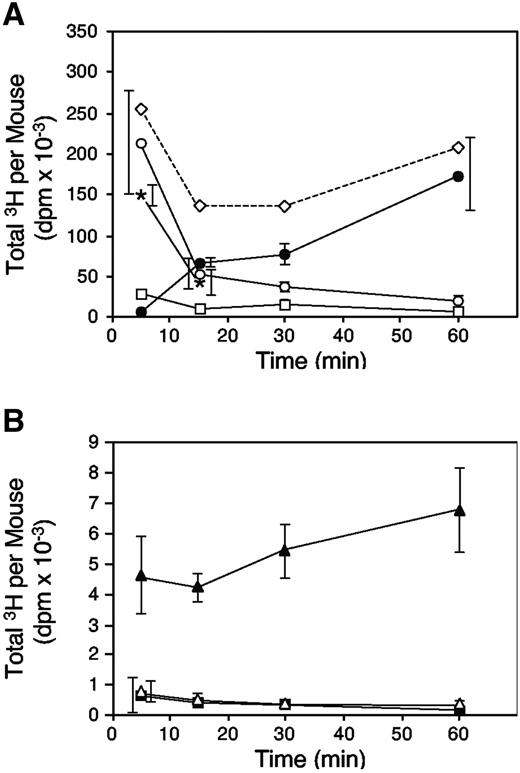
As shown in Figure 3A, 67% of the 3H-labeled tracer was detectable in the arterial circulation 5 minutes after injection of a dose of 10 mg/kg dermatan sulfate. The amount present in plasma decreased to 17% at 15 minutes and 7% at 60 minutes. Thus, the antithrombotic activity increased as the concentration of dermatan sulfate in plasma at the onset of endothelial injury was falling (compare Figure 2, solid circles with Figure 3A, open circles). This observation argues against temporary sequestration of dermatan sulfate in the pulmonary vasculature. An alternative explanation for the absence of an antithrombotic effect when dermatan sulfate was given 5 minutes, but not 15 minutes, before rose bengal is that rose bengal retarded the delivery of dermatan sulfate to its target(s) in vivo. To test this hypothesis, we administered rose bengal simultaneously with 3H-labeled dermatan sulfate and measured clearance of the tracer from plasma (Figure 3A, asterisks). At 5 minutes after injection, the amount of 3H-labeled dermatan sulfate in plasma was 214 000 ± 64 000 dpm without rose bengal and 149 000 ± 13 000 dpm with rose bengal (P = .16). After 15 minutes, the amounts were 54 000 ± 19 000 dpm without rose bengal and 42 000 ± 16 000 dpm with rose bengal (P = .45). Thus, rose bengal did not significantly alter the pharmacokinetics of dermatan sulfate.
Pharmacokinetics of porcine skin dermatan sulfate in HCII+/+ mice. Mice were injected intravenously with 320 000 dpm 35H-labeled dermatan sulfate at time = 0 minute and were killed at the times indicated. Total radioactivity present in plasma and urine was determined. The mice were then perfused with 50 mL PBS, and total radioactivity present in the kidneys, heart, lungs, and liver was determined. In a separate experiment, plasma was collected from mice simultaneously injected with 35H-labeled dermatan sulfate and rose bengal. The data represent the mean ± SD of 3 animals studied at each time point (overlapping error bars are offset horizontally). (A) Plasma (○); urine (•); kidneys (□); and plasma (*) from mice injected with rose bengal. (B) Heart (▪), lungs (▵), and liver (▴). The dashed line in panel A indicates the sum of the mean radioactivity recovered at each time point.
Pharmacokinetics of porcine skin dermatan sulfate in HCII+/+ mice. Mice were injected intravenously with 320 000 dpm 35H-labeled dermatan sulfate at time = 0 minute and were killed at the times indicated. Total radioactivity present in plasma and urine was determined. The mice were then perfused with 50 mL PBS, and total radioactivity present in the kidneys, heart, lungs, and liver was determined. In a separate experiment, plasma was collected from mice simultaneously injected with 35H-labeled dermatan sulfate and rose bengal. The data represent the mean ± SD of 3 animals studied at each time point (overlapping error bars are offset horizontally). (A) Plasma (○); urine (•); kidneys (□); and plasma (*) from mice injected with rose bengal. (B) Heart (▪), lungs (▵), and liver (▴). The dashed line in panel A indicates the sum of the mean radioactivity recovered at each time point.
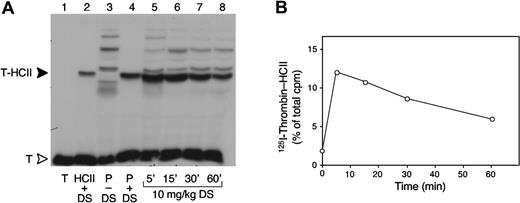
To determine the activity of the circulating dermatan sulfate, plasma samples were obtained from mice at various times after injection of a 10-mg/kg dose and were incubated with 125I-thrombin in vitro. As indicated by formation of 125I-thrombin-HCII complexes, the activity of dermatan sulfate was maximal 5 minutes after injection and decreased thereafter (Figure 4). This result suggests that dermatan sulfate was not metabolized to a more active form in the circulation. Furthermore, gel filtration and ion-exchange chromatography of plasma samples obtained 5 and 30 minutes after injection showed no alteration in the size or charge of the 3H-labeled dermatan sulfate (data not shown).
Formation of 125I-thrombin-HCII complexes ex vivo. (A) Plasma was obtained from HCII+/+ mice 5 to 60 minutes after intravenous injection of 10 mg/kg porcine skin dermatan sulfate (DS) (lanes 5-8). The plasma samples were incubated for 2 minutes with 125I-thrombin (T), and formation of 125I-thrombin-HCII complexes (T-HCII) was assayed by SDS-PAGE and autoradiography. A sample containing only 125I-thrombin is shown in lane 1. The thrombin-HCII complex was identified by incubating 125I-thrombin with purified mouse HCII (5 μM) (lane 2) or with mouse plasma (P) (lane 4) in the presence of 50 μg/mL dermatan sulfate. 125I-thrombin was also incubated with mouse plasma in the absence of dermatan sulfate (lane 3). Radioactive bands above T-HCII probably represent complexes of thrombin with α2-macroglobulin; bands below T-HCII probably represent thrombin-antithrombin or partially degraded complexes. (B) 125I-thrombin-HCII bands from the gel in panel A were cut out, and the radioactivity was measured in a gamma counter.
Formation of 125I-thrombin-HCII complexes ex vivo. (A) Plasma was obtained from HCII+/+ mice 5 to 60 minutes after intravenous injection of 10 mg/kg porcine skin dermatan sulfate (DS) (lanes 5-8). The plasma samples were incubated for 2 minutes with 125I-thrombin (T), and formation of 125I-thrombin-HCII complexes (T-HCII) was assayed by SDS-PAGE and autoradiography. A sample containing only 125I-thrombin is shown in lane 1. The thrombin-HCII complex was identified by incubating 125I-thrombin with purified mouse HCII (5 μM) (lane 2) or with mouse plasma (P) (lane 4) in the presence of 50 μg/mL dermatan sulfate. 125I-thrombin was also incubated with mouse plasma in the absence of dermatan sulfate (lane 3). Radioactive bands above T-HCII probably represent complexes of thrombin with α2-macroglobulin; bands below T-HCII probably represent thrombin-antithrombin or partially degraded complexes. (B) 125I-thrombin-HCII bands from the gel in panel A were cut out, and the radioactivity was measured in a gamma counter.
Approximately 55% of the injected dose of 3H-labeled dermatan sulfate was present in the urine after 60 minutes (Figure 3A). Despite excretion of more than half of the dermatan sulfate, antithrombotic activity was maintained when endothelial injury was initiated at this time point (compare Figure 2). Small but measurable amounts of tritium remained in the heart, lung, liver, and kidney at all time points after injection of 3H-labeled dermatan sulfate, even after arterial perfusion of the animal with 50 mL phosphate-buffered saline (Figure 3A-B). The amount present in heart and lung did not decrease with time in proportion to the plasma radioactivity, and the amount present in liver appeared to increase, indicating that some of the 3H-labeled dermatan sulfate remained in these tissues after clearance of most of the circulating material.
Antithrombotic effects of glycosaminoglycans in HCII-/- mice
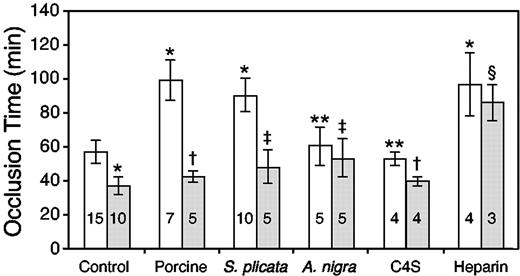
We previously reported that the time to thrombotic occlusion of the carotid artery was significantly shorter in HCII-/- mice than in HCII+/+ mice at both the F1 and N6F1 generation.12 Similarly, HCII-/- mice at the N10F1 generation, which are essentially congenic with inbred C57BL/6 animals, had significantly shorter occlusion times than their HCII+/+ counterparts (37 ± 5 minutes vs 57 ± 7 minutes, P < .0001; Figure 5). Porcine skin dermatan sulfate (10 mg/kg) did not prolong the occlusion time of HCII-/- mice (P = .08; Figure 5). To confirm that prolongation of the occlusion time was HCII dependent, we administered purified HCII intravenously to HCII-/- mice to achieve a plasma concentration of approximately 0.3 μM HCII 15 minutes prior to the injection of dermatan sulfate. Reconstitution of plasma HCII resulted in prolongation of the occlusion time (88 ± 7 minutes, n = 4) to the same degree as that observed in HCII+/+ mice (P = .12). In these and all subsequent experiments, the glycosaminoglycan was administered 15 minutes prior to injection of rose bengal. Heparin prolonged the occlusion time of HCII+/+ mice in a dose-dependent manner (data not shown), reaching a maximum value of 97 ± 18 minutes at a dose of 0.125 mg/kg, and prolonged the occlusion time of HCII-/- mice to a similar degree (P = .4; Figure 5). Thus, HCII is required for the antithrombotic activity of porcine skin dermatan sulfate but plays no role in the antithrombotic activity of heparin in this experimental model.
Antithrombotic effects of glycosaminoglycans. Porcine skin, S plicata and A nigra dermatan sulfate, and chondroitin 4-sulfate (C4S) were administered intravenously at a dose of 10 mg/kg to HCII+/+ (□) or HCII-/- (▦) mice. Porcine intestinal heparin was administered at a dose of 0.125 mg/kg. The glycosaminoglycan or an equal volume of 0.15 M NaCl (control) was given 15 minutes prior to rose bengal. The number within each column indicates the number of mice studied. *P < .0001 versus HCII+/+ control; **P > .2 versus HCII+/+ control; †P ≥ .08 versus HCII-/- control; ‡P < .02 versus HCII-/- control; and §P = .4 versus HCII+/+ heparin.
Antithrombotic effects of glycosaminoglycans. Porcine skin, S plicata and A nigra dermatan sulfate, and chondroitin 4-sulfate (C4S) were administered intravenously at a dose of 10 mg/kg to HCII+/+ (□) or HCII-/- (▦) mice. Porcine intestinal heparin was administered at a dose of 0.125 mg/kg. The glycosaminoglycan or an equal volume of 0.15 M NaCl (control) was given 15 minutes prior to rose bengal. The number within each column indicates the number of mice studied. *P < .0001 versus HCII+/+ control; **P > .2 versus HCII+/+ control; †P ≥ .08 versus HCII-/- control; ‡P < .02 versus HCII-/- control; and §P = .4 versus HCII+/+ heparin.
We also determined the antithrombotic activities of dermatan sulfates isolated from 2 marine invertebrates, Styela plicata and Ascidia nigra. At a dose of 10 mg/kg, S plicata dermatan sulfate prolonged the occlusion time of HCII+/+ mice to the same degree as did porcine skin dermatan sulfate, while A nigra dermatan sulfate was much less effective (Figure 5). The antithrombotic activity of S plicata dermatan sulfate was largely dependent on the presence of HCII, although the occlusion time of HCII-/- mice treated with S plicata dermatan sulfate was slightly longer than that of untreated HCII-/- mice (48 ± 10 minutes vs 37 ± 5 minutes, P = .016). Similarly, the occlusion time of HCII-/- mice treated with A nigra dermatan sulfate was slightly longer than that of untreated HCII-/- mice (53 ± 11 minutes vs 37 ± 5 minutes, P = .002). Increasing the dose of S plicata or A nigra dermatan sulfate to 20 mg/kg did not significantly prolong the occlusion time above that of the 10-mg/kg dose in HCII-/- mice (57 ± 4 minutes vs 48 ± 10 minutes, P = .16, for S plicata; and 62 ± 5 minutes vs 48 ± 10 minutes, P = .20, for A nigra). Thus, both of the invertebrate dermatan sulfates had weak, HCII-independent antithrombotic activity in this experimental model. Chondroitin 4-sulfate did not prolong the occlusion time of HCII+/+ or HCII-/- mice.
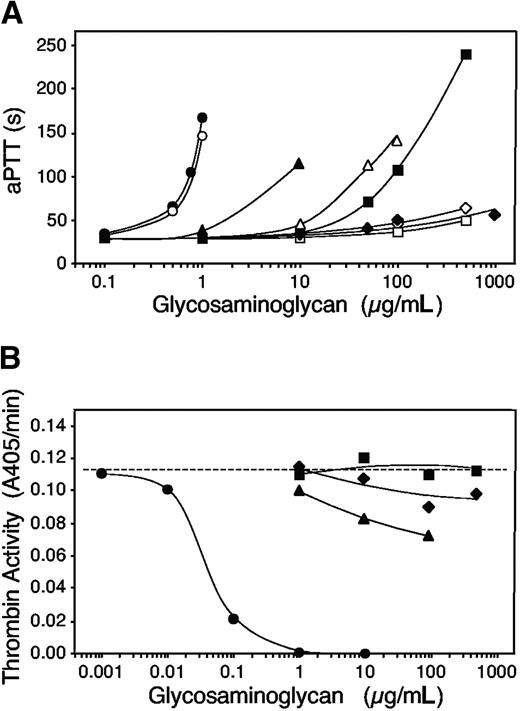
The in vitro anticoagulant activities of the glycosaminoglycans in mouse plasma are shown in Figure 6A. S plicata dermatan sulfate, porcine skin dermatan sulfate, and heparin prolonged the aPTT of plasma obtained from HCII+/+ mice, but A nigra dermatan sulfate did not (Figure 6A, solid symbols). In agreement with previous results obtained with human plasma, S plicata dermatan sulfate was about 10 times more potent than porcine skin dermatan sulfate and 10 times less potent than heparin.14 Neither A nigra nor porcine skin dermatan sulfate prolonged the aPTT of HCII-/- mouse plasma, but S plicata dermatan sulfate was active when present at about 10 times the concentration needed to prolong the aPTT of HCII+/+ plasma (Figure 6A, open symbols). Heparin was equally effective in plasma from both HCII+/+ and HCII-/- mice.
In vitro anticoagulant assays. (A) aPTT assays of HCII+/+ (solid symbols) or HCII-/- (open symbols) mouse plasma were performed in the presence of glycosaminoglycans at the final concentrations indicated. Circles indicate porcine intestinal heparin; squares, porcine skin dermatan sulfate; triangles, S plicata dermatan sulfate; and diamonds, A nigra dermatan sulfate. (B) Purified mouse thrombin (16 nM) and mouse antithrombin (50 nM) were incubated in the presence of glycosaminoglycans for 60 seconds, and the remaining thrombin activity was determined by hydrolysis of a chromogenic substrate. The dashed line indicates the activity of thrombin in buffer alone. Symbols are as defined in panel A.
In vitro anticoagulant assays. (A) aPTT assays of HCII+/+ (solid symbols) or HCII-/- (open symbols) mouse plasma were performed in the presence of glycosaminoglycans at the final concentrations indicated. Circles indicate porcine intestinal heparin; squares, porcine skin dermatan sulfate; triangles, S plicata dermatan sulfate; and diamonds, A nigra dermatan sulfate. (B) Purified mouse thrombin (16 nM) and mouse antithrombin (50 nM) were incubated in the presence of glycosaminoglycans for 60 seconds, and the remaining thrombin activity was determined by hydrolysis of a chromogenic substrate. The dashed line indicates the activity of thrombin in buffer alone. Symbols are as defined in panel A.
Figure 6B shows the ability of each glycosaminoglycan to stimulate inhibition of mouse thrombin by mouse antithrombin in vitro. Heparin was active at final concentrations of 0.1 μg/mL or higher, while porcine skin dermatan sulfate was completely inactive at the highest concentration tested (500 μg/mL). S plicata dermatan sulfate had demonstrable activity at 10 to 100 μg/mL, but inhibition of thrombin was incomplete under the conditions of the assay. A nigra dermatan sulfate had barely detectable activity at concentrations of 100 μg/mL or higher.
Discussion
Intravenous administration of porcine skin dermatan sulfate to HCII+/+ mice doubled the time to complete thrombotic occlusion of the carotid artery after endothelial injury but did not prolong the occlusion time of HCII-/- mice (Figure 5). These results provide the first direct evidence that HCII is required for antithrombotic activity of dermatan sulfate in vivo and suggest that other potential mechanisms of action of dermatan sulfate, such as release of tissue plasminogen activator,10,11 are less important. Heparin inhibited thrombosis equally well in HCII+/+ and HCII-/- mice, presumably by activation of plasma antithrombin. In these experiments, endothelial injury was induced by singlet oxygen generated from rose bengal dye (3′,4′,5′,6′-tetrachloro-2,4,5,7-tetraiodofluorescein) in the bloodstream when exposed to green light transmitted through the vessel. Electron microscopy has shown that this procedure causes rapid detachment of the arterial endothelium, leaving the elastic lamina and underlying smooth muscle cells intact, followed by platelet and fibrin deposition.19 Although it is likely that HCII also mediates the antithrombotic effect of dermatan sulfate in models of stasis-induced venous thrombosis, our experiments did not address this issue.
The lowest dose of porcine skin dermatan sulfate that caused maximal prolongation of the thrombotic occlusion time in HCII+/+ mice was 10 mg/kg (Figure 1). Theoretically, a 10-mg/kg bolus of dermatan sulfate will produce an initial plasma concentration of 317 μg/mL, assuming a plasma volume of 31.5 mL/kg body weight. This concentration of porcine skin dermatan sulfate was sufficient to prolong the aPTT of plasma from HCII+/+ mice but did not prolong the aPTT of plasma from HCII-/- mice (Figure 6). In contrast, a much lower dose of heparin (0.125 mg/kg or 23 U/kg), corresponding to an initial plasma concentration of 4 μg/mL (0.7 U/mL), prolonged the occlusion time of both HCII+/+ and HCII-/- mice to the same degree. In vitro, heparin prolonged the aPTT of plasma from both HCII+/+ and HCII-/- mice (Figure 6).
Dermatan sulfate is synthesized as a repeating polymer of d-glucuronic acid and N-acetyl-d-galactosamine (GalNAc), which is then modified by epimerization of d-glucuronic acid to l-iduronic acid (IdoA), sulfation of the 2-OH group of IdoA, and sulfation of the 4- and/or 6-OH groups of GalNAc.20 Incomplete epimerization and O-sulfation lead to microheterogeneity of the dermatan sulfate polymers. The smallest fragment of porcine skin dermatan sulfate that bound to HCII was a hexasaccharide composed of 3 Ido 2-sulfate-GalNAc 4-sulfate disaccharides.13 The disaccharide subunit of the HCII-binding hexasaccharide is a minor component of porcine skin dermatan sulfate, comprising only 5% of the total disaccharides. Although this hexasaccharide increased the rate of thrombin inhibition approximately 100-fold, dermatan sulfate chains 12 to 14 residues in length were necessary for maximal stimulation of HCII (∼ 10 000-fold).21,22 In the current study, we used unfractionated porcine skin dermatan sulfate in which the majority of chains contain more than 14 residues.
We obtained information about the structural features of dermatan sulfate required for antithrombotic activity by comparing mammalian and invertebrate preparations of known composition. The dermatan sulfates isolated from S plicata and A nigra both have a higher content of IdoA 2-sulfate residues (66%-80%) than do typical mammalian dermatan sulfates (5%-7%), and they differ in the pattern of sulfation of GalNAc.14 The GalNAc residues of S plicata dermatan sulfate are 94% 4-sulfated and 6% 6-sulfated, whereas those of A nigra are 100% 6-sulfated. Despite comparable overall degrees of sulfation, A nigra dermatan sulfate stimulated thrombin inhibition by purified HCII only at very high concentrations, whereas S plicata dermatan sulfate was 1000 times more active.14 This observation confirmed the importance of GalNAc 4-sulfate residues for stimulation of HCII. However, the presence of GalNAc 4-sulfate was not sufficient for stimulation of HCII, because the repeating polymer of glucuronic acid and GalNAc 4-sulfate (ie, chondroitin 4-sulfate) was inactive.1 In comparison with mammalian dermatan sulfate, S plicata dermatan sulfate contains approximately 10 times more IdoA 2-sulfate and was 10 times more active with purified HCII. Thus, there is a good correlation between the abundance of residues found in the HCII-binding hexasaccharide (IdoA 2-sulfate and GalNAc 4-sulfate) and the ability of dermatan sulfate both to stimulate HCII in vitro1,5,14 and to produce an antithrombotic effect in vivo (Figure 5).
Although the anticoagulant activity of S plicata dermatan sulfate in vitro was approximately 10 times greater than that of porcine skin dermatan sulfate (Figure 6),14 a 1-mg/kg dose of S plicata dermatan sulfate did not prolong the occlusion time of HCII+/+ mice (data not shown). Similarly, Vicente et al found that S plicata dermatan sulfate was less effective than mammalian dermatan sulfate in a stasis-induced thrombosis model in rats.5 These results suggest that there may be important differences in the pharmacokinetics and/or pharmacodynamics of porcine skin and S plicata dermatan sulfate. Moreover, S plicata dermatan sulfate had substantial activity in the aPTT assay with plasma from HCII-/- mice and caused a small but significant prolongation of the thrombotic occlusion time in HCII-/- mice. A nigra dermatan sulfate also prolonged the occlusion time slightly in HCII-/- mice but had no activity in the aPTT assay. Although neither S plicata nor A nigra dermatan sulfate stimulated inhibition of factor Xa or thrombin by human antithrombin,14 we found that both of the invertebrate dermatan sulfates weakly stimulated inhibition of mouse thrombin by mouse antithrombin (Figure 6B). Therefore, stimulation of the thrombin-antithrombin reaction could contribute to the weak antithrombotic activity of these glycosaminoglycans in HCII-/- mice.
The pharmacokinetic behavior of dermatan sulfate in HCII+/+ mice is complex and remains incompletely understood. We observed that the antithrombotic activity of dermatan sulfate did not correspond to the amount or activity of the glycosaminoglycan present in plasma (Figures 2, 3, 4) at the onset of endothelial injury. The amount of 3H-labeled dermatan sulfate recovered in plasma and urine during the interval from 15 to 60 minutes was substantially less (40%-60%) than the total amount injected (Figure 3). Examination of saline-perfused heart, lung, and liver indicated that a small percentage of the administered dermatan sulfate (∼ 4%) was detectable in these organs 60 minutes after injection. Presumably, the remainder of the dermatan sulfate was sequestered in other tissues. Similar experiments demonstrating rapid plasma clearance, urinary excretion, and deposition of radiolabeled dermatan sulfate in tissues have been performed in rabbits and rats.23,24 These observations suggest that porcine skin dermatan sulfate expresses antithrombotic activity after being transferred from the plasma to sites in the vessel wall that remain to be identified. The relatively low specific radioactivity that we were able to achieve by tritium labeling of dermatan sulfate (∼10 000 dpm per microgram) precluded detection in an isolated arterial segment, whose mass is miniscule in comparison with that of the heart, lung, or liver.
In conclusion, we have demonstrated that porcine skin dermatan sulfate administered intravenously to mice inhibits thrombosis of the carotid artery after endothelial injury and that the antithrombotic effect requires HCII. By contrast, the antithrombotic effect of S plicata dermatan sulfate depends mainly, but not entirely, on the presence of HCII; the weak HCII-independent activity of S plicata and A nigra dermatan sulfate in the thrombosis model may be related to their much greater charge density in comparison with porcine skin dermatan sulfate. It remains to be determined whether dermatan sulfate molecules present endogenously in the vessel wall also stimulate HCII to inhibit thrombus formation.
Prepublished online as Blood First Edition Paper, August 17, 2004; DOI 10.1182/blood-2004-02-0598.
Supported by grants from the National Heart, Lung and Blood Institute (R01 HL55520), the Edward Mallinckrodt Jr Foundation, the American Heart Association, and the National Institutes of Health Fogarty International Center (R03 TW05775).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal