Abstract
PU.1 is a member of the ETS family of transcription factors and is required for the development of multiple hematopoietic lineages. PU.1-/- mice die from hematopoietic failure at about embryonic day 18.5 (e18.5) and show a complete absence of B cells, mature T cells, and macrophages. This phenotype suggests that PU.1 may function at the level of the hematopoietic stem cell (HSC) or a multilineage progenitor. To investigate the role of PU.1 in the regulation of HSCs, PU.1-/- embryos were analyzed at various stages of embryonic development. The absolute number and frequency of HSCs were determined by flow cytometric analysis of c-Kit+Thy-1.1loLin-Sca-1+ (KTLS) cells. We found that KTLS cells were absent or severely reduced in PU.1-/- fetal liver from e12.5 to e15.5. Progenitor cells with a c-Kit+Lin-AA4.1+ and c-Kit+Lin-CD34+ phenotype were also severely reduced. In addition, PU.1-/- fetal liver at e14.5 lacked common myeloid progenitors (CMPs) and granulocyte-macrophage progenitors (GMPs) but retained megakaryocyteerythroid progenitors (MEPs). Consistent with the loss of HSC activity, a 10-fold reduction in erythroid progenitors (mature erythroid burst-forming units [BFUEs]) was observed between e14.5 and e16.5. These data suggest that PU.1 plays an important role in the maintenance or expansion of HSC number in murine fetal liver. (Blood. 2004;104:3894-3900)
Introduction
The formation of blood cells in mice is first observed in the extraembryonic blood islands of the yolk sac at embryonic day 7.5 (e7.5) and within the embryo proper in the paraaortic splanchnopleura (PAS) and the aorta-gonad-mesonephros (AGM) regions between e7.5 and e11.0.1,2 Definitive hematopoiesis, which is characterized by enucleated red blood cells expressing fetal and adult-type hemoglobin genes, is established in the fetal liver when hematopoietic stem cells (HSCs) emigrate in waves from the AGM region between e10.0 and e11.5.3-5 Proper homing of HSCs to the fetal liver is dependent on β1 integrin expression, in that chimeric embryos generated from β1 integrin-deficient embryonic stem (ES) cells showed high levels of circulating β1 null HSCs and no detectable hematopoiesis from β1 null cells in the fetal liver.6,7
Definitive HSCs that colonize the fetal liver have been purified to varying degrees using a variety of cell surface markers. All of the long-term repopulating HSC (LT-HSC) activity in murine fetal liver was found in the AA4.1+ population, which comprises about 1% of fetal liver.8 Additional studies have shown that LT-HSCs could be further enriched within the AA4.1+Sca-1+Lin- fraction of e14.0 fetal liver, which represents approximately 0.05% to 0.08% of total liver cells.9,10 Fetal liver LT-HSCs have also been found exclusively in the CD34+ subset at e1211 or e14.12 In another study, 6 e14.5 fetal liver cells of the phenotype Sca-1+Lin-/loMac-1+Thy-1.1lo were sufficient to long-term reconstitute lethally irradiated hosts.13 All long-term reconstituting cells were c-kit+ in that analysis. Characterization of the Sca-1+Lin-/loThy-1.1lo fraction of fetal liver showed that these cells were more proliferative than their adult bone marrow counterparts, with 40% of the cells having greater than 2N DNA content based on Hoechst 33342 staining.14 The Sca-1+Lin-/loMac-1+Thy-1.1lo cells double daily until they reach a peak at about e14.5, after which time a large fraction of the long-term self-renewing HSC population emigrates to other sites that will support hematopoiesis in the adult animal including the spleen, thymus, and bone marrow.5,13
The generation of definitive hematopoietic cells in the fetal liver is dependent on the function of a number of transcription factors. Embryos deficient in either the DNA-binding subunit of core-binding factor, acute myeloid leukemia 1 (AML-1)/RUNX1, or the non-DNA-binding subunit that interacts with RUNX1, core binding factor β (CBFβ), have no definitive hematopoietic cells and die at e12.5 from intracranial hemorrhaging.15-18 The absence of definitive hematopoiesis in the Runx1 and CBFβ knockouts may be due to defective formation of AGM stem cells in the dorsal aorta prior to HSC colonization of the fetal liver.19 Embryos that lack the transcription factors GATA-2 and c-myb also exhibit severe cell autonomous defects in definitive hematopoiesis where all hematopoietic lineages are affected.20,21 GATA-2-deficient embryos die at e10 to e11 of severe anemia and exhibit abnormalities in primitive erythropoiesis. In the case of c-myb, CD34+c-kit+ progenitor cells derived from c-myb-/- ES cells were found in the fetal livers of chimeric embryos, but these cells could not expand like phenotypically similar wild-type progenitors.22 Therefore, the GATA-2 and c-myb mutations most likely affect the maintenance, proliferation, and/or differentiation potential of HSCs or very primitive multilineage progenitor cells.
The absence of the ETS family transcription factor PU.1 also has profound effects on hematopoietic development.23,24 In one PU.1 knockout model,23 embryos die at about e18.5 and show a complete absence of B-lymphoid and myeloid lineage cells. Fetal thymic T-cell development was blocked at the precommitment CD44+CD25-CD3-CD4-CD8- stage,25 although PU.1-/- thymocytes from e14.5 embryos could generate mature T cells in fetal thymic organ cultures.26 Although erythropoiesis and megakaryopoiesis were apparently normal in the PU.1-/- embryos, analysis of chimeric animals derived from PU.1-/- ES cells showed an absence of PU.1-/- adult erythrocytes, indicating that erythroid development may be severely impacted by the absence of PU.1.27 The embryonic lethality associated with the loss of PU.1 was shown to be due to hematopoietic failure, in that in utero transplantation of wild-type HSCs completely rescued PU.1-/- embryos from death.28
The multilineage phenotype associated with the loss of PU.1 and the hematopoietic failure observed in the PU.1-/- embryos by e18.5 suggest that PU.1 may regulate the maintenance or function of LT-HSCs or primitive progenitor cells in the fetal liver. In order to investigate the role of PU.1 in the regulation of HSC function, we characterized the c-Kit+Thy-1.1loLin-/loSca-1+ (KTLS) LT-HSC compartment in PU.1-/- embryos by flow cytometry (fluorescence-activated cell sorter [FACS]). FACS analysis showed that KTLS cells were severely reduced or completely absent in PU.1-/- fetal liver from e12.5 to e15.5. In addition, the number of c-Kit+Lin- hematopoietic progenitor cells do not expand after e13.5 in PU.1-/- versus wild-type control embryos. The loss in the absolute numbers of progenitor cells could not be accounted for by an enhanced rate of apoptotic cell death or to decreased proliferation kinetics within the c-Kit+Lin- population at any embryonic day. Methylcellulose assays also demonstrated that erythroid progenitor cells responsive to erythropoietin (EPO) or a combination of EPO and stem cell factor (SCF) stimulation at e14.5 or e16.5 were significantly reduced compared with controls. These observations are consistent with the hypothesis that PU.1 is necessary for maintenance or expansion of LT-HSCs in murine fetal liver and suggest that hematopoietic failure in PU.1-/- embryos could be due to differentiation and/or loss of HSC activity during fetal liver hematopoiesis.
Materials and methods
Mouse strains
Preparation of fetal liver
Timed pregnancies were set up in the late afternoon and the appearance of a vaginal plug was designated as 0.5 days after coitus (dpc). Pregnant females were killed at different time points from e12.5 to e15.5. Livers were quantitatively dissected from embryos and a single-cell suspension of each liver was made by passing the cells through a nylon cell strainer. The absolute number of fetal liver cells was then determined by standard cell counting. Genomic DNA was isolated from the remaining embryo for genotyping by polymerase chain reaction (PCR) and Southern blot analysis.
PCR and Southern blot analysis
Screening of PU.1 mice was done by PCR using 2 primer sets, one specific for the neomycin gene and the other for the wild-type PU.1 locus, as previously described.30 PCR genotypes of the transgenic animals were confirmed by Southern blot analysis as described in the original characterization of the knockout animals.23
Staining of fetal liver cells and FACS analysis
After initial counting, cells were pelleted and then stained with a blocking antibody to the FcγII/III receptor (2.4G2; BD Biosciences-Pharmingen, San Diego, CA) for 20 minutes at 4°C. After blocking, cells were stained for 25 minutes at 4°C with c-KitAPC (2B8), Sca-1Texas Red (E13-161.7), LinPE, and Thy-1.1FITC (19XE5). The Lin cocktail included antibodies to CD3 (145-2C11), CD4 (GK1.5), CD5 (53-7.3), CD8 (53-6.7), Gr-1 (8C5), B220 (6B2), and Ter119. Further characterization of the fetal liver progenitor compartment was done by staining with CD34FITC or biotinylated AA4.1 followed by secondary staining with avidin-Texas Red (Caltag, Burlingame, CA). The common myeloid progenitor (CMP), granulocyte-macrophage progenitor (GMP), and megakaryocyte-erythroid progenitor (MEP) subsets were analyzed by staining with biotinylated Lin antibodies (CD3, CD4, CD5, CD8, B220, CD19 [1D3], immunoglobulin M [IgM; AF6-78], Ter119, interleukin-7 receptor α [IL-7Rα; B12-1], and AA4.1) visualized with streptavidin cychrome, c-KitAPC, Sca-1Texas Red, CD34FITC (RAM34), and CD16/CD32PE (2.4G2) as previously described.31 Stem cell analysis was performed on a triple laser Mo-Flo cell sorter (Cytomation, Fort Collins, CO) configured with argon, krypton, and rhodamine dye lasers. Dead cells were excluded from the analysis by propidium iodide staining. All antibodies were obtained from BD Pharmingen (San Jose, CA) unless otherwise noted.
Erythroid progenitor assay
The e14.5 and e16.5 fetal liver was isolated and single-cell suspensions were diluted with Iscoves Modified Dulbecco Media (IMDM) with 2% fetal bovine serum (FBS) at 2 × 106 cells/mL. Cells (6 × 105) were then added to 3.0 mL of methylcellulose (Methocult 3334; Stem Cell Technologies, Vancouver, BC, Canada) and mixed by vortexing. Cell and methylcellulose mixture (1.1 mL) was dispensed into 10-mm culture dishes using a 3-mL syringe and a 16-gauge blunt-end needle. Dishes were gently rotated to spread the methylcellulose evenly and placed in a 100-mm Petri dish containing 4 mL of sterile water. Mature erythroid burst-forming unit (BFU-E) colonies were counted 5 to 7 days after plating. Stem cell factor (50 ng/mL; R&D Systems, Minneapolis, MN) was added to some platings to stimulate erythroid progenitors.
Cell cycle analysis and apoptosis
For cell cycle analysis, cells were harvested from the fetal liver and washed with cold phosphate-buffered saline (PBS). Cell pellets were resuspended in Hoechst 33342 (Molecular Probes, Eugene, OR) at a final concentration of 10 μg/mL at 1 × 106 cells/mL and incubated at 37°C for 30 minutes. Verapamil (50 μg/mL; Sigma, St Louis, MO), which is an inhibitor of multidrug efflux pump activity, was added to prevent efflux of Hoechst dye during all subsequent stainings. For apoptosis analysis, cells were collected by centrifugation at 500g for 10 minutes and then washed by resuspending in 500 μL of cold PBS followed by centrifugation. Each pellet was gently resuspended in 100 μL of incubation buffer (100 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.0; 1.5 M NaCl; 50 mM KCl; 10 mM MgCl2; 18 mM CaCl2; 0.025 μg of annexin-FITC) and incubated in the dark for 15 minutes at room temperature. Cells were collected and washed with 300 μL of incubation buffer without annexin-FITC and then resuspended in 100 μL of PBS with propidium iodide for FACS analysis.
Results
Absence of c-Kit+Thy-1.1loLin-Sca-1+ (KTLS) cells in PU.1-/-fetal liver
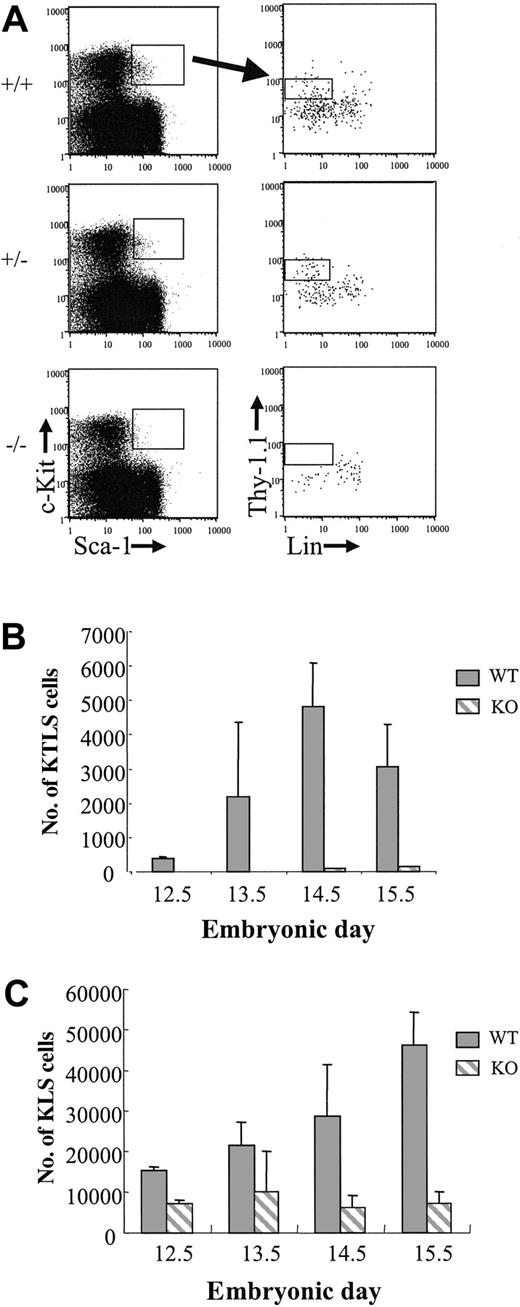
Previous studies showed that PU.1 knockout embryos die in utero from hematopoietic failure at about e18.5.23,28 In light of these observations, we wished to characterize the LT-HSC compartment in PU.1 knockout fetal liver in more detail. PU.1 knockout mice were backcrossed onto a C57BL/Ka-Thy-1.1 background for more than 11 generations and then phenotyped for the stem cell markers c-Kit, Thy-1.1, Lin (a cocktail including CD3, CD4, CD5, CD8, Ter119, B220, and Gr-1 antibodies), and Sca-1. Six KTLS cells that also express the Mac-1 marker can long-term reconstitute lethally irradiated congenic animals.13 Since PU.1 regulates Mac-1 expression (CD11b), we were unable to use Mac-1 as a marker of LT-HSCs in PU.1 knockout embryos.23,32,33 To generate timed pregnancies, heterozygous PU.1+/- animals were crossed and the appearance of a vaginal plug was considered e0.5. Fetal livers dissected from embryos between e12.5 and e15.5 were then stained for the stem cell phenotype. Genotyping of embryos was done by both PCR and Southern blot analysis (see “Materials and methods”). Representative FACS analysis and gating for all embryos is shown in Figure 1A for e13.5 embryos. The absolute number of Sca-1+c-Kit+Lin- cells was reduced in all PU.1-/- embryos (n = 2, e12.5; n = 6, e13.5; n = 8, e14.5; and n = 6, e15.5) compared with either PU.1+/+ or PU.1+/- embryos, regardless of stage (Figure 1C). At e15.5, there was a 6.6-fold reduction in Sca-1+c-Kit+Lin- cells comparing wild-type fetal liver with PU.1-/- fetal liver. The presence of a significant percentage of Sca-1+c-Kit- cells in all embryos (Figure 1A) suggests that the reduction in Sca-1+c-Kit+Lin- cells was not due to PU.1 regulation of the Sca-1 marker in the knockout embryos. In addition, there were no detectable Thy-1.1lo cells among the gated Sca-1+c-Kit+ cells, although Thy-1.1-Lin- cells were always present, albeit at reduced numbers compared with control embryos (Figure 1A-B). The absolute number of KTLS cells in wild-type fetal liver increased 2- to 3-fold daily to a peak at e14.5, which was consistent with observations made by others.13 Heterozygous PU.1 fetal livers showed an intermediate phenotype with respect to absolute numbers of KTLS cells, which suggests that PU.1 dosage may be critical in the maintenance of this cell population (data not shown).
KTLS cells in e13.5 fetal liver from +/+, +/-, and -/- PU.1 embryos. (A) Cells obtained from representative e13.5 fetal livers were incubated with a blocking antibody to Fc receptor (2.4G2) and then were stained with Sca-1TexasRed, c-KitAPC, LinPE, and Thy-1.1FITC. Cells expressing Sca-1 and c-Kit (left) were analyzed for Thy-1.1 and lineage marker expression (right). (B) The absolute number of KTLS and (C) KLS cells in PU.1+/+ and PU.1-/- fetal livers is indicated (for -/- embryos, n = 2, e12.5; n = 6, e13.5; n = 8, e14.5; and n = 6, e15.5; for +/+ embryos, n = 2, e12.5; n = 2, e13.5; n = 6, e14.5; and n = 5, e15.5). Error bars indicate ± SEM.
KTLS cells in e13.5 fetal liver from +/+, +/-, and -/- PU.1 embryos. (A) Cells obtained from representative e13.5 fetal livers were incubated with a blocking antibody to Fc receptor (2.4G2) and then were stained with Sca-1TexasRed, c-KitAPC, LinPE, and Thy-1.1FITC. Cells expressing Sca-1 and c-Kit (left) were analyzed for Thy-1.1 and lineage marker expression (right). (B) The absolute number of KTLS and (C) KLS cells in PU.1+/+ and PU.1-/- fetal livers is indicated (for -/- embryos, n = 2, e12.5; n = 6, e13.5; n = 8, e14.5; and n = 6, e15.5; for +/+ embryos, n = 2, e12.5; n = 2, e13.5; n = 6, e14.5; and n = 5, e15.5). Error bars indicate ± SEM.
c-Kit+Lin- progenitors are reduced in PU.1-/- fetal liver
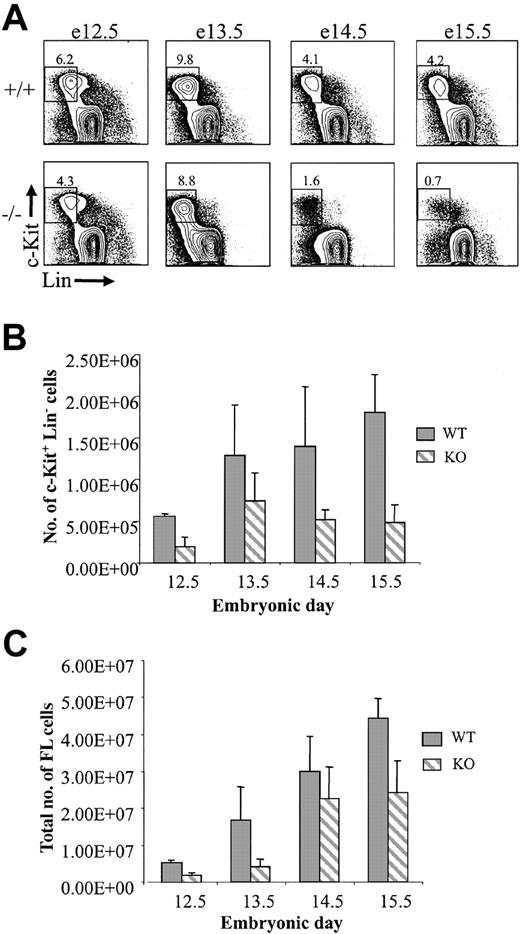
It might be anticipated that the absence or severe reduction in KTLS cells in PU.1-/- embryos between e12.5 and e15.5 would lead to reduced numbers of hematopoietic progenitor cells (c-Kit+Lin-). At early stages following stem cell seeding of the fetal liver at about e11.0, there was a modest but statistically significant (P < .03 at e12.5; and P < .002 at e13.5) difference between the absolute numbers of c-Kit+Lin- cells in wild-type or PU.1-/- embryos (e12.5, 2.5-fold; e13.5, 1.7-fold; Figure 2A-B). Beginning at e14.5, there was a marked reduction of c-Kit+Lin- cells compared with wild-type embryos at the same developmental stage (a 2.7-fold reduction at e14.5; and 3.8-fold at e15.5). Wild-type fetal livers showed a progressive increase in c-Kit+Lin- cells between e12.5 and e15.5, whereas c-Kit+Lin- cells in PU.1-/- embryos decreased after a peak at e13.5. The decrease in the absolute numbers of c-Kit+Lin- cells in PU.1-/- fetal liver between e13.5 and either e14.5 or e15.5 was modest, although statistically significant (P < .003 and P < .006, respectively), and seemed to plateau between e14.5 and e15.5 (P = .427). This indicates that the c-Kit+Lin- population ceases to expand after e13.5 in PU.1-/- embryos but is not entirely lost, which may be due to the half-life of cells within the c-Kit+Lin- population. The reduction in the absolute number of c-Kit+Lin- cells in PU.1-/- fetal livers could not be completely accounted for by a similar reduction in fetal liver cellularity at e14.5 and e15.5. Fetal liver cellularity at e14.5 was reduced 1.3-fold in PU.1-/- embryos compared with wild-type controls and 1.8-fold reduced at e15.5 (Figure 2C).
Progressive decline in c-Kit+Lin- progenitor cells in PU.1-/- embryos. (A) Representative FACS analysis of wild-type and PU.1 knockout embryos between e12.5 and e15.5. The number of embryos representing each genotype is indicated in the legend of Figure 1. Numbers in each plot indicate the percentage of cells within the gated populations. (B) Differences in c-Kit+Lin- cells between +/+ and -/- embryos were statistically significant (P < .05) at all days indicated. (C) Reduction in the total fetal liver cellularity in PU.1-/- embryos. The number of embryos representing each genotype is indicated in the legend of Figure 1. Error bars indicate SEM.
Progressive decline in c-Kit+Lin- progenitor cells in PU.1-/- embryos. (A) Representative FACS analysis of wild-type and PU.1 knockout embryos between e12.5 and e15.5. The number of embryos representing each genotype is indicated in the legend of Figure 1. Numbers in each plot indicate the percentage of cells within the gated populations. (B) Differences in c-Kit+Lin- cells between +/+ and -/- embryos were statistically significant (P < .05) at all days indicated. (C) Reduction in the total fetal liver cellularity in PU.1-/- embryos. The number of embryos representing each genotype is indicated in the legend of Figure 1. Error bars indicate SEM.
c-Kit+Lin-CD34+ and c-Kit+Lin-AA4.1+ progenitors are significantly reduced in e14.5 PU.1-/- embryos
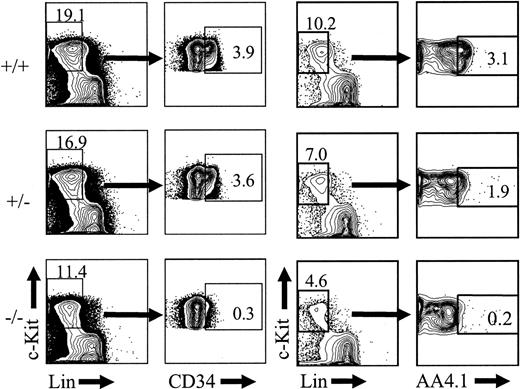
The severe reduction or absence of HSCs of the KTLS phenotype in PU.1-/- embryos could be accounted for by the regulation of the Sca-1 antigen by PU.1 specifically in HSCs. As stated previously, Sca-1 is expressed in the Lin+ fraction of PU.1-/- fetal liver, which indicates that the loss of PU.1 does not result in complete loss of Sca-1 expression in the entire fetal liver (Figure 1A). Loss of HSCs in PU.1-/- embryos would predict that other markers of primitive HSCs, like CD34 and AA4.1, would also be reduced in PU.1-/- embryos. To address this, fetal liver cells isolated at e14.5 were stained with antibodies to lineage markers, c-Kit, and either CD34 or AA4.1. As shown in Figure 3, there were severe reductions in both c-Kit+Lin-CD34+ and c-Kit+Lin-AA4.1+ cells in PU.1-/- embryos. There was a 9.1-fold reduction in the absolute numbers of c-Kit+Lin-CD34+ cells and a 6.4-fold reduction in c-Kit+Lin-AA4.1+ cells when PU.1+/+ embryos (n = 7) were compared with PU.1-/- embryos (n = 3) in 2 independent experiments. The reduction in c-Kit+Lin-AA4.1+ cells is consistent with previous observations showing a 5-fold reduction in AA4.1+Lin- cells in PU.1-/- embryos.23 These data further support the hypothesis that the absence of PU.1 results in the loss or severe reduction of definitive HSCs in PU.1-/- fetal liver, since it would not be expected that PU.1 regulates multiple LT-HSC markers including Sca-1, Thy-1.1, AA4.1, and CD34.
Severe reduction in c-Kit+Lin-CD34+ and c-Kit+Lin-AA4.1+ cells in PU.1-/- embryos. Single-cell suspensions from representative e14.5, PU.1+/+ (n = 7), or PU.1-/- (n = 3) embryos were stained with c-KitAPC, LinPE, and either CD34FITC or AA4.1biotin/avidin-TexasRed and then analyzed by FACS. The percentage of each subset among total fetal liver cells is indicated. The average fold difference in absolute numbers of c-Kit+Lin-CD34+ and c-Kit+Lin-AA4.4+ cells between wild-type and PU.1-/- fetal liver at e14.5 was 9.1-fold and 6.4-fold, respectively.
Severe reduction in c-Kit+Lin-CD34+ and c-Kit+Lin-AA4.1+ cells in PU.1-/- embryos. Single-cell suspensions from representative e14.5, PU.1+/+ (n = 7), or PU.1-/- (n = 3) embryos were stained with c-KitAPC, LinPE, and either CD34FITC or AA4.1biotin/avidin-TexasRed and then analyzed by FACS. The percentage of each subset among total fetal liver cells is indicated. The average fold difference in absolute numbers of c-Kit+Lin-CD34+ and c-Kit+Lin-AA4.4+ cells between wild-type and PU.1-/- fetal liver at e14.5 was 9.1-fold and 6.4-fold, respectively.
CMPs and GMPs are absent from e14.5 PU.1-/- embryos
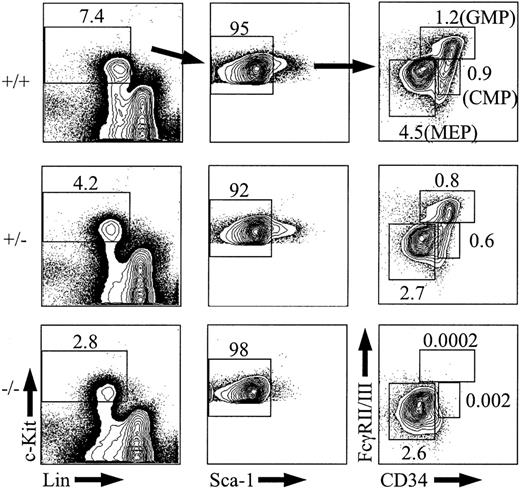
If long-term repopulating HSCs are absent or severely reduced in PU.1 knockout embryos, then it might be expected that myeloid progenitor populations including CMPs, GMPs, and MEPs could also be reduced if their in vivo half-lives were sufficiently short in the absence of self-renewal within these subsets. To analyze CMPs, GMPs, and MEPs, e14.5 fetal liver cells were stained with antibodies to mature lineage antigens (Lin) along with c-Kit, Sca-1, CD34, and FcγRII/III.31 Analysis of PU.1+/+ or PU.1+/- embryos showed the expected frequencies of CMPs, GMPs, and MEPs at e14.5 (Figure 4; n = 2 for +/+ and n = 5 for +/-).31 In PU.1-/- fetal liver, there were no detectable CMPs or GMPs at e14.5 (n = 3). Even though CMPs were completely absent in the PU.1 knockout embryos, the absolute number of MEPs was not changed compared with MEPs from PU.1+/+ or PU.1+/- fetal liver (average absolute number of 275 824 for PU.1+/+ and 226 330 for PU.1-/-). These results suggest that CMPs have all differentiated by e14.5 in the absence of PU.1 or that CMPs are never generated and MEPs are derived from a distinct hematopoietic intermediate that remains uncharacterized in murine fetal liver.
Analysis of CMPs, GMPs, and MEPs from e14.5 fetal liver. Representative e14.5, PU.1+/+ (n = 2), PU.1+/-(n = 5), or PU.1-/- (n = 3) fetal liver samples were stained with c-KitAPC, Sca-1TexasRed, Linbiotin/streptavidinPE-Cy5, FcγII/IIIPE, and CD34FITC and then analyzed by FACS. The percentage of CMPs, GMPs, and MEPs among total fetal liver cells is indicated. The percentages of Sca-1-cells are given as a percentage of the c-Kit+Lin- subset.
Analysis of CMPs, GMPs, and MEPs from e14.5 fetal liver. Representative e14.5, PU.1+/+ (n = 2), PU.1+/-(n = 5), or PU.1-/- (n = 3) fetal liver samples were stained with c-KitAPC, Sca-1TexasRed, Linbiotin/streptavidinPE-Cy5, FcγII/IIIPE, and CD34FITC and then analyzed by FACS. The percentage of CMPs, GMPs, and MEPs among total fetal liver cells is indicated. The percentages of Sca-1-cells are given as a percentage of the c-Kit+Lin- subset.
PU.1-/- embryos have reduced numbers of erythroid progenitors compared with PU.1+/+ or PU.1+/- controls
Previous studies showed that the number of erythroid progenitors (erythroid colony-forming units [CFU-Es]) in PU.1-/- fetal liver at e15.5 were normal.27 Although fetal erythropoiesis was apparently unimpaired in these studies, adult erythropoiesis derived from PU.1-/- cells was absent in the context of chimeric animals. Additional studies showed that at e16.5, erythroid progenitors in the fetal liver failed to respond synergistically to the cytokines EPO and SCF in methylcellulose assays, which suggests that some defect may be present in fetal erythropoiesis in PU.1 knockout animals.28 To address whether erythroid progenitor cells were reduced in PU.1-/- embryos, we compared responsiveness to EPO and SCF at early (e14.5) and late (e16.5) stages in fetal hematopoiesis. Similar to previous results,28 we found a marked inability of e16.5 PU.1-/- progenitors to respond synergistically to EPO and SCF signaling compared with control (PU.1+/-) fetal liver samples when each was plated in duplicate in methylcellulose (Table 1). Control platings showed an 11-fold increase in colony numbers in the presence of both EPO and SCF at e16.5 compared with colony numbers seen with EPO stimulation alone. A small difference (2-fold) in the number of colonies that responded to EPO alone was evident in PU.1-/- and PU.1+/- embryos when an equivalent number of cells (6 × 105) were plated. In contrast to e16.5, mature BFU-Es derived from plating 6 × 105 fetal liver cells at e14.5 showed that EPO-responsive progenitors were reduced approximately 4- to 5-fold (P < .01) compared with PU.1+/+ or PU.1+/- controls (Table 1). EPO+SCF-responsive progenitors in e14.5 PU.1-/- fetal liver were also reduced between 5.2-fold (PU.1+/-) and 9.6-fold (PU.1+/+), indicating an overall reduction in both EPO-responsive and EPO + SCF-responsive progenitor cells. Importantly, PU.1-/- erythroid progenitors at e14.5 were able to respond synergistically to EPO and SCF to a similar degree as controls. This may indicate that the lack of responsiveness to both EPO and SCF at e16.5 could be due to the absence of a specific erythroid progenitor population by this stage in the PU.1-/- embryos and not the failure to respond to proper signaling through the c-Kit receptor.
Characterization of erythroid progenitor responsiveness to EPO and EPO/SCF stimulation
Embryonic day and genotype (no. embryos) . | EPO only, no. colonies . | EPO + SCF, no. colonies . |
|---|---|---|
| e14.5 | ||
| +/+ (2) | 23 ± 3.9 | 490 ± 15.7 |
| +/− (4) | 23 ± 9.0 | 267 ± 23.5 |
| −/− (2) | 5 ± 3.5 | 51 ± 30.4 |
| e16.5 | ||
| +/+ (3) | 4 ± 1.6 | 45 ± 10.4 |
| −/− (2) | 2 ± 1.2 | 3 ± 2.2 |
Embryonic day and genotype (no. embryos) . | EPO only, no. colonies . | EPO + SCF, no. colonies . |
|---|---|---|
| e14.5 | ||
| +/+ (2) | 23 ± 3.9 | 490 ± 15.7 |
| +/− (4) | 23 ± 9.0 | 267 ± 23.5 |
| −/− (2) | 5 ± 3.5 | 51 ± 30.4 |
| e16.5 | ||
| +/+ (3) | 4 ± 1.6 | 45 ± 10.4 |
| −/− (2) | 2 ± 1.2 | 3 ± 2.2 |
PU.1-/- fetal liver cells do not exhibit decreased proliferation or increased sensitivity to cell death
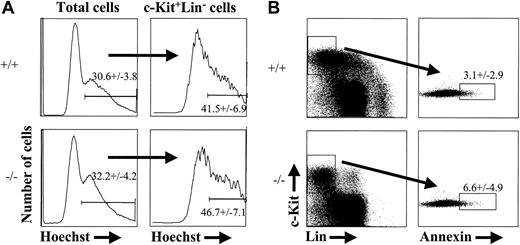
The reduction in progenitor cells characterized as c-Kit+Thy-1.1loLin-Sca-1+, c-Kit+Lin-AA4.1+, or c-Kit+Lin-CD34+, the lack of detectable CMPs/GMPs at e14.5, and the reduction in EPO+SCF-responsive erythroid progenitor cells by e16.5 could be due to the absence or severe reduction in KTLS stem cells. Alternatively, the reduction in progenitor subsets might be caused by decreased proliferation or increased sensitivity to cell death among cells that lack PU.1. To address the latter possibility, we stained e14.5 PU.1-/- and control fetal liver cells with the cell-cycle dye, Hoechst 33342.34 Hoechst staining discriminates between cells that contain 2N or greater than 2N DNA content and therefore defines cells that are in S-G2-M phases of the cell cycle. We chose to analyze e14.5 embryos, given the observation that c-Kit+Lin- progenitor cells began to decline in absolute number by that stage (Figure 2B). As shown in Figure 5A, there was no significant difference in the percentage of cells in S-G2-M phases of the cell cycle between PU.1+/+ and PU.1-/- c-Kit+Lin- progenitor populations at e14.5 (n = 4, PU.1+/+; n = 3, PU.1-/-). To address the issue of whether there might be an increased frequency of c-Kit+Lin- cells undergoing cell death in PU.1-/- embryos, we stained e14.5 fetal liver with annexin V. Annexin V stains phosphatidyl serine that has become exposed on the outer leaflet of the plasma membrane during the initiation of apoptosis.35,36 Again, we observed no significant increase in the frequency of c-Kit+Lin- cells undergoing apoptosis in the absence of PU.1 (Figure 5B; n = 3, PU.1+/+; n = 4, PU.1-/-). These results support the notion that decreased progenitor cell numbers in PU.1 knockout fetal liver are due to the loss of LT-HSC activity that would normally function to maintain the progenitor cell population.
Cell cycle and apoptosis status of PU.1-deficient fetal liver cells. Cells from e14.5 wild-type or PU.1-/- fetal liver were incubated with Hoechst 33342 for 30 minutes at 37°C and then stained for expression of c-Kit and lineage markers. The data shown are representative of 4 wild-type and 3 PU.1-/- embryos analyzed for Hoechst 33342 staining (A) and 3 wild-type and 4 knockout embryos for annexin V (B). Annexin V staining was done using a combination of Annexin VFITCc-KitAPCLinPE. The numbers indicated in both (A) and (B) represent percentages of cells that fall within the gate plus or minus the standard deviation from the mean.
Cell cycle and apoptosis status of PU.1-deficient fetal liver cells. Cells from e14.5 wild-type or PU.1-/- fetal liver were incubated with Hoechst 33342 for 30 minutes at 37°C and then stained for expression of c-Kit and lineage markers. The data shown are representative of 4 wild-type and 3 PU.1-/- embryos analyzed for Hoechst 33342 staining (A) and 3 wild-type and 4 knockout embryos for annexin V (B). Annexin V staining was done using a combination of Annexin VFITCc-KitAPCLinPE. The numbers indicated in both (A) and (B) represent percentages of cells that fall within the gate plus or minus the standard deviation from the mean.
Discussion
Given previous observations that PU.1 knockout embryos die at approximately e18.5 of hematopoietic failure, we sought to determine the status of the LT-HSC population during fetal liver hematopoiesis in the embryo. Contrary to our expectations, LT-HSCs of the KTLS phenotype were absent or severely reduced even at the earliest time point analyzed (e12.5). Between 300 and 500 KTLS cells could be found in the PU.1+/+ fetal liver at this stage (Figure 1). Since definitive erythrocytes and megakaryocytes are present in the knockout embryos,23,24 definitive stem cells from the AGM (or extraembryonic yolk sac) regions would have seeded the fetal liver, although the absolute number of LT-HSCs that initiated definitive hematopoiesis in PU.1-/- embryos is not known due to the difficulty in determining the absolute number of LT-HSCs prior to e12.5. One possibility for the apparent lack of KTLS cells could be due to the regulation of either Sca-1 or Thy-1.1 by PU.1, which would not permit an accurate phenotypic characterization of the stem cell population in the PU.1 knockout embryos. Although we cannot formally rule out this possibility, the observation of a significant percentage of Sca-1+ cells in the PU.1-/- embryos (Figure 1) suggests that PU.1 does not regulate Sca-1 expression in a large fraction of cells. Although expression of the murine Thy-1 promoter is not thought to be regulated by PU.1,37 this analysis alone does not rule out direct or indirect regulation of Thy-1 by PU.1 in LT-HSCs. Further analysis of other markers of primitive fetal liver HSCs, including CD34 and AA4.1, indicates that there is a severe reduction in all cells that express markers of primitive HSCs. Given these results, it would seem highly unlikely that PU.1 regulates multiple fetal liver HSC markers. The inability to competitively reconstitute lethally irradiated mice with PU.1-/- fetal liver cells (Fisher et al28 ; data not shown) further supports the hypothesis that primitive HSCs are not present by e14.5. However, our transplantation experiments do not rule out the possibility that HSCs with a highly altered cell surface marker expression pattern exist in the knockout fetal livers and cannot properly home to the bone marrow after intravenous injection. A previous study has suggested that PU.1 may play a critical role in HSC homing to bone marrow, although homing alone would probably not account for the lack of donor reconstitution in these studies, since large numbers of hematopoietic progenitor cells (1 × 105 to 2 × 105 AA4.1+ cells) were injected and the homing efficiency was reduced to just 10% to 45% of wild-type fetal liver cells.28 These results predict that a sufficient number of HSCs for long-term multilineage reconstitution would have properly homed to bone marrow.
Asecond possible explanation for the absence of KTLS cells in PU.1 knockout embryos is that PU.1 might be required for the maintenance or expansion of definitive HSCs in the fetal liver. In this scenario, definitive HSCs from the AGM/yolk sac regions properly seed the fetal liver and rapidly differentiate into the pool of progenitor cells that initially expand and generate the definitive lineages present in the knockout embryos. In the absence of self-renewal in the long-term self-renewing HSC compartment, exhaustion of hematopoiesis occurs as the progenitor population becomes depleted during further differentiation. Evidence that argues for this possibility is the reduction in progenitor cells characterized as c-Kit+Lin-Sca-1+, c-Kit+Lin-AA4.1+, and c-Kit+Lin-CD34+, the absence of both CMPs and GMPs by e14.5, and the dramatic reduction of erythroid progenitor activity in the PU.1-/- embryos at both e14.5 and e16.5 (Figures 2, 3, 4; Table 1). The absence of KTLS cells in PU.1-/- fetal liver would also be consistent with observations made from PU.1-/- chimeric studies, where a contribution to erythropoiesis could be detected from PU.1-/- ES cells in e16.5 fetal chimeras but not in adult chimeric animals.27 It would not be expected that cells of all progenitor phenotypes be completely absent due to the loss of KTLS stem cells, since the half-lives of these subsets in vivo are presently unknown.
Curiously, we observed normal numbers of MEPs at e14.5 in the PU.1-/- fetal livers. This may suggest that fetal liver MEPs are not entirely derived from CMPs or that the loss of PU.1 activates/permits an alternative pathway that is equally efficient at generating MEPs. Previous studies have shown that the loss of PU.1 results in the severe reduction in expression of the Fc receptor for IgG (CD16/CD32), so it is possible that CMPs exist but cannot be characterized by flow cytometry in the PU.1 knockout embryos.30,38 Although this is a formal possibility, the lack of all CD34+ cells within the c-Kit+Lin-Sca-1- progenitor subset would further support the absence of CMPs. Even though the absolute numbers of MEPs were normal at e14.5, we noted reduced BFU-Es between e14.5 and e16.5. One explanation for this potential inconsistency would be that the MEP subset, which represents 2% to 3% of total fetal liver cells,31 is not a quantitative measure of the cell that actually reads out as a BFU-E colony in an in vitro assay. The MEP subset has an in vitro cloning efficiency of approximately 75% and only a small percentage of colonies (about 10%) give an erythroid-only readout, with the other colonies being megakaryocyte or megakaryocyte/erythrocyte colonies.31 Therefore, the reduction of BFU-Es might not be apparent by FACS characterization of the MEP subset. A recent study by Fisher et al39 using the same PU.1 knockout animals also notes a reduction in colonies that respond to EPO alone or to EPO + SCF from e16.5 PU.1-/- fetal liver compared with platings of wild-type fetal liver cells, although they interpret their findings as a loss of synergy between EPO and other cytokine signaling pathways and not the reduction or loss of a progenitor subset that responds to multiple cytokine stimulatory signals.
If LT-HSCs are lost from PU.1-deficient embryos at very early times following stem cell seeding of the fetal liver, then what might account for the ability to rescue multilineage developmental potential (B-lymphoid and granulocytic development) under defined in vitro culture conditions?30,40,41 Culture of PU.1-/- cells from e14.5 fetal liver30 or from neonatal liver40 in the cytokine IL-3 alone, or in IL-6 plus SCF, allowed for rescue of granulocytic development in methylcellulose assays. Furthermore, retroviral transduction of the IL-7 receptor alpha chain into cytokine-stimulated PU.1-/- fetal liver cells isolated from e14.5 embryos rescued early B-lineage developmental potential on the S17 stromal cell line in the presence of IL-7 (DeKoter et al,41 Sheetal Purohit and C.A.K., unpublished observations, June 2002). These results indicate that progenitor cells capable of lymphoid- and myeloidlineage development are present in e14.5 fetal livers deficient in PU.1 and/or that cytokine signaling through the IL-7 receptor, the IL-3 receptor, or the combined activity of IL-6 and SCF can reprogram multipotentiality in an otherwise developmentally restricted PU.1-/- progenitor cell. Even though cytokine signaling can rescue cells that were otherwise blocked in their developmental potential in the absence of PU.1, knockout cells rescued by retroviral expression of PU.1 fail to reconstitute lethally irradiated mice in vivo (data not shown). These results further support the hypothesis that long-term self-renewing HSCs are absent or severely reduced in PU.1-/- fetal liver.
An interpretation of the data that would be consistent with the loss of KTLS cells and LT-HSC activity in PU.1-/- fetal liver would be that multipotential cells with very limited or no self-renewal potential are present at e14.5 and it is these cells that are being rescued by cytokine signaling in vitro. It is also formally possible that there are other LT-HSC cell surface phenotypes that may reflect unique stages in stem cell development or phenotypic heterogeneity in the stem cell compartment. In support of the latter possibility, it was noted that a small but significant fraction of long-term repopulating HSC activity exists in the Thy-1.1- portion of fetal liver.13 In that study, 4 of 13 animals reconstituted with Thy-1.1- cells were long-term multilineage repopulated with donor cells. In our own hands, we have also observed LT-HSC repopulating activity in the c-Kit+Thy-1.1-Lin-Sca-1+ fraction of wild-type fetal liver when 120 cells were coinjected into irradiated recipient mice with 2 × 105 recipient-type bone marrow cells (data not shown). As seen in Figure 1A, c-Kit+Thy-1.1-Lin-Sca-1+ cells are present in PU.1-/- fetal livers, albeit at a reduced frequency compared with PU.1+/+ or PU.1+/- controls.
As noted earlier in this section, there are a number of non-mutually exclusive explanations for the hematopoietic failure observed during definitive hematopoiesis in PU.1-deficient fetal liver. Understanding the nature of the lethal hematopoietic phenotype is further complicated by other phenotypes associated with the loss of PU.1 activity, including defects in osteoclast development and proper bone formation, that could affect the hematopoietic microenvironment in the adult and stem cell homing abnormalities, which can best be attributed to altered homing receptor expression in the PU.1 knockout embryos.28,42 The absence of stem cells of the KTLS phenotype from e12.5 to e15.5 and the progressive decline in hematopoietic progenitor activity during embryonic development support the supposition that PU.1 may play an important regulatory role in maintaining the LT-HSC phenotype at the initiation of definitive hematopoiesis in the mouse.
Prepublished online as Blood First Edition Paper, August 24, 2004; DOI 10.1182/blood-2002-08-2425.
Supported by a Howard Hughes Medical Institute (HHMI) faculty development award (C.A.K.; grant no. 53000281). H.-G.K. is supported by National Institutes of Health (NIH) grant no. RO1DK54766. C.G.d.G. was supported by the Molecular and Viral Oncology Training Grant (5T32CA09467). C.S.S. was supported by the Immunologic Diseases and Basic Immunology Training Grant (T32 AI07051).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Dr John Kearney for use of the FACS Calibur and dissecting microscope and Dr Delicia Carey in Comprehensive Cancer Center Biostatistics Division for the statistical analyses. We also wish to thank Dr Harinder Singh (University of Chicago), Dr Robert Fisher (University of Florida), and members of the Klug laboratory for critical reading of the manuscript and helpful discussions. We thank Dr Max Cooper and members of the Division of Developmental and Clinical Immunology for their support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal