Abstract
The Lyn tyrosine kinase plays essential inhibitory signaling roles within hematopoietic cells by recruiting inhibitory phosphatases such as SH2-domain containing phosphatase-1 (SHP-1), SHP-2, and SH2-domain containing 5′-inositol phosphatase (SHIP-1) to the plasma membrane in response to specific stimuli. Lyn-deficient mice display a collection of hematopoietic defects, including autoimmune disease as a result of autoantibody production, and perturbations in myelopoiesis that ultimately lead to splenomegaly and myeloid neoplasia. In this study, we demonstrate that loss of Lyn results in a stem/progenitor cell-intrinsic defect leading to an age-dependent increase in myeloid, erythroid, and primitive hematopoietic progenitor numbers that is independent of autoimmune disease. Despite possessing increased numbers of erythroid progenitors, and a more robust expansion of these cells following phenylhydrazine challenge, Lyn-deficient mice are more severely affected by the chemotherapeutic drug 5-fluorouracil, revealing a greater proportion of cycling progenitors. We also show that mice lacking SHIP-1 have defects in the erythroid and myeloid compartments similar to those in mice lacking Lyn or SHP-1, suggesting an intimate relationship between Lyn, SHP-1, and SHIP-1 in regulating hematopoiesis. (Blood. 2004;104:3901-3910)
Introduction
The production and lineage commitment of hematopoietic cells is governed by the actions of a multitude of cytokines, hormones, and growth factors that bind to cell surface receptors activating signal transduction cascades that ultimately regulate the expression of genes that control cell fate and effector function.1 Signal propagation in these cells is actively counterbalanced by several families of inhibitory gene products including protein tyrosine phosphatases,2 phosphatidyl-inositol phosphatases,3 the suppressors of cytokine signaling (SOCS) proteins,4 and receptors bearing immunoreceptor tyrosine-based inhibitory motifs (ITIMs).5 The central role played by tyrosine phosphorylation is exemplified by mutations in particular genes that lead to deregulation of hematopoiesis. For example, mutational activation of either the Abl6,7 or Janus tyrosine kinases8-10 leads to leukemogenesis. Loss of appropriate negative regulation of signaling may also have catastrophic consequences. For example, loss-of-function mutations within the inhibitory phosphatase Src homology 2 (SH2)-containing phosphatase-1 (SHP-1)11-13 in motheaten and motheaten viable mice (Mev), or disruption of the murine SH2-domain containing 5′-inositol phosphatase (SHIP-1)14,15 gene, lead to severe perturbations in hematopoiesis with myeloid cell consolidation of the lungs of deficient mice leading to premature death.14-16 Thus, the appropriate balance of positive and negative elements of signal transduction is essential for maintaining normal hematopoietic cell self-renewal, differentiation, and immune cell function.
Although clearly involved in initiating tyrosine-phosphorylation cascades following hematopoietic cell stimulation,17 Lyn has emerged as a critical enzyme responsible for establishing signaling thresholds in B cells,18-21 myelomonocytic cells,22,23 and mast cells.24-28 Indeed, loss of Lyn kinase leads to defects in activation of inhibitory phosphatases that likely underlies the hypersensitivity of deficient cells to immunoreceptor and cytokine stimulation.20,22,29,30 In the case of B cells and mast cells, Lyn deficiency is associated with impaired activation of spleen tyrosine kinase (Syk), but also with enhanced immunoreceptor-dependent activation of AKT and mitogen-activated protein (MAP) kinases, as well as heightened growth factor or immunoreceptor-dependent proliferation.18,19,24-27,31 In macrophages, Lyn deficiency leads to enhanced sensitivity to granulocyte-macrophage colony-stimulating factor (GM-CSF) and macrophage-CSF (M-CSF), diminished phosphorylation of SHIP-1 and SHP-1, enhanced AKT activity, and improved survival in cytokine-reduced conditions.22,23 Similarly, loss of either SHIP-1 or SHP-1 leads to enhanced hypersensitivity to growth factors and resistance to cytokine withdrawal-induced cell death.14,15,32,33
We have previously shown that Lyn-/- mice exhibit hematopoietic system defects that lead to an age-dependent increase in extramedullary myelopoiesis and widely disseminated myeloid neoplasia. In this study, we have characterized the temporal development of perturbed hematopoiesis, identified an increase in erythroid progenitor numbers in these mice, and conducted a comparative analysis of the Lyn-/- phenotype with both Mev/Mev and SHIP-1-/- mice. We show that Lyn-/- mice develop a similar, although much less severe, defect in hematopoiesis than Mev/Mev and SHIP-1-/- mice, which is characterized by dramatically increased splenic progenitors and progeny of the myeloid and erythroid lineages. Furthermore, we show that these characteristics of Lyn-deficient mice are transplantable with bone marrow (BM) and independent of B cells and autoimmune disease.
Materials and methods
Mice
Lyn-/-34 (mixed 129Ola × C57BL/6 intercross or C57BL/6 generation 20 background), SHIP-1-/-15 (mixed 129 × C57BL/6 intercross), Mev/Mev (C57BL/6), μMT/μMT35 (C57BL/6), and Lyn-/-;μMT/μMT (mixed 129Ola × C57BL/6) mice were housed in microisolation units. Animal experimentation was performed in accordance with National Health and Medical Research Council of Australia (NHMRC) guidelines.
Progenitor and colony-forming unit-spleen 12 (CFU-S12) assays
Myeloid colony-forming numbers were determined in semisolid agar.22 Erythroid progenitors assays were conducted in methylcellulose (Methocult; StemCell Technologies, Vancouver, BC) in 4 units/mL erythropoietin (EPO, Eprex 1000; Janssen-Cilag, North Ryde, Australia) alone (colony-forming unit-erythroid [CFU-e]) or EPO, stem cell factor (SCF, 50 ng/mL), and interleukin-3 (IL-3, 10 ng/mL) for burst-forming unit-erythroid (BFU-e). Colonies were scored on day 2 (CFU-e's) or day 10 (BFU-e's) following staining with benzidine dihydrochloride (Sigma, St Louis, MO). Day-12 colony-forming unit-spleen assays were performed by intravenously injecting 75 000 BM or 375 000 spleen cells from matched C57BL/6 Lyn+/+ and Lyn-/- mice into lethally irradiated C57BL/6 recipients (11 Gy). Donor samples were pooled from 3 mice/genotype and injected into 6 recipients. At 12 days after injection, spleens were fixed in Bouin solution, and macroscopic colonies enumerated.
Treatment with 5-fluorouracil and phenylhydrazine
Matched Lyn+/+ and Lyn-/- C57BL/6 background mice (5 mice/time point) were intravenously injected with 150 mg/kg 5-fluorouracil (5-FU). Blood was analyzed on an ADVIA 120 hematology system (Bayer Australia, Pymble, Australia).22 For phenylhydrazine challenge, mice were intraperitoneally injected on days 1 and 2 with 60 mg/kg per mouse of phenylhydrazine hydrochloride in phosphate-buffered saline (PBS). Reticulocyte numbers (1000 cells/mouse) were determined by staining with New methylene blue (Sigma).
Transplantation experiments
B6.SJL-Ptprca (Ly5.1) congenic mice were intravenously injected with 106 BM cells from Ly5.2 C57BL/6 background Lyn+/+ or Lyn-/- donors following lethal irradiation with 11 Gy of gamma irradiation given in 2 doses 3 hours apart. BM from 3 donor mice was pooled before injection, and 4 to 6 recipients were injected with each donor population/experiment. Irradiated recipient mice were maintained on enrofloxacin (170 mg/L) for 2 weeks. Assessment of engraftment was achieved by fluorescence-activated cell-sorter (FACS) analysis of peripheral blood, BM, and spleen preparations 10 to 12 weeks after transplantation using the Ly5.1/5.2 markers CD45.1/CD45.2, respectively.
Flow cytometry and ELISA assays
Single-cell suspensions were stained with Ter119 (Ly-76), CD71 (C2), CD45.1 (A20), CD45.2 (104), Mac-1 (M1/70), Gr-1 (Rb6-8C5), or isotype control rat immunoglobulin G2bκ (IgG2bκ, A95-1).22,36 Assessment of bromodeoxyuridine (BrdU) incorporation into cycling cells was determined according to the manufacturer's instructions (BD Pharmingen, San Diego, CA). Annexin V-fluorescein isothiocyanate (FITC) staining of fetal liver and BM erythroid cells37 and measurement of total immunoglobulin34,36 were carried out as described. Antinuclear antibody (ANA) enzyme-linked immunosorbent assays (ELISAs) were conducted using a commercially available human ANA detection kit (Binding Site, Birmingham, England) adapted to mouse ANA detection.
Purification of CD71+ cells
Spleens were harvested from phenylhydrazine-treated mice at day 5 or 6 after injection, homogenized, and then incubated in ice-cold Tris (tris(hydroxymethyl)aminomethane)-buffered NH4Cl at 37°C to lyse red blood cells (RBCs). Cell suspensions were then passed through a 40-μm nylon cell strainer after washing in PBS, 0.5% fetal calf serum (FCS), 2 mM EDTA (ethylenediaminetetraacetic acid) (magnetic-activated cell separation [MACS] buffer). Cells were then labeled with 7.5 μL CD71-biotin (C2; BD Pharmingen) and purified using streptavidin microbeads and LS cell separation columns according to the manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of CD71+/Ter119+ cells was always more than 90% as judged by FACS analysis. Cell yields were routinely between 1.5 and 3 × 108 cells/spleen.
Cell stimulation and lysis
Cells (1-5 × 108) were resuspended in Tyrode solution (124 mM NaCl, 4 mM KCl, 0.64 mM NaH2PO4, 1.6 mM CaCl2, 1 mM MgCl2, 10 mM NaHCO3, 5.5 mM glucose, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 5 mM MES [2-(N-morpholino)ethanesulfonic acid], 0.1% gelatin; pH 7.65). Samples were preheated at 37°C for 10 minutes before stimulation. Cells were washed in ice-cold Tyrode solution before lysis.22 Antibodies (Abs) used included anti-PY (4G10; UBI, Lake Placid, NY), anti-EPOr (M-20; Santa Cruz Biotechnology, Santa Cruz, CA), Janus kinase 2 (Jak2, C-20; Santa Cruz Biotechnology), and signal transducer and activator of transcription 5 (STAT5, N-20; Santa Cruz Biotechnology). Phospho-STAT5 and phospho-extracellular signal-related kinase 1/2 (ERK1/2) Abs were from Cell Signaling Technology (Beverly, MA). Rabbit antibodies against SHIP-1 and Lyn were produced by immunizing rabbits with a glutathione-S-transferase (GST) fusion protein containing residues 953 to 1122 of murine SHIP-1 or the SH3/SH2 domains of Lyn (provided by P. Lock, Department of Surgery, University of Melbourne, Australia). Blots were processed as described elsewhere.22
Results
Age-dependent increase of myeloid progenitors in Lyn-/- mice
We have previously demonstrated that Lyn-/- mice develop splenomegaly characterized by a dramatic increase in hematopoietic progenitors responsive to cytokines such as GM-CSF, IL-3, M-CSF, and SCF. Moreover, Lyn-/- mice develop a myeloproliferative disease characterized by the accumulation of myelomonocytic cells widely disseminated throughout the animals.22
In order to understand the temporal progression of this phenotype, we investigated hematopoiesis in embryonic day-14 (E14) fetal liver and 4- and 16-week-old mice. This analysis revealed increased numbers of myeloid progenitors in the spleens of Lyn-/- mice at both 4 and 16 weeks of age (Figure 1A-B), although, the magnitude of the increase in GM-CSF- and M-CSF-responsive progenitors was enhanced in older mice (GM-CSF/M-CSF progenitors were 13- and 35-fold higher, respectively, in 16-week-old Lyn-/- mice compared with Lyn+/+ mice, and 4- and 6-fold higher, respectively, in 4-week-old mice). This increase in splenic myeloid progenitors does not appear to be due to an impairment of BM myelopoiesis, as the number of myeloid progenitors in the BM of Lyn-/- mice was similar to that in wild-type mice at all ages (Figure 1A-B). While splenic myelopoiesis was enhanced in 4- and 16-week-old mice, analysis of E14 fetal liver revealed no difference in myeloid progenitor numbers between Lyn+/+ and Lyn-/- mice (Figure 1C).
Normal fetal and bone marrow myelopoiesis but enhanced splenic myelopoiesis in 4- and 16-week-old Lyn-/- mice. Progenitors responsive to GM-CSF, IL-3, or M-CSF in the spleen and BM of (A) 16-week-old or (B) 4-week-old sex-matched Lyn+/+ and Lyn-/- mice were assessed by in vitro semisolid agar assays. Data presented in panels A-B correspond to the mean (± SEM) for 2 to 4 experiments using 2 to 3 mice per experiment. (C) Day-14 myeloid fetal liver progenitor populations in Lyn+/+ (n = 9), Lyn+/- (n = 17), or Lyn-/- (n = 8) mice were assessed in the presence of the indicated cytokines (mean ± SEM). Fetal livers were derived from day-14 embryos obtained from time-mated Lyn+/- mice. The splenic myeloid data presented in panel A (left panel) have been reported previously22 (reprinted from
Normal fetal and bone marrow myelopoiesis but enhanced splenic myelopoiesis in 4- and 16-week-old Lyn-/- mice. Progenitors responsive to GM-CSF, IL-3, or M-CSF in the spleen and BM of (A) 16-week-old or (B) 4-week-old sex-matched Lyn+/+ and Lyn-/- mice were assessed by in vitro semisolid agar assays. Data presented in panels A-B correspond to the mean (± SEM) for 2 to 4 experiments using 2 to 3 mice per experiment. (C) Day-14 myeloid fetal liver progenitor populations in Lyn+/+ (n = 9), Lyn+/- (n = 17), or Lyn-/- (n = 8) mice were assessed in the presence of the indicated cytokines (mean ± SEM). Fetal livers were derived from day-14 embryos obtained from time-mated Lyn+/- mice. The splenic myeloid data presented in panel A (left panel) have been reported previously22 (reprinted from
Increased numbers of immature erythroid cells and their progenitors in Lyn-/- mice
Analysis of histologic sections of spleens from Lyn-/- mice older than 12 weeks revealed an increase in the number of erythroid cells (not shown). These data, and reports showing increased percentages of Ter119 staining cells in the spleens of Lyn-/- mice,38 prompted us to investigate whether erythroid (RBC) development was also perturbed in Lyn-deficient mice. We used the transferrin receptor monoclonal antibody (mAb) CD71 and the erythroid lineage-restricted marker Ter119, as described in the characterization of STAT5A-/-5B-/- mice,39-41 to identify erythroblasts in Lyn-/- spleen. Staining with these Abs revealed a large increase in the proportion of CD71+Ter119+ erythroblasts in the spleens of Lyn-/- mice (Figure 2A). To investigate whether there was a corresponding increase in primitive erythroid progenitors in the spleens of Lyn-/- mice, we assessed the numbers of colony- and burst-forming unit erythroid progenitors (CFU-e's and BFU-e's, respectively).42,43 While there was a slight increase in CFU-e's in the spleens of 8-week-old Lyn-/- mice, a dramatic increase in CFU-e's became evident by 16 weeks of age (Figure 2B). In the BM, we observed an increase in the number of CFU-e progenitors in both 8- and 16-week-old mice (Figure 2C). By contrast, the numbers of BM primitive BFU-e progenitors were similar at both time points. Similarly, enumeration of BM and spleen mixed erythroid/myeloid colonies generated in a cytokine cocktail of EPO, IL-3, and SCF revealed no difference in the number of these progenitors in 8-week-old Lyn+/+ and Lyn-/- BM (Lyn+/+, 22.3 ± 3.5; Lyn-/-, 22.5 ± 2.3; n = 12 mice ± SEM/2 × 104 cells) or spleen (Lyn+/+, 2.3 ± 0.6; Lyn-/-, 3.1 ± 0.5; n = 12 mice ± SEM/105 cells). As was the case with fetal liver myeloid progenitors, no difference in either CFU-e's or BFU-e's was detected in E14 fetal livers (Figure 2D). Additionally, no significant difference in either CD71+/Ter119+ cell numbers or numbers of apoptotic CD71+ cells were observed in either fetal liver or adult BM (Figure 2E, and not shown). Thus, Lyn-/- mice develop a significant age-dependent increase in myeloid and erythroid progenitors with the expansion most apparent in relatively mature lineage-restricted progenitors.
Expansion of immature erythroid cells in Lyn-/- spleen and increased erythroid CFU-e numbers in BM and spleen of Lyn-/- mice. (A) A 3-color flow cytometric analysis of 8-week-old Lyn+/+ and Lyn-/- spleen populations stained with CD71 and Ter119. Propidium iodide (PI)-positive and mature RBCs were excluded from the analysis. Relative percentages (± SD) of immature erythroid (upper right quadrant) and more mature erythroid (lower right) cells of 3 mice of each genotype are indicated. Erythropoiesis in (B) spleen, (C) BM of 8- and 16-week-old Lyn+/+ and Lyn-/- mice, and (D) embryonic day-14 fetal liver was assessed by methylcellulose culture. Mature erythroid (CFU-e) and immature erythroid (BFU-e) progenitors were determined at day 2 and day 10, respectively. (E) Relative percentages (± SD) of annexin-FITC-positive erythroid cells in fetal liver (n = 7-8/genotype) and adult bone marrow (n = 5/genotype) are indicated.
Expansion of immature erythroid cells in Lyn-/- spleen and increased erythroid CFU-e numbers in BM and spleen of Lyn-/- mice. (A) A 3-color flow cytometric analysis of 8-week-old Lyn+/+ and Lyn-/- spleen populations stained with CD71 and Ter119. Propidium iodide (PI)-positive and mature RBCs were excluded from the analysis. Relative percentages (± SD) of immature erythroid (upper right quadrant) and more mature erythroid (lower right) cells of 3 mice of each genotype are indicated. Erythropoiesis in (B) spleen, (C) BM of 8- and 16-week-old Lyn+/+ and Lyn-/- mice, and (D) embryonic day-14 fetal liver was assessed by methylcellulose culture. Mature erythroid (CFU-e) and immature erythroid (BFU-e) progenitors were determined at day 2 and day 10, respectively. (E) Relative percentages (± SD) of annexin-FITC-positive erythroid cells in fetal liver (n = 7-8/genotype) and adult bone marrow (n = 5/genotype) are indicated.
Perturbed hematopoiesis is transplanted with Lyn-/- bone marrow
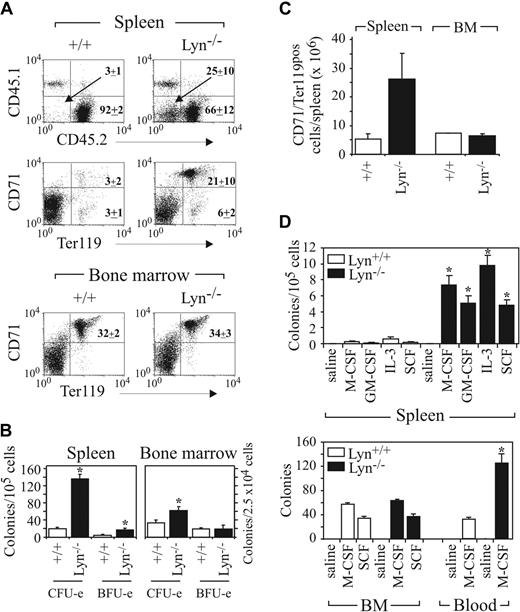
To investigate whether the hematopoietic compartment perturbation in Lyn-/- mice is intrinsic to the stem/progenitor cell, or due to microenvironmental differences, we conducted BM transplantations. Irradiated recipient (Ly5.1) mice received transplants of either Lyn+/+ or Lyn-/- (Ly5.2) BM. Mice were then analyzed at between 10 to 12 weeks after transplantation for donor cell repopulation and transplantability of perturbed hematopoiesis. Engraftment was assessed by staining peripheral blood, spleen, and BM with the Ly5.2-specific mAb CD45.2 (donor) and Ly5.1 mAb CD45.1 (recipient), which revealed effective reconstitution with donor BM (Figure 3A, and not shown). Analysis of peripheral blood revealed similar percentages of donor-derived CD45.2+ cells (Lyn+/+, 94.7 ± 2.4%; Lyn-/-, 89.3 ± 5.3%; n = 4). However, numbers of CD19+ B cells in the peripheral blood (Lyn+/+, 4.2 ± 0.5 × 103/μL; Lyn-/-, 0.6 ± 0.2 × 103/μL) and spleen (Lyn+/+, 13.0 ± 1.8 × 107; Lyn-/-, 2.7 × 107 ± 0.4 × 107; n = 6) were dramatically reduced following transplantation with Lyn-/- BM, as previously described.20 Interestingly, analysis of recipients of Lyn-/- BM revealed a significant population of cells in the spleen (25% ± 10%) that failed to stain with either CD45.1 or CD45.2 (Figure 3A). Further analysis of this CD45- population revealed that they were CD71+Ter119+ and lacked c-Kit expression, consistent with our previous analysis of the spleens of Lyn-/- donors (Figures 2A, 3A, and not shown). This population was increased approximately 5-fold in the spleens of Lyn-/- BM recipients compared with Lyn+/+ transplants. By contrast, the percentages and absolute numbers of CD71+Ter119+ cells in the BM of Lyn-/- and Lyn+/+ BM recipients were similar (Figure 3A,C). Analysis of erythroid and myeloid progenitors in spleen revealed that, like Lyn-/- donors, irradiated recipients of Lyn-/- BM displayed dramatically increased myeloid and erythroid progenitor numbers (Figure 3B,D). Although there was a modest increase in CFU-e's in the BM of Lyn-/- BM recipients, the number of myeloid progenitors and BFU-e's in the BM was similar (Figure 3B,D). Increased numbers of progenitors responsive to M-CSF in peripheral blood were also detected in the transplants, a phenotype we previously observed in 8-week-old Lyn-/- mice22 (Figure 3B,D). Collectively, these results reveal that the age-dependent defect observed in Lyn-/- donors is recapitulated in Lyn-/- BM recipients.
Perturbed myelo/erythropoiesis is transplantable with Lyn-/- BM. (A) BM from C57BL/6 Ly5.2 background donor Lyn+/+ or Lyn-/- mice was transplanted into irradiated C57BL/6 Ly5.1 mice. Engraftment was assessed by identifying donor and recipient cells by FACS analysis with CD45.2 (donor) and CD45.1 (recipient) mAbs. Alternatively, immature erythroid lineage cells in spleen and BM were assessed by staining with CD71 and Ter119 mAbs. Both PI-positive and mature RBCs were excluded from the analysis. The relative percentages of each population (± SD) in 4 recipient mice of each genotype are presented. (B) CFU-e and BFU-e content in the spleen and BM of recipients of either Lyn+/+ or Lyn-/- BM are shown. (C) The relative number of CD71/Ter119 double-positive erythroid cells in spleen and BM assessed 10 to 12 weeks after transplantation. (D) Myeloid progenitors in spleen, BM, and peripheral blood responsive to the indicated cytokines were determined in mice reconstituted with either Lyn+/+ or Lyn-/- BM. Progenitors were scored following plating of 105 spleen cells, 2.5 × 105 BM cells, or 1 to 2 μL peripheral blood. Data presented in panels B and D correspond to the mean (± SEM) for 3 experiments using 2 mice/experiment (*P < .01, Student t test). Data presented in panel C were obtained from the analysis of 4 mice in 2 experiments (mean ± SEM).
Perturbed myelo/erythropoiesis is transplantable with Lyn-/- BM. (A) BM from C57BL/6 Ly5.2 background donor Lyn+/+ or Lyn-/- mice was transplanted into irradiated C57BL/6 Ly5.1 mice. Engraftment was assessed by identifying donor and recipient cells by FACS analysis with CD45.2 (donor) and CD45.1 (recipient) mAbs. Alternatively, immature erythroid lineage cells in spleen and BM were assessed by staining with CD71 and Ter119 mAbs. Both PI-positive and mature RBCs were excluded from the analysis. The relative percentages of each population (± SD) in 4 recipient mice of each genotype are presented. (B) CFU-e and BFU-e content in the spleen and BM of recipients of either Lyn+/+ or Lyn-/- BM are shown. (C) The relative number of CD71/Ter119 double-positive erythroid cells in spleen and BM assessed 10 to 12 weeks after transplantation. (D) Myeloid progenitors in spleen, BM, and peripheral blood responsive to the indicated cytokines were determined in mice reconstituted with either Lyn+/+ or Lyn-/- BM. Progenitors were scored following plating of 105 spleen cells, 2.5 × 105 BM cells, or 1 to 2 μL peripheral blood. Data presented in panels B and D correspond to the mean (± SEM) for 3 experiments using 2 mice/experiment (*P < .01, Student t test). Data presented in panel C were obtained from the analysis of 4 mice in 2 experiments (mean ± SEM).
The hematopoietic defect in Lyn-/- mice is not B-cell or autoantibody dependent
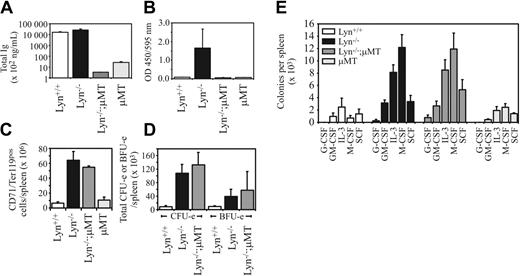
Lyn-/- mice have a B-cell defect that results in hyper-IgM and autoantibody production leading to glomerulonephritis and premature death.34,44 To exclude the possibility that deregulated hematopoiesis is due to underlying autoimmune disease, we eliminated autoantibody production by crossing Lyn-/- mice with μMT/μMT mice, which have a mutation in the B-cell receptor (BCR) μ-chain leading to impaired B-cell maturation.35 The levels of total immunoglobulin were reduced more than 5000-fold, and antinuclear antibodies were undetectable in Lyn-/-;μMT/μMT double-mutant mice (Figure 4A-B). However, the number of CD71+Ter119+ cells previously observed in Lyn-/- mice was not altered in the double mutant, with both Lyn-/- and Lyn-/-;μMT/ μMT 9-month-old mice containing approximately 8- to 10-fold more splenic erythroid cells (Figure 4C). The total numbers of CFU-e's and BFU-e's in the spleens of Lyn-/- and Lyn-/-;μMT/ μMT mice were also correspondingly elevated. Likewise, myeloid progenitors were increased in both Lyn-/- and Lyn-/-;μMT/μMT mice (Figure 4D-E). Thus, B-cell-dependent autoimmune disease is not a major contributing factor to this phenotype.
B-cell developmental defects and autoimmune disease are not linked to the hematopoietic changes observed in Lyn-/- mice. (A) Lyn+/+, Lyn-/-, Lyn-/-;μMT/μMT, and μMT/μMT control mice were assessed for total immunoglobulin levels by ELISA, or (B) antinuclear antibody titers using DNA and nuclear antigen-coated ELISA plates incubated with a 1:500 dilution of sera from mice of the indicated genotypes. Relative optical densities are shown. (C) Total numbers of CD71+Ter119+ cells were enumerated following FACS analysis of spleen cell preparations from mice of the indicated genotypes. (D) Splenic CFU-e's and BFU-e's in mice of the indicated genotypes. Data in panels C-D are means (± SD) for 2 mice per genotype. Mean spleen weights were Lyn+/+, 0.11 ± 0.01 g; Lyn-/-, 0.31 ± 0.08 g; and Lyn-/- μMT, 0.14 ± 0.05 g. (E) Splenic myeloid progenitor numbers responsive to the indicated cytokines in mice of the indicated genotypes. Data represent the mean (± SEM) for 2 mice per genotype per experiment in 2 separate experiments.
B-cell developmental defects and autoimmune disease are not linked to the hematopoietic changes observed in Lyn-/- mice. (A) Lyn+/+, Lyn-/-, Lyn-/-;μMT/μMT, and μMT/μMT control mice were assessed for total immunoglobulin levels by ELISA, or (B) antinuclear antibody titers using DNA and nuclear antigen-coated ELISA plates incubated with a 1:500 dilution of sera from mice of the indicated genotypes. Relative optical densities are shown. (C) Total numbers of CD71+Ter119+ cells were enumerated following FACS analysis of spleen cell preparations from mice of the indicated genotypes. (D) Splenic CFU-e's and BFU-e's in mice of the indicated genotypes. Data in panels C-D are means (± SD) for 2 mice per genotype. Mean spleen weights were Lyn+/+, 0.11 ± 0.01 g; Lyn-/-, 0.31 ± 0.08 g; and Lyn-/- μMT, 0.14 ± 0.05 g. (E) Splenic myeloid progenitor numbers responsive to the indicated cytokines in mice of the indicated genotypes. Data represent the mean (± SEM) for 2 mice per genotype per experiment in 2 separate experiments.
Increased numbers of splenic multipotent progenitors in Lyn-/- mice
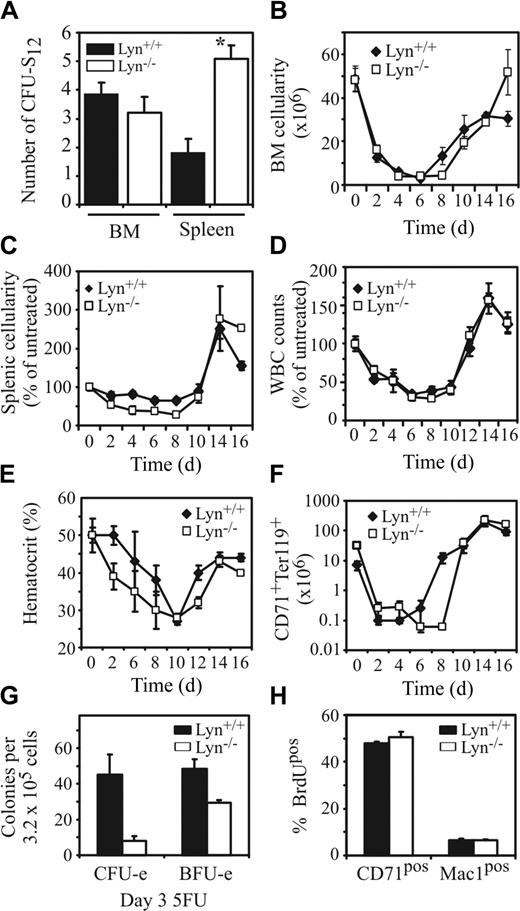
To quantify the number of more primitive progenitor populations, we conducted a day-12 colony-forming unit-spleen assay.45 While the spleens of Lyn-/- mice contained approximately 3-fold more CFU-S12, the BM contained equivalent numbers of these progenitors (Figure 5A). Thus, in addition to an increase in more mature lineage-restricted progenitors, Lyn-/- mice possess increased numbers of primitive progenitors in the spleen without a corresponding alteration in BM progenitors.
Lyn-/- mice have elevated numbers of CFU-S12 in the spleen but are more severely affected by the cytotoxic drug 5-FU. (A) Numbers of primitive hematopoietic progenitors in the spleen and BM of Lyn+/+ and Lyn-/- mice capable of forming macroscopic colonies in the spleens of irradiateSGGd recipient mice 12 days after injection of BM or spleen cell populations (*P < .01, Student t test). Total cellularity of the (B) BM, (C) spleen, (D) peripheral blood, and (E) hematocrits of Lyn+/+ and Lyn-/- was determined at 2-day intervals following intravenous injection of the cytotoxic drug 5-FU. Results for spleen and blood are shown as percent of untreated, since young C57BL/6 background Lyn-/- mice have diminished splenic and white blood cell (WBC) counts compared with control mice. (F) The total cell number of CD71+Ter119+ double-positive cells in the spleen of 5-FU-treated Lyn+/+ and Lyn-/- mice determined by FACS analysis at 2-day intervals over 16 days. In experiments depicted in panels B-F, 5 age- and sex-matched mice per genotype were analyzed at each time point. Data presented are the mean (± SEM). (G) Numbers of BM CFU-e's and BFU-e's were assessed 3 days after challenge with 5-FU. Data represent the mean (± SD) of 3 to 4 mice/genotype. (H) Percentage BrdUpos erythroid (CD71+) and myeloid (Mac-1+) cells in BM 2.5 hours after injection of BrdU (40 mg/kg). Data presented are the mean (± SD) of 3 mice per genotype with similar results observed in 2 independent experiments.
Lyn-/- mice have elevated numbers of CFU-S12 in the spleen but are more severely affected by the cytotoxic drug 5-FU. (A) Numbers of primitive hematopoietic progenitors in the spleen and BM of Lyn+/+ and Lyn-/- mice capable of forming macroscopic colonies in the spleens of irradiateSGGd recipient mice 12 days after injection of BM or spleen cell populations (*P < .01, Student t test). Total cellularity of the (B) BM, (C) spleen, (D) peripheral blood, and (E) hematocrits of Lyn+/+ and Lyn-/- was determined at 2-day intervals following intravenous injection of the cytotoxic drug 5-FU. Results for spleen and blood are shown as percent of untreated, since young C57BL/6 background Lyn-/- mice have diminished splenic and white blood cell (WBC) counts compared with control mice. (F) The total cell number of CD71+Ter119+ double-positive cells in the spleen of 5-FU-treated Lyn+/+ and Lyn-/- mice determined by FACS analysis at 2-day intervals over 16 days. In experiments depicted in panels B-F, 5 age- and sex-matched mice per genotype were analyzed at each time point. Data presented are the mean (± SEM). (G) Numbers of BM CFU-e's and BFU-e's were assessed 3 days after challenge with 5-FU. Data represent the mean (± SD) of 3 to 4 mice/genotype. (H) Percentage BrdUpos erythroid (CD71+) and myeloid (Mac-1+) cells in BM 2.5 hours after injection of BrdU (40 mg/kg). Data presented are the mean (± SD) of 3 mice per genotype with similar results observed in 2 independent experiments.
Heightened sensitivity of Lyn-/- mice to 5-FU
The increase in hematopoietic progenitors in Lyn-/- mice coupled with previous findings illustrating an essential inhibitory signaling role for Lyn within hematopoietic cells led us to speculate that loss of Lyn might enhance the ability of the hematopoietic system to recover from insults that are hematoablative.46 To test this hypothesis, we challenged mice with the chemotherapeutic drug 5-FU, which is cytotoxic to cells in cycle. The cellularity of the indicated compartments was then assessed at 2-day intervals for 16 days following 5-FU challenge. Surprisingly, the total cellularity of each tissue analyzed was similar irrespective of genotype (Figure 5B-D). However, 5-FU had a more severe effect on the hematocrit of Lyn-/- mice than that of control mice (Figure 5E). This, together with the observation that Lyn-deficient mice showed a lag of approximately 2 days in the mobilization/expansion of immature CD71+Ter119+ erythroblasts in the spleen following 5-FU treatment (Figure 5F), may suggest a greater percentage of progenitor cells in cycle and thus heightened 5-FU sensitivity. Alternatively, an underlying defect in erythropoiesis in Lyn-/- mice may lead to an impaired rate of RBC production. To investigate these possibilities we challenged Lyn+/+ and Lyn-/- mice with 5-FU and looked for differential sensitivity of erythroid progenitors to 5-FU. Interestingly, Lyn-/- mice, which normally contain between 3- to 5-fold more CFU-e's in BM than Lyn+/+ mice (Figure 2C), exhibited approximately 5-fold fewer CFU-e's than Lyn+/+ mice following 5-FU challenge (Figure 5G). However, analysis of BrdU uptake into more mature CD71+ erythroblasts or Mac-1+ myeloid cells in untreated mice revealed similar levels of BrdU incorporation (Figure 5H). Thus, Lyn-/- mice contain an increase in both the absolute number of erythroid progenitors, and an increase in the cycling status of these progenitors, without a corresponding increase in BrdU incorporation into more mature BM erythroblasts.
Similarities between Lyn-/-, Mev/Mev, and SHIP-1-/- mice
We and others have defined a relationship between Lyn's inhibitory signaling role and its ability to recruit and activate SHP-1 and SHIP-1 by membrane localization and/or phosphorylation.20,22,29,30,47 Recent studies have also revealed that Lyn and SHIP-1 act together to negatively regulate M-CSF-dependent AKT activity,23 a finding supported by our previous results showing that SHIP-1 is a target of Lyn-dependent phosphorylation in primary macrophages and B cells,22,36 and results illustrating coordinated roles for both Lyn and SHIP-1 in establishing Fcγ receptor IIb1 (FcγRIIb1)-dependent inhibitory signaling in B cells and mast cells.18,19,24,31 To investigate this relationship further, we analyzed both Mev/Mev and SHIP-1-deficient mice to see if the phenotype of Lyn-/- mice might be explained by deregulation of either SHP-1 or SHIP-1. We have previously highlighted the similarities between Lyn-/-, Mev/Mev, and SHIP-1-/- mice in that they develop splenomegaly, increased splenic and peripheral blood myeloid progenitors, and in the case of Lyn-/- and Mev/Mev mice, enhanced sensitivity to M-CSF and GM-CSF.22,32,33
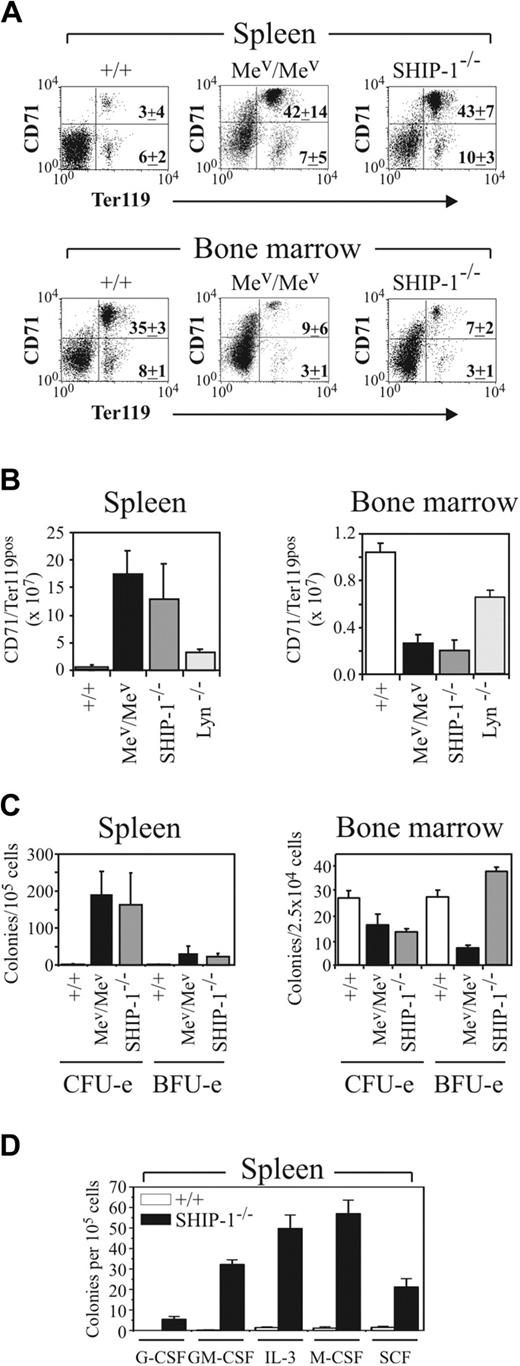
To further our characterization of Lyn-/-, Mev/Mev, and SHIP-1-/- mice, we conducted a limited analysis of the hematopoietic compartments of the 3 mutants and analyzed erythropoiesis in age-matched 6- to 8-week-old mice. Results presented in Table 1 reveal that both Mev/Mev and SHIP-1-/- mice develop similar degrees of splenomegaly, while Mev/Mev mice exhibit dramatically reduced BM cellularity and more severe anemia than do age-matched SHIP-1-/- mice. By contrast, Lyn-/- mice do not show evidence of splenomegaly until 10 to 13 weeks of age22 (Table 1). Interestingly, the spleens of Mev/Mev, like Lyn-/- mice, contained a large proportion of CD71+Ter119+ cells (Figure 6A), which, taking into account the splenomegaly in Mev/Mev mice, corresponded to a 30-fold increase in this population (Figure 6B). Indeed, this population is expanded to a similar degree as that of the Mac-1+ myelomonocytic/granulocytic population in the spleens of Mev/Mev mice (not shown). In keeping with the increase in CD71+Ter119+ cells in the spleen, there was a corresponding increase in splenic CFU-e's, and to a lesser extent, BFU-e's, in Mev/Mev mice (Figure 6C), as previously described.11 Thus, our results showing significant increases in erythroid progenitors in Lyn-/- mice highlight another similarity between these 2 mouse strains. However, splenic extramedullary hematopoiesis in Mev/Mev mice is much more severe than that observed in Lyn-/- mice of similar age. The BM of Mev/Mev mice also exhibited a more dramatic reduction in the proportion and number of CD71+Ter119+ cells than that observed in Lyn-/- mice, and a corresponding decrease in CFU-e's and BFU-e's, not observed in Lyn-/- mice (Figure 6A-C).
Analysis of wild-type, SHIP-1−/−, Mev/Mev, and Lyn−/− mouse HCT, spleen weight, and BM and spleen cellularity
Genotype (no. mice) . | HCT, % . | Spleen weight, g . | Spleen cellularity, × 106 . | BM cellularity/femur, × 106 . |
|---|---|---|---|---|
| Wild-type (8) | 52.4 ± 1.6 | 0.085 ± 0.012 | 135 ± 53 | 17.0 ± 2.1 |
| SHIP-1−/− (7) | 50.8 ± 4.6 | 0.213 ± 0.064 | 300 ± 93 | 13.4 ± 3.0 |
| Mev/Mev (4-6) | 43.7 ± 3.7 | 0.236 ± 0.091 | 298 ± 124 | 4.3 ± 0.9 |
| Lyn−/− (5) | 50.1 ± 2.4 | 0.087 ± 0.019 | 117 ± 9 | 18.2 ± 1.9 |
Genotype (no. mice) . | HCT, % . | Spleen weight, g . | Spleen cellularity, × 106 . | BM cellularity/femur, × 106 . |
|---|---|---|---|---|
| Wild-type (8) | 52.4 ± 1.6 | 0.085 ± 0.012 | 135 ± 53 | 17.0 ± 2.1 |
| SHIP-1−/− (7) | 50.8 ± 4.6 | 0.213 ± 0.064 | 300 ± 93 | 13.4 ± 3.0 |
| Mev/Mev (4-6) | 43.7 ± 3.7 | 0.236 ± 0.091 | 298 ± 124 | 4.3 ± 0.9 |
| Lyn−/− (5) | 50.1 ± 2.4 | 0.087 ± 0.019 | 117 ± 9 | 18.2 ± 1.9 |
All characterization was performed on mice of 6 to 8 weeks of age. Hematocrit (HCT) was determined by analysis of blood obtained from the retro-orbital venous plexus on an ADVIA 120 hematology system. Values represent the mean ± SD for the number of mice indicated.
Motheaten viable and SHIP-1-/- mice exhibit a similar but more severe hematopoietic phenotype than Lyn-/- mice. (A) FACS profiles of 6- to 8-week-old wild-type (+/+), Mev/Mev, and SHIP-1-/- spleen and BM populations stained with CD71 and Ter119 mAbs. PI-positive and mature RBCs were excluded from the analysis. Relative percentages of immature erythroid (upper right quadrant) and more mature erythroid (lower right) cells are indicated for 4 mice of each genotype (± SD). (B) Total numbers of CD71+Ter119+ cells in the spleen and BM of +/+, Mev/Mev, SHIP-1-/-, and Lyn-/- mice are shown. Values represent the mean (± SD) of 5 C57BL/6 background mice per group, except Mev/Mev, for which 2 mice per group are shown. (C) Mature erythroid (CFU-e) and immature erythroid (BFU-e) progenitors in +/+, Mev/Mev, and SHIP-1-/- spleen and BM were determined at day 2 and day 10, respectively. Data represent the mean (± SEM) for 2 Mev/Mev mice and 4 +/+ and SHIP-1-/- mice. (D) Myeloid progenitor content in the spleens of 8-week-old SHIP-1-/- mice was assessed as in Figure 1 in the presence of the indicated cytokines. Values represent the mean (± SEM) for 4 animals in 2 experiments.
Motheaten viable and SHIP-1-/- mice exhibit a similar but more severe hematopoietic phenotype than Lyn-/- mice. (A) FACS profiles of 6- to 8-week-old wild-type (+/+), Mev/Mev, and SHIP-1-/- spleen and BM populations stained with CD71 and Ter119 mAbs. PI-positive and mature RBCs were excluded from the analysis. Relative percentages of immature erythroid (upper right quadrant) and more mature erythroid (lower right) cells are indicated for 4 mice of each genotype (± SD). (B) Total numbers of CD71+Ter119+ cells in the spleen and BM of +/+, Mev/Mev, SHIP-1-/-, and Lyn-/- mice are shown. Values represent the mean (± SD) of 5 C57BL/6 background mice per group, except Mev/Mev, for which 2 mice per group are shown. (C) Mature erythroid (CFU-e) and immature erythroid (BFU-e) progenitors in +/+, Mev/Mev, and SHIP-1-/- spleen and BM were determined at day 2 and day 10, respectively. Data represent the mean (± SEM) for 2 Mev/Mev mice and 4 +/+ and SHIP-1-/- mice. (D) Myeloid progenitor content in the spleens of 8-week-old SHIP-1-/- mice was assessed as in Figure 1 in the presence of the indicated cytokines. Values represent the mean (± SEM) for 4 animals in 2 experiments.
Surprisingly, analysis of SHIP-1-/- mice revealed a phenotype very similar to that of Mev/Mev mice, and to a lesser degree, Lyn-/- mice. Staining with CD71 and Ter119 revealed a previously unrecognized increase in erythroblasts in the spleens of SHIP-1-/- mice. The absolute number of these cells was increased to a similar extent to that observed in 6- to 8-week-old Mev/Mev mice (Figure 6A-B), and to a lesser extent, Lyn-/- mice (Figures 4C, 6B). Analysis of erythroid progenitor numbers revealed a large increase in CFU-e's in spleens of SHIP-1-/- mice (Figure 6C). Comparison of total CFU-e spleen content revealed Mev/Mev 1634 × 103 (± 1252 × 103) more than SHIP-1-/- 490 × 103 (± 232 × 103) more than Lyn-/- 107 × 103 (± 26 × 103) more than wild-type 6 × 103 (± 1 × 103). Like Mev/Mev mice, SHIP-1-/- BM contained a dramatically increased proportion of Mac-1-positive myeloid cells (wild-type, 34.8% ± 1.9%; SHIP-1-/-, 76.7% ± 5.2%; Mev/Mev, 90.3% ± 2.8%; mean ± SD, n = 4/genotype) and a correspondingly reduced proportion and number of CD71+Ter119+ cells (Figure 6A-B). In keeping with this reduction in BM erythroid cells, SHIP-1-/- mice also have fewer CFU-e progenitors (Figure 6C) as previously reported.14 In terms of CFU-e content per femur, wild-type mice contained 19 515 (± 2241) progenitors versus 6651 (± 1222) for SHIP-1-/- mice. By contrast, BM BFU-e content per femur was not significantly different between SHIP-1-/- and wild-type mice (wild-type, 19 487 ± 1162 vs SHIP-1-/-, 18 681 ± 2062 per femur) (Figure 6C). The number of progenitors responsive to G-CSF, GM-CSF, IL-3, M-CSF, or SCF was dramatically increased in the spleens of SHIP-1-/- mice in keeping with previous characterization of GM-CFC content14 (Figure 6D). Consistent with the increased numbers of myeloid cells in SHIP-1-/- BM, we observed 2-fold increases in the numbers of BM GM-CSF, M-CSF, and IL-3 CFCs (not shown). Thus, like Lyn-/- mice, both SHIP-1-/- and Mev/Mev mice develop splenomegaly characterized by increased numbers of immature erythroblasts and myeloid cells in addition to enhanced numbers of their respective progenitors, with the latter 2 mutants both more severely affected than Lyn-/- mice. The BM compartments of the 3 mutants, however, are significantly different both in terms of CD71+Ter119+ erythroid cell numbers and alterations in CFU-e and BFU-e progenitor content.
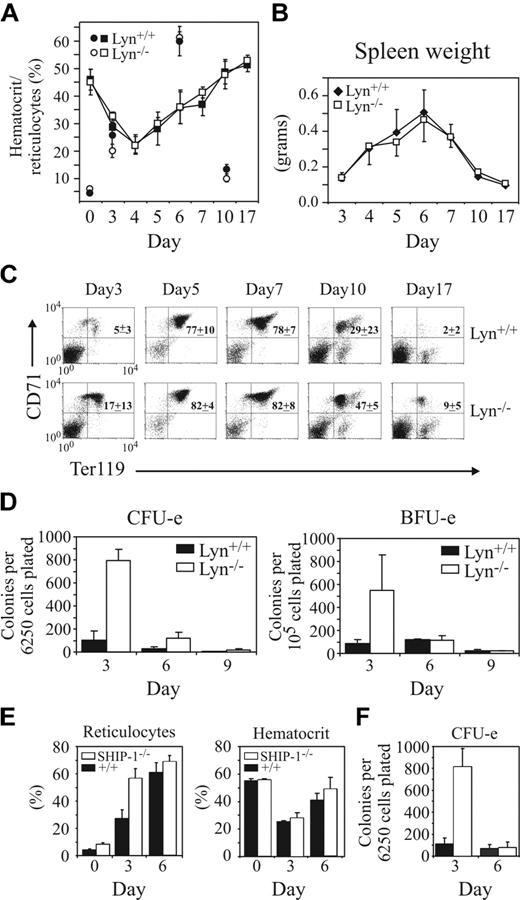
Lyn-/- and SHIP-1-/- mice exhibit enhanced production of erythroid progenitors but normal recovery of hematocrit following erythropoietic stress
The RBC cytotoxic drug phenylhydrazine induces anemia in mice that is reversed by a wave of erythropoiesis characterized by an accumulation of CD71+Ter119+ cells in the spleen preceding the restoration of normal RBC numbers and hematocrits. A comparison of the response of Lyn+/+ and Lyn-/- mice to phenylhydrazine revealed a similar rate of hematocrit recovery, reticulocyte production, splenomegaly, and increase in splenic CD71+Ter119+ cellularity, despite the fact that Lyn-/- mice exhibited dramatically increased splenic CFU-e and BFU-e progenitor numbers following phenylhydrazine challenge (Figure 7A-D). Interestingly, comparison of SHIP-1-/- with Lyn-/- mice revealed a similar increase in CFU-e's at day 3 after phenylhydrazine challenge in both mutants, although SHIP-1-/- mice also showed increased numbers of reticulocytes at day 3 (Figure 7E-F). However, this increase in splenic erythroid progenitors and peripheral blood reticulocyte numbers (for SHIP-1-/- but not Lyn-/- mice) did not result in a statistically significant enhancement in hematocrit recovery from phenylhydrazine challenge in either strain.
Lyn-/- mice exhibit normal hematocrit recovery following phenylhydrazine challenge but show enhanced erythroid progenitor expansion. (A) Changes in hematocrit and percentages of reticulocytes over a 17-day period were assessed in Lyn+/+ and Lyn-/- mice after 2 consecutive intraperitoneal injections of phenylhydrazine (60 mg/kg) on days 1 and 2. Mean hematocrit (squares) and reticulocyte (circles) values for 3 to 8 mice per time point (± SD) are shown. (B) Changes in spleen weights in Lyn+/+ and Lyn-/- mice were recorded following phenylhydrazine challenge. Values represent the mean (± SD) for 3 to 5 mice per time point, excepting days 5 to 7, where 6 to 19 mice per point are shown. (C) Representative 2-color FACS profiles of spleen cell populations from phenylhydrazine-treated Lyn+/+ and Lyn-/- mice stained with the mAbs CD71 and Ter119. Mature RBCs and PI-positive cells were excluded from the analysis. (D) Numbers of splenic CFU-e's and BFU-e's in Lyn+/+ and Lyn-/- mice were determined 3, 6, and 9 days after phenylhydrazine challenge. Values are the mean (± SD) of 5 mice per genotype. (E) The response of SHIP-1+/+ and SHIP-1-/- mice to phenylhydrazine challenge was determined by assessing reticulocyte and hematocrit levels at days 0, 3, and 6 after challenge. Values are the mean (± SD) of 4 mice per genotype. (F) Numbers of splenic CFU-e's in SHIP-1+/+ (▪) and SHIP-1-/- mice (□) were determined 3 and 6 days after phenylhydrazine challenge. Values are the mean (± SD) of 4 mice/genotype.
Lyn-/- mice exhibit normal hematocrit recovery following phenylhydrazine challenge but show enhanced erythroid progenitor expansion. (A) Changes in hematocrit and percentages of reticulocytes over a 17-day period were assessed in Lyn+/+ and Lyn-/- mice after 2 consecutive intraperitoneal injections of phenylhydrazine (60 mg/kg) on days 1 and 2. Mean hematocrit (squares) and reticulocyte (circles) values for 3 to 8 mice per time point (± SD) are shown. (B) Changes in spleen weights in Lyn+/+ and Lyn-/- mice were recorded following phenylhydrazine challenge. Values represent the mean (± SD) for 3 to 5 mice per time point, excepting days 5 to 7, where 6 to 19 mice per point are shown. (C) Representative 2-color FACS profiles of spleen cell populations from phenylhydrazine-treated Lyn+/+ and Lyn-/- mice stained with the mAbs CD71 and Ter119. Mature RBCs and PI-positive cells were excluded from the analysis. (D) Numbers of splenic CFU-e's and BFU-e's in Lyn+/+ and Lyn-/- mice were determined 3, 6, and 9 days after phenylhydrazine challenge. Values are the mean (± SD) of 5 mice per genotype. (E) The response of SHIP-1+/+ and SHIP-1-/- mice to phenylhydrazine challenge was determined by assessing reticulocyte and hematocrit levels at days 0, 3, and 6 after challenge. Values are the mean (± SD) of 4 mice per genotype. (F) Numbers of splenic CFU-e's in SHIP-1+/+ (▪) and SHIP-1-/- mice (□) were determined 3 and 6 days after phenylhydrazine challenge. Values are the mean (± SD) of 4 mice/genotype.
Lyn kinase is dispensable for EPO-dependent activation of Jak/STAT/MAPK pathways and SHIP-1 phosphorylation in primary erythroblasts
Given that the loss of Lyn lowers the threshold for B cell,18-21 mast cell,25-28 and macrophage stimulation,22,23 and the reported role for Lyn in EPO receptor phosphorylation and STAT5 activation,48 we investigated whether loss of Lyn might modulate EPO-dependent signaling in primary erythroid cells. We purified CD71+ erythroblasts from spleens of phenylhydrazine-challenged mice and showed that they were predominantly basophilic and early polychromatophilic erythroblasts that expressed both CD71 and Ter119 (Supplemental Figure 1A-B, available by clicking the Supplemental Figure link at the top of the online article on the Blood website), but not c-Kit, or CD45 (not shown). Stimulation of these cells with EPO led to dose-dependent tyrosine phosphorylation of both the EPO receptor and Jak2, and corresponding activation of STAT5 and MAP kinases (Supplemental Figure 1C). However, comparison of EPO-dependent signaling in Lyn+/+ and Lyn-/- cells revealed no detectable modulation in either the dose response or time course of STAT5 or MAP kinase activation (Supplemental Figure 1D). Moreover, unlike the case in B cells36 and macrophages,22 loss of Lyn did not alter the tyrosine phosphorylation of SHIP-1 or SHP-1 in erythroblasts (Supplemental Figure 1E, and not shown). Thus, Lyn is dispensable for EPO-dependent activation of the JAK/STAT and MAP kinase pathways and the tyrosine phosphorylation of SHP-1 and SHIP-1 in erythroblasts.
Discussion
Lyn plays a critical role in myelomonocytic cells as a regulator of SHP-1, SHP-2, SHIP-1, and the ITIM-containing inhibitory receptors paired immunoglobulin-like receptor B (PIR-B) and signal regulatory protein α (SIRPα).22 Failure to appropriately engage inhibitory signaling in the absence of Lyn is thought to underlie the extramedullary hematopoiesis observed in these mice and the hypersensitivity of Lyn-deficient macrophages to proliferation and survival signals provided by GM-CSF and M-CSF. Here, we have further defined the temporal progression of perturbations in hematopoiesis that develop as a consequence of Lyn deficiency. We have also identified a novel defect in erythropoiesis that results in a significant increase in erythroid progenitors and erythroblasts in Lyn-/- spleen. This characteristic becomes more severe with age and coincides temporally with the development of splenomegaly. By contrast, significant increases in splenic myeloid progenitor numbers were apparent in mice as young as 4 weeks of age and preceded splenomegaly.
In light of the superficial similarities between Lyn-/-, SHIP-1-/-, and Mev/Mev mice, and Lyn's critical role in regulating the tyrosine phosphorylation and plasma membrane mobilization of SHIP-1 and SHP-1, we compared hematopoiesis in these mice. Interestingly, the composition of the spleens of both SHP-1- and SHIP-1-deficient mice was remarkably similar in terms of the degree of splenomegaly and erythroblast/erythroid progenitor composition. The BM of both mutants also showed a dramatic reduction in immature erythroid cells and an expansion of myeloid populations. Thus, the phenotypes of SHIP-1-/- and Mev/Mev mice were similar, albeit more severe, than similarly aged Lyn-/- mice. However, it should be noted that Lyn-/- mice do develop splenomegaly of a similar magnitude to that observed in Mev/Mev and SHIP-1-/- mice as they age.22 Comparison of Lyn-/- and SHIP-1-/- splenic myeloid colony numbers also revealed that myeloid progenitors are dramatically increased in SHIP-1-/- mice. This increase in myelopoiesis in SHIP-1-/- mice again is more severe than that observed in Lyn-/- mice when the degree of splenomegaly is taken into account. There were also notable differences between Lyn-/-, Mev/Mev, and SHIP-1-/- mice including a diminution in CFU-e and BFU-e (Mev/Mev) numbers in the BM not observed in Lyn-/- mice. However, the dramatic expansion of myeloid populations in the BM of Mev/Mev and SHIP-1-/- mice, and thus potential limitations in stromal-cell support, may be a contributing factor to these BM progenitor population perturbations.
Splenomegaly may arise for a variety of reasons, including loss of signal inhibition, such as in SHP-1-12,13 or SHIP-1-deficient mice,14,15 or be due to the expression of oncogenes such as BCR/Abl.6,7 Splenomegaly may also reflect an underlying defect resulting in hematologic stress. For example, mice lacking STAT5A/5B39,40 or Bcl-x49 exhibit a large increase in splenic erythropoiesis as a compensatory response to impaired RBC production in the BM due to impaired induction of Bcl-XL and survival of erythroblasts.50-52 Impaired terminal differentiation of erythroid cells, such as that observed in mice lacking GATA-1, may also result in splenomegaly due to a dramatic expansion of erythroid progenitors.53-55 Thus, we reasoned that splenomegaly in Lyn-/- mice might be due to Lyn's role in signal inhibition, or an as-yet-unrecognized role for Lyn in the production, survival, or differentiation of erythroblasts. Severe defects in erythropoiesis in mice are most readily apparent during fetal development, a period of high erythropoietic rate.39 However, analysis of fetal liver hematopoiesis in Lyn-/- mice failed to show alterations in either myeloid or erythroid progenitor numbers, and quantitation of neonatal erythroblasts revealed no significant difference between Lyn+/+ and Lyn-/- mice (not shown). This is in keeping with our previous analysis of aged Lyn+/+ and Lyn-/- mice, which failed to show evidence of anemia.22 A thorough analysis of apoptosis in immature erythroblasts in adult BM and embryonic day-14 fetal liver also revealed no differences between Lyn+/+ and Lyn-/- mice, in keeping with our finding that Lyn deficiency does not alter EPO-dependent STAT5 phosphorylation. However, the BM of Lyn-/- mice did display a modest decrease in immature erythroid cells and an increase in CFU-e's, suggesting an impairment in the expansion/differentiation of CFU-e progenitors into erythroblasts. Interestingly, Helgason et al14 have speculated that the diminution of BM CFU-e's and Ter119+ cells in SHIP-1-/- BM may indicate a positive role for SHIP-1 in erythropoiesis. Our data suggesting an underlying defect in erythropoiesis in Lyn-/- mice were supported by our finding that 5-FU-dependent anemia56 was more severe, and the anemia-induced expansion of the splenic CD71+/Ter119+ erythroblasts was delayed in Lyn-/- mice. However, the heightened sensitivity of Lyn-deficient mice to 5-FU was likely due to a greater proportion of cycling Lyn-/- progenitor cells, as assessment of CFU-e and BFU-e erythroid progenitor numbers 3 days after 5-FU challenge revealed a more severe diminution of erythroid progenitors in Lyn-/- versus Lyn+/+ mice. Interestingly, the hematopoietic progenitor cell compartment in SHIP-1-/- mice also exhibits heightened sensitivity to 5-FU and diminished stem cell competitive repopulation ability.57
The similarity between Lyn+/+, Lyn-/-, and SHIP-1-/- mice in hematocrit recovery following treatment with the RBC-depleting drug phenylhydrazine argues against a major defect in erythropoiesis in either Lyn-/- or SHIP-1-/- mice. Indeed, assessment of erythroid progenitor numbers in the spleens of Lyn+/+, Lyn-/-, and SHIP-1-/- mice after phenylhydrazine treatment revealed a more robust expansion of this lineage in both Lyn-/- and SHIP-1-/- mice. It is surprising, however, that the large increase in erythroid progenitors in the spleens of both Lyn-/- and SHIP-1-/- mice did not ultimately lead to an enhanced recovery from phenylhydrazine. Indeed, this observation raises the possibility that Lyn and SHIP may act as negative regulators during early myelo/erythropoiesis, but may be required for appropriate differentiation of hematopoietic cells at later stages of development.
Given the similarities between Lyn-/-, Mev/Mev, and SHIP-1-/- mice, together with studies showing a role for Lyn in EPO-dependent signaling,48 and the reported role of SHP-1 as a direct antagonist of EPO receptor and Jak2 phosphorylation/activation,58,59 we investigated the role of Lyn in EPO receptor signaling in primary erythroblasts. We found no significant difference in either the dose response or time course of activation of STAT5 or MAP kinase following EPO stimulation. Surprisingly, Lyn was also dispensable for both SHIP-1 and SHP-1 (not shown) tyrosine phosphorylation in these cells. Thus, Lyn does not play a significant role in the EPO-dependent activation of the JAK/STAT or MAP kinase pathways, or in SHP-1 and SHIP-1 tyrosine phosphorylation in erythroblasts at this stage of development. These results suggest that the increased erythropoiesis observed in Lyn-/- mice is unlikely to be a simple consequence of heightened EPO sensitivity of Lyn-/- erythroblasts. However, Lyn-dependent regulation of other EPO-dependent signaling pathways, or regulation of EPO signaling at other stages of erythroid development, cannot be ruled out. Inhibitory roles for Lyn in signaling from other receptors expressed within this lineage, functions for Lyn in differentiation or mobilization of erythroid cells,60-62 and roles for Lyn in more primitive progenitor populations also remain to be explored. Regardless, it is clear that Lyn plays a critical role in signaling required for appropriate development of the myeloid, erythroid, and B-lymphoid arms of the hematopoietic system. Analysis of combined loss-of-function mutants of Lyn, SHP-1, and/or SHIP-1 will be an important step in assessing the genetic interactions between these signal transduction regulators in hematopoietic stem/progenitor cell biology.
Prepublished online as Blood First Edition Paper, August 31, 2004; DOI 10.1182/blood-2003-12-4396.
Supported in part by funds from the Cancer Council of Victoria (K.W.H. and M.L.H.); the Cooperative Research Centre for Cellular Growth Factors, the National Health and Medical Research Council of Australia; Terry Fox Postdoctoral and Research Fellowships from the National Cancer Institute of Canada (K.W.H.); and the National Health and Medical Research Council of Australia (M.L.H. and D.M.T.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Drs D. Hilton, W. Alexander, and N. Nicola for their generous gift of cytokines and assistance with the ADVIA hematology system blood analyses. Thanks also to D. L. Krebs and A. W. Burgess for critical review of the manuscript. The authors are grateful to J. Corbin for help with blood analysis, P. Tilbrook for advice on benzidine staining of erythroid colonies, T. Gonda for advice on the methylcellulose assays, S. Jackson for SHIP-1-/- mice, and T. Thorne, M. Arnold, and J. Cohen for their work in the animal facility. We would also like to thank A. R. Dunn and G. S. Hodgson for valuable discussions.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal