Abstract
Tumor suppressor p53 plays an important role in regulating cell cycle progression and apoptosis. Here we applied RNA interference to study the role of p53 in human hematopoietic development in vivo. An siRNA construct specifically targeting the human tumor-suppressor gene p53 was introduced into human CD34+ progenitor cells by lentivirus-mediated gene transfer, which resulted in more than 95% knockdown of p53. We adapted the human-SCID mouse model to optimize the development of hematopoietic cells, particularly of T cells. This was achieved by the intraperitoneal injection of CD34+ precursor cells into newborn Rag2-/- γc-/- mice that lack T, B, and NK cells. Robust development of T cells was observed in these mice, with peripheral T-cell repopulation 8 weeks after injection of the precursor cells. Other lymphocyte and myeloid subsets also developed in these mice. Injecting p53 siRNA-transduced CD34+ cells resulted in stable expression and down-modulation of p53 in the mature T-cell offspring. Inactivating p53 did not affect the development of CD34+ cells into various mature leukocyte subsets, including T cells, but it conferred resistance to γ-irradiation and other p53-dependent apoptotic stimuli to the T cells. (Blood. 2004;104:3886-3893)
Introduction
The tumor suppressor p53 plays an important role in regulating the cell cycle and apoptosis in response to DNA damage caused by irradiation or exposure to genotoxic mediators. In addition, p53 can mediate several cellular responses, including cell cycle arrest, senescence, differentiation, and apoptosis, depending on the cell type and the microenvironment.1 Although mutations occur in the gene encoding p53 in human cancers, including tumors of hematopoietic origin, its function in normal human hematopoietic development remains largely unexplored.
Recently, we obtained evidence that p53 plays a role in regulating the replicative lifespan of mature human T cells in vitro through the suppression of human telomerase reverse transcriptase (hTERT) (R.G., E.W., R. Beijersbergen, and H.S., manuscript in preparation). Telomeres are DNA repeats at the distal ends of the chromosomes that protect against chromosome end-to-end fusion.2 Telomeres are shortened at each cell division, and cells with critically short telomeres undergo cell cycle arrest and become senescent.3-5 hTERT, which prevents telomere shortening, is transiently up-regulated in T cells on stimulation through the T-cell receptor (TCR),4,5 and expression of a dominant-negative mutant of hTERT significantly decreased the lifespan of CD4+ and CD8+ T cells,5 indicating that hTERT plays a regulatory role in the lifespan of human T cells. Recently, we observed that the down-regulation of p53 by RNA interference (RNAi) extends the lifespan of mature human T cells and neutralizes the inhibition by dominant-negative hTERT, indicating that p53 regulates hTERT expression in primary human T cells. Given the function of p53 in mature human T cells, we asked whether the p53 loss would affect the homeostatic proliferation of T cells. Mice deficient in p53 did not show obvious defects in T-cell homeostasis. However, T cells from inbred mouse strains have longer telomeres than T cells from humans, resulting in a delay in the onset of replicative senescence and in an extended lifespan of these murine T cells. It is, therefore, of interest to test the effect of p53 inactivation on human T-cell homeostasis.
In addition, we examined the role of p53 in thymic T-cell development because studies in the mouse have revealed that p53 is induced after the initiation of TCR rearrangement6 and that it plays an important role in early T-cell development, specifically in pre-T-cell receptor signaling.7 Results obtained in mice cannot always be extrapolated to humans, and the role of p53 in human T-cell development in the thymus is still unknown. To inactivate p53 in developing T cells, we used RNA interference (RNAi), which has emerged as a powerful method to silence target genes. This method makes use of the property of short double-stranded RNA to target mRNA for degradation.8 The recent demonstration that stable expression of short-interfering RNA (siRNA) can be achieved by retrovirus-mediated9 or lentivirus-mediated10 gene transfer allows for silencing of genes in primary mammalian cells.
We used an alymphoid mouse strain, RAG2-/- γc-/- double knockout mice,11 to monitor the development of p53 siRNA-transduced human stem cells into T cells and other lymphoid cells in vivo. Using this model, we investigated the consequences of knockdown of p53 on human hematopoietic development. We injected p53 siRNA- and control-transduced CD34+ cells intraperitoneally into newborn RAG2-/- γc-/- mice and observed a robust development of human mononuclear cells, including T cells in animals injected with p53 siRNA- and control-transduced CD34+ cells. In the mature T-cell progeny that developed from the p53 siRNA-transduced CD34+ cells, p53 remained inactivated. We did not observe obvious effects of p53 knockdown on the development of human T cells or other hematopoietic cells in comparison with untransduced or control-transduced CD34+ cells. In contrast, we noticed that the p53 siRNA-expressing T cells were more resistant to p53-dependent apoptotic stimuli than untransduced or control-transduced T cells. Besides giving specific information about the possible role of p53 in T-cell development and homeostasis, these experiments present a versatile, easy-to-manipulate model in which the effects of gene knockdown on human hematopoietic development in vivo can be examined.
Materials and methods
Mice
H-2d RAG2-/- mice (kindly provided by Dr Antonius Rolink and Dr Shunichi Takeda, Basel Institute for Immunology, Switzerland12 ) were crossed with interleukin-2 receptor (IL-2R) γc-/- mice13 to obtain H-2d RAG2-/- IL-2Rγc-/- mice (further referred to as RAG2-/- γc-/- mice).11,14 These mice are immunodeficient because they show a total absence of T, B, and NK lymphocytes.13 Mice were bred and maintained in isolators and were fed autoclaved food and water. All manipulations were performed under laminar flow.
Fetal liver cell preparation
Human fetal liver was obtained from elective abortions. Gestational ages ranged from 14 to 20 weeks. The use of this tissue was approved by the medical ethical committees of our institutions and was contingent on informed consent. Human fetal liver cells were isolated by gentle disruption of the tissue, followed by density gradient centrifugation over Ficoll-Hypaque (Lymphoprep; Nycomed Pharma, Oslo, Norway). Single-cell suspensions were prepared by mincing tissues and pressing them through a stainless steel mesh. Large aggregates were removed, and cells were washed with medium before CD34+ cells were isolated.
Transplantation of human CD34+ cells into RAG2-/- γc-/- mice and evaluation of human cell engraftment
Enrichment of CD34+ cells (more than 98% pure, as assessed by flow cytometry; data not shown) was performed using the CD34 Progenitor Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). CD34+ cells (0.2-2 million cells) were transplanted intraperitoneally into sublethally irradiated (3.50 Gy) newborn RAG2-/- γc-/- mice (younger than 1 week of age). Peripheral blood (PB) was collected from the tail vein every 3 to 4 weeks after transplantation to determine the kinetics of human cell engraftment. Mice were killed at different time points after transplantation, and PB, liver, lung, spleen, bone marrow (BM), and thymus were evaluated for the presence of human cells. Total body irradiation (TBI) was required because nonirradiated mice do not show repopulation with human cells (data not shown). Mononuclear cells were isolated by density gradient centrifugation over Ficoll-Hypaque and were stained with anti-human CD45 (Becton Dickinson, San Jose, CA) and other markers. Expression of these markers was measured on a FACSCalibur (Becton Dickinson) and analyzed with the FCS express program (Denovosoftware, Ontario, Canada). The grafted human mononuclear population was defined based on forward and side scatter parameters, and the percentage of positive cells for a given marker was determined for cells falling within the leukocyte and CD45+ gates. There were no cells present that reacted with the anti-human CD45 in RAG2-/- γc-/- mice that did not undergo transplantation. Specific subsets of human cells were quantified by staining with anti-human specific monoclonal antibodies (mAbs): CD11c, CD19, CD34, and CD45RA (Coulter-Immunotech, Luminy, France); CD3, CD4, CD8, CD10, CD14, CD16, CD20, CD56, CD83, and CD123 (Becton Dickinson); CD38 and CD45RO (DAKO, Glostrup, Denmark), and BDCA-2 (Miltenyi Biotec), conjugated with different fluorochromes.
Lentivirus production
Replication-defective self-inactivating HIV vectors were produced by transient transfection of 293T cells using FUGENE (Roche, Nutley, NJ) and 3 different plasmids. The plasmids used were the VSV-G envelope coding plasmid pMD.G; the packaging plasmid pCMVDR8.91, designed to provide the Gag, Pol, Tat, and Rev proteins to produce the virus particle; and the transfer vector pTRIPΔU3-E1α.15 pTRIPΔU3-E1α has been modified to include the siRNA cassette from the pSUPER vector containing the human p53 targeting sequence 5′GACTCCAGTGGTAATCTAC, described by Brummelkamp et al,9 and it carries the enhanced GFP gene driven by the elongation factor-1α promoter. Twenty hours after transfection, medium was replaced by Yssel medium,16 and 2 samples of virus were collected at 24 and 48 hours. The virus-containing supernatants were centrifuged for 10 minutes at 1800 rpm to remove cells and then were passed through a 0.22-mm filter and kept at -80°C until use.
Transduction protocol
Transduction of CD34+ human cells was performed by 1 cycle of overnight exposure to viral supernatant on retronectin (Takara Shuzo, Otsu, Japan)-coated 24-well plates in the absence of cytokines. The next day, cells were washed and injected intraperitoneally into mice or kept in culture in Yssel medium in the presence of thrombopoietin, stem cell factor, and IL-7 at 10 ng/mL each (all from Peprotech). The efficiency of transduction was estimated by determining the percentage of green fluorescence protein (GFP)-positive cells, 2 to 3 days later, by flow cytometry.
Western blot analysis
Total cellular extracts were prepared by lysing the cells in RIPA buffer for 30 minutes on ice. Equivalent amounts of proteins, determined by the Bio-Rad Protein Assay (Bio-Rad, Munich, Germany), were loaded onto 10% sodium dodecyl sulfate (SDS) gels. p53 protein was detected using the monoclonal antibody DO-1 (Santa Cruz Biotechnology, Santa Cruz, CA) and β-actin as a loading control using the polyclonal antibody I-19 (Santa Cruz Biotechnology).
Human T-cell culture and analysis of the TCR repertoire
The expansion of human T cells from blood and spleen of RAG2-/- γc-/- mice, reconstituted with human transduced stem cells, was performed by stimulation with a feeder mix consisting of irradiated allogeneic human peripheral blood leukocytes (PBLs) from 2 different donors and an irradiated Epstein-Barr virus (EBV)-transformed B-cell line (JY), phytohemagglutinin (PHA; Gibco, Grand Island, NY), and recombinant human IL-2 (rhIL-2; Roche, Nutley, NJ), as described.17 All the cultures involving human cells were grown in Yssel medium. Analysis of the repertoire was performed as described in Kostense et al.18
Detection of apoptotic cells
Cellular viability was analyzed by flow cytometry, before or 24 hours after gamma irradiation (30 Gy) or after treatment with staurosporine (1 μM) or fludarabine (5 μM). Cells were harvested and washed in ice-cold HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (10 mM HEPES, 150 mM KCl, 1 mM MgCl2, and 1.3 mM CaCl2, pH 7.4) and then were incubated with allophycocyanin (APC)-labeled Annexin V (Becton Dickinson) for 20 minutes. Just before analysis of the samples by flow cytometry, propidium iodide (PI; Sigma, St Louis, MO) was added (final concentration, 5 μg/mL). Viable cells were defined as negative for Annexin V and PI staining.
Results
Introduction of siRNA into human CD34+ cells by lentivirus-mediated gene transfer
Lentiviruses have 2 key advantages over other gene delivery systems: they can infect noncycling cells, and they are not silenced during development. Several groups have described the use of small nuclear RNA promoters (H19 and U619 ) for the expression of RNAi in mammalian cells. The H1 promoter was used to drive expression of siRNA, designed to target the human p53 protein, and lentiviral vectors expressing the siRNA cassette were prepared (Figure 1A). A highly enriched population of CD34+ cells was isolated from human fetal liver and was subsequently transduced with the siRNA/GFP-expressing lentiviral vectors. Transduction efficiencies routinely ranged between 20% and 50%, as indicated by the levels of GFP expression determined by flow cytometry. No obvious effect on cell growth or survival of the transduced cells was observed in vitro after culture for 3 weeks in the presence of cytokines, as described in “Materials and methods.” The proportion of CD34+-derived cells expressing GFP remained comparable to that of the initial population (data not shown).
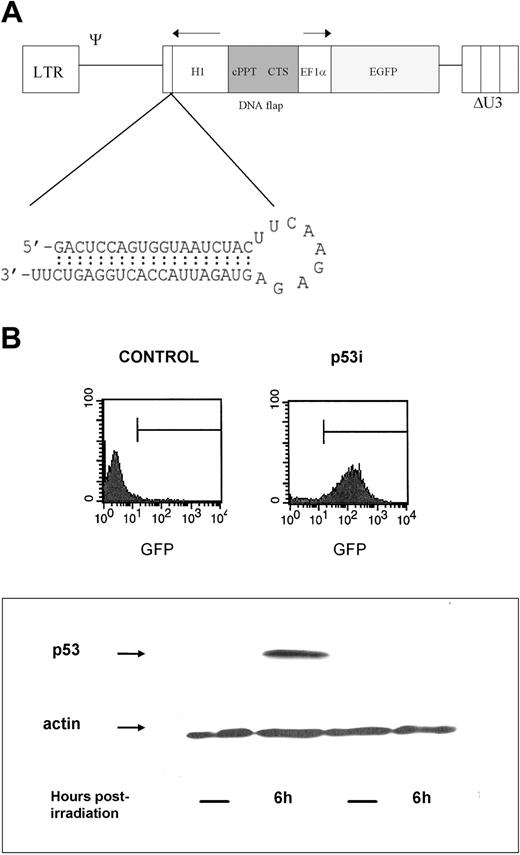
p53 knockdown in human hematopoietic precursors. (A) Schematic representation of the lentiviral RNA interference vector pTRIPΔU3-EF1α p53. The predicted short hairpin RNA targeting the human p53 is shown. (B) Human hematopoietic progenitors (CD34+) were isolated and transduced with the lentiviral vector pTRIPΔU3-EF1α p53 and, after 1 week in culture with cytokines, were sorted based on the expression of GFP. Cells were then γ-irradiated. Six hours later, whole-cell extracts were prepared, separated using 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and immunoblotted to detect human p53. The blot was reprobed with an antibody against β-actin as a loading control.
p53 knockdown in human hematopoietic precursors. (A) Schematic representation of the lentiviral RNA interference vector pTRIPΔU3-EF1α p53. The predicted short hairpin RNA targeting the human p53 is shown. (B) Human hematopoietic progenitors (CD34+) were isolated and transduced with the lentiviral vector pTRIPΔU3-EF1α p53 and, after 1 week in culture with cytokines, were sorted based on the expression of GFP. Cells were then γ-irradiated. Six hours later, whole-cell extracts were prepared, separated using 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and immunoblotted to detect human p53. The blot was reprobed with an antibody against β-actin as a loading control.
To demonstrate that the introduced construct was active and able to knock down the expression of p53 in these cells, we sorted the CD34+ cells after transduction based on their GFP expression. The expression of p53, which is usually low, is up-regulated quickly after exposure of cells to several genotoxic stimuli, including γ-irradiation. Untransduced and control-transduced (not shown) cells responded to irradiation by increasing the levels of p53, whereas the cells expressing the p53 siRNA construct did not (Figure 1B), demonstrating that the construct was able to silence gene expression in hematopoietic stem cells.
Effect of p53 knockdown on T-cell development and homeostasis
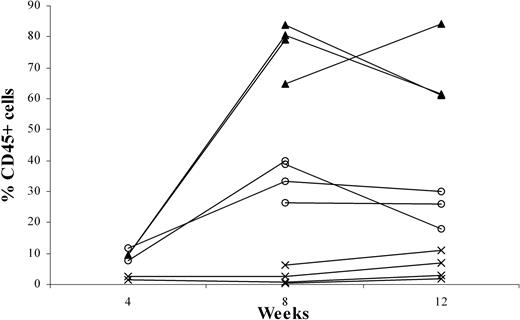
To examine the effects of p53 knockdown on T-cell development, we used the human-SCID mouse model pioneered by Dick and colleagues.20 This model is based on the capacity of these primitive cells (termed SCID-repopulating cells) to repopulate the BM of C.B-17-Prkdcscid (SCID)21 mice and, more recently, NOD/LtSz-Prkdcscid (NOD/SCID)22,23 mice with multilineage lymphoid and myeloid cells. However, for the study of T-cell development, these mice have limitations. T cell development was only occasionally observed, apparently because of the presence of an active innate immune system in the mice. Recently, we reported that CD34+ cells—on intravenous injection into RAG2-/- γc-/- mice, which lack T, B, and NK cells—can develop into B cells and plasmacytoid dendritic cells (pDCs).11 In addition, we observed that T cells develop in these adult mice after intravenous injection of CD34+ cells (results not shown). Although the success rate was 80%, it took an average of 12 to 16 weeks to observe consistent T-cell engraftment in the periphery. Moreover, the thymi of these mice remained small (fewer than 0.8 × 106 human cells). Considering that the thymic microenvironment of the 6- to 8-week-old RAG2-/- γc-/- mice might not be optimal for human T-cell development, we decided to inject human precursors at an earlier time point. Newborn (younger than 1 week), 1-week-old, and 2-week-old mice were injected intraperitoneally with fetal liver-derived CD34+ cells, and PB was analyzed 8 and 12 weeks after injection. Figure 2 shows that engraftment with human cells in the periphery is higher in the youngest animals. Injection of 1-week-old mice also resulted in significant engraftment, but in 2-week-old mice human cells did not develop after intraperitoneal injection of CD34+ cells (Figure 2). Percentages of the CD3+ population in the PB of 1-day-old mice and 1-week-old mice did not differ significantly at 8 (7.7% ± 4.7% and 4.3% ± 2.9%, respectively) and 12 weeks (7.3% ± 2.4% and 7.1% ± 1.5%, respectively). The same was true for the CD19+ populations (87.2% ± 5.4% and 89.3% ± 3.7% at 8 weeks and 86.7% ± 4.5% and 78.3% ± 8.4% at 12 weeks). Thus, the recovery of T cells and B cells was increased in mice injected at 1 day of age compared with those injected at 1 week. In the 2-week-old mice, the percentages of CD45+ cells were too low to discriminate between CD3+ and CD19+ cells.
Intraperitoneally engraftment of newborn RAG2-/- γc-/- mice at 1 day, 1 week, or 2 weeks of age, with CD34+ fetal liver (FL) cells. Three groups each of 4 newborn RAG2-/- γc-/- mice—1 day (▴), 1 week (○), or 2 weeks (×) of age—were intraperitoneally grafted with 0.5 × 106 MACS-selected CD34+ FL cells. At 4, 8, and 12 weeks after engraftment, all mice were bled, and the blood was subjected to fluorescence-activated cell sorter (FACS) analysis to determine the percentage of CD45+ human cells.
Intraperitoneally engraftment of newborn RAG2-/- γc-/- mice at 1 day, 1 week, or 2 weeks of age, with CD34+ fetal liver (FL) cells. Three groups each of 4 newborn RAG2-/- γc-/- mice—1 day (▴), 1 week (○), or 2 weeks (×) of age—were intraperitoneally grafted with 0.5 × 106 MACS-selected CD34+ FL cells. At 4, 8, and 12 weeks after engraftment, all mice were bled, and the blood was subjected to fluorescence-activated cell sorter (FACS) analysis to determine the percentage of CD45+ human cells.
Analysis of 78 mice that were injected intraperitoneally between day 1 and 3 after birth revealed reconstitution with human CD45+ cells (Table 1) in 64 (83%) mice. Thirty-two mice showed more than 50% reconstitution, and the rest showed between 10% and 50% reconstitution. Any mouse with human CD45+ cells in the PB also had T cells in the thymus and other organs.
Summary of results from 12 experiments
Human CD45+ cells in PB, % . | No. mice (%) . |
|---|---|
| Less than 10 | 13 (16) |
| 10-30 | 16 (20) |
| 30-50 | 17 (22) |
| More than 50 | 32 (42) |
Human CD45+ cells in PB, % . | No. mice (%) . |
|---|---|
| Less than 10 | 13 (16) |
| 10-30 | 16 (20) |
| 30-50 | 17 (22) |
| More than 50 | 32 (42) |
Seventy-eight newborn mice (younger than 1 week of age) were intraperitoneally injected with 0.5 to 2.0 × 106 CD34+ fetal liver cells. Mice were bled 6 to 11 weeks after injection, and blood was analyzed using FACS. Percentages of CD45+ cells in the lymphocyte gate are given.
Thymic structure of mice injected intraperitoneally 1 day after birth was examined by confocal microscopy 10 weeks after transplantation. A differentiated thymus was observed with CD1+CD4+ and CD4+CD8+ cells in the cortexlike region, and single-positive CD4+ and CD8+ T cells were observed in the medulla-like region (results not shown).
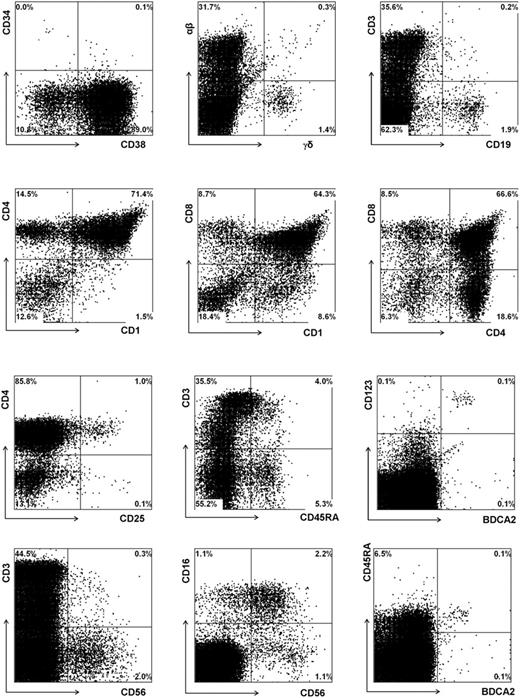
To further validate the newborn mouse model, we first analyzed the T cells in the thymus and various peripheral organs in a large series of mice injected with untransduced CD34+ fetal liver cells. In addition we analyzed these mice for the presence of other leukocyte subsets. Eight to 10 weeks after injection, 2 to 10 million cells could be recovered from the thymus. Extensive flow cytometric analysis (Figure 3) revealed the presence of all T-lineage subsets, including double-positive CD4+CD8+ cells and single-positive CD4+ and CD8+ T cells. Furthermore, a subset of the CD3+high cells expressed CD45RA+. This population represents T cells just before emigration to the periphery.24 Interestingly, CD25 was expressed on a subset of CD4+ T cells that were likely regulatory T (Treg) cells previously described by Stephens et al.25 The observation that these CD25+CD4+ cells are also highly positive for glucocorticoid-induced tumor necrosis factor receptor family-related gene (GITR) and cytoplasmic CTLA-4 (results not shown) support the notion that these cells represent Treg cells. In addition to TCR-αβ+ cells and CD3+CD56+ T cells, the thymus also contained substantial numbers of CD3-CD56+ NK cells, with approximately half expressing CD16. The percentage of NK cells (2%) was much higher than what is normally found in the thymi of children (less than 0.1%). It is possible that the mouse thymus environment favors the generation of NK cells. Alternatively, the much higher proportion of NK cells may be a consequence of a lower expansion rate of TCR-αβ+ cells than occurs in a normal human thymic microenvironment. We also observed relatively high proportions of TCR-γδ+ cells (1.5% vs less than 0.1% in a normal thymus) and B cells (2.2% vs less than 0.1% in a normal thymus), and these high percentages may be also a consequence of a low expansion rate of TCR-αβ+ cells. In contrast to the increased percentages of NK, B, and TCR-γδ cells, that of BDCA2+CD123high pDCs was within the normal range (0.1%).
Repopulation of thymus of RAG2-/- γc-/- mice, 11 weeks after intraperitoneal injection with CD34+ hematopoietic stem cells from fetal liver. FACS profiles of thymi. Thymocytes were stained with antibodies against stem cell markers CD34 and CD38, T-cell markers TCR αβ and TCR γδ, CD1, CD3, CD4, CD8, CD25, CD45RA, NK cell markers CD16 and CD56, plasmacytoid DC markers CD123 and BDCA2, and B-cell marker CD19. Cells in the analysis of CD16 and CD56 were gated not only on CD45+ cells but also on CD3- cells.
Repopulation of thymus of RAG2-/- γc-/- mice, 11 weeks after intraperitoneal injection with CD34+ hematopoietic stem cells from fetal liver. FACS profiles of thymi. Thymocytes were stained with antibodies against stem cell markers CD34 and CD38, T-cell markers TCR αβ and TCR γδ, CD1, CD3, CD4, CD8, CD25, CD45RA, NK cell markers CD16 and CD56, plasmacytoid DC markers CD123 and BDCA2, and B-cell marker CD19. Cells in the analysis of CD16 and CD56 were gated not only on CD45+ cells but also on CD3- cells.
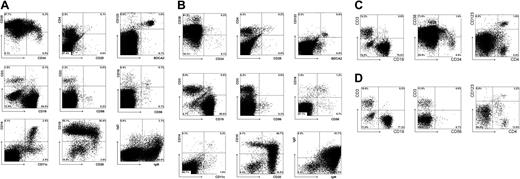
The T cells that develop in the thymus migrate to multiple anatomic locations. Variable percentages of T cells were found not only in the thymus but also in BM, spleen, liver, and lungs (Figure 4). The BM had a low proportion (1%) of T cells, whereas in the lung the percentage was 11%. The liver and spleen contained comparable percentages of T cells. The CD4/CD8 ratios (3:1 to 4:1) were similar in all organs and were within the range normally observed in humans. Interestingly, in the spleen and BM, a population of CD3+CD4+CD25+ cells could be detected that presumably contained Treg cells and recently activated CD4+ T cells. Our results show that intraperitoneal injection of CD34+ cells into newborn mice results in a relatively rapid reconstitution of human T cells in most mice.
Repopulation of BM, spleen, liver, and lung of RAG2-/- γc-/- mice 11 weeks after intraperitoneal injection with CD34+ hematopoietic stem cells from fetal liver. (A) FACS profiles of BM. BM cells were stained with antibodies against stem cell markers CD34 and CD38; T-cell markers CD3, CD4, CD25; NK cell markers CD16 and CD56; plasmacytoid DC markers CD123 and BDCA2; B cell markers CD11, CD19, CD20; IgM and IgD; and myeloid cells markers CD11c and CD14. Cells in the analysis of IgM and IgD are not only gated on CD45+ cells but also on CD19+ cells. (B) FACS profiles of spleen. Spleen cells were stained with the same antibodies as BM cells. (C) FACS profiles of liver. Liver cells were stained with antibodies against stem cell markers CD34 and CD38, T cell marker CD3, plasmacytoid DC markers CD123 and CD4, and B cell marker CD19. (D) FACS profiles of lung. Lung cells were stained with antibodies against T-cell marker CD3, plasmacytoid DC markers CD123 and CD4, and B-cell marker CD19.
Repopulation of BM, spleen, liver, and lung of RAG2-/- γc-/- mice 11 weeks after intraperitoneal injection with CD34+ hematopoietic stem cells from fetal liver. (A) FACS profiles of BM. BM cells were stained with antibodies against stem cell markers CD34 and CD38; T-cell markers CD3, CD4, CD25; NK cell markers CD16 and CD56; plasmacytoid DC markers CD123 and BDCA2; B cell markers CD11, CD19, CD20; IgM and IgD; and myeloid cells markers CD11c and CD14. Cells in the analysis of IgM and IgD are not only gated on CD45+ cells but also on CD19+ cells. (B) FACS profiles of spleen. Spleen cells were stained with the same antibodies as BM cells. (C) FACS profiles of liver. Liver cells were stained with antibodies against stem cell markers CD34 and CD38, T cell marker CD3, plasmacytoid DC markers CD123 and CD4, and B cell marker CD19. (D) FACS profiles of lung. Lung cells were stained with antibodies against T-cell marker CD3, plasmacytoid DC markers CD123 and CD4, and B-cell marker CD19.
As has been published by others using adult NOD/SCID mice26 and NOD/SCIDγc-/- mice,27 CD34+ cells developed into multiple lineages when injected into newborn RAG2-/- γc-/- mice. B cells were the most dominant cell population that developed in the PB and in various organs in mice injected 1 day after birth. The highest percentage of CD19+ B cells was found in the BM (Figure 4A). Not unexpectedly, most B cells in the spleen (Figure 4B) were CD10+CD20+ cells and coexpressed immunoglobulin M (IgM) and IgD. In the BM, most B cells were CD20-CD10+, suggesting that the mouse BM is the site for human B-cell development. Inspection of the BM (Figure 4A) revealed the presence of CD34+ cells, a small percentage of which expressed only low levels of CD38, suggesting that some primitive CD34+CD38dim progenitor cells remain in their undifferentiated state.
As described earlier,11 significant numbers of pDCs were found in all organs; pDCs were most clearly present in the BM (Figure 4A) and liver (Figure 4C) but were also present in the lungs (Figure 4D). NK cells could also be detected in PB and various organs, but in general the percentages were much lower than in the thymus. CD11c+ and CD14+ monocytes were observed in the organs (Figure 4), indicating that not only human lymphoid but also human myeloid development took place in these mice.
We then injected a series of mice with CD34+ fetal liver cells transduced with p53 siRNA or with an empty vector. Table 2 shows that the percentages of thymocytes in GFP+ and GFP- cells were similar in mice injected with p53 siRNA and with control-transduced CD34+ cells. Similarly, we could not detect significant differences in the peripheries of these animals when liver or spleen cells were analyzed. Thus, we conclude that inactivating p53 does not lead to a survival advantage of p53 siRNA-expressing peripheral T cells.
T-cell development
. | . | . | . | CD3+, % . | . | |
|---|---|---|---|---|---|---|
| Mouse . | Construct . | CD45+, % . | GFP+ in CD45+, % . | GFP+ . | GFP− . | |
| 1 | p53i | 80.3 | 6.6 | 25.2 | 22.1 | |
| 2 | p53i | 60.0 | 66.0 | 97.2 | 94.8 | |
| 3 | p53i | 99.4 | 94.2 | 87.7 | 96.3 | |
| 4 | p53i | 97.5 | 60.6 | 99.4 | 94.0 | |
| 5 | p53i | 97.4 | 25.1 | 56.7 | 33.2 | |
| 6 | p53i | 97.6 | 85.9 | 68.3 | 85.8 | |
| 7 | p53i | 98.5 | 29.0 | 98.7 | 53.6 | |
| 8 | Control | 45.2 | 5.2 | 25.9 | 32.4 | |
| 9 | Control | 63.0 | 36.5 | 97.4 | 95.5 | |
| 10 | Control | 99.0 | 99.0 | 95.7 | 76.0 | |
. | . | . | . | CD3+, % . | . | |
|---|---|---|---|---|---|---|
| Mouse . | Construct . | CD45+, % . | GFP+ in CD45+, % . | GFP+ . | GFP− . | |
| 1 | p53i | 80.3 | 6.6 | 25.2 | 22.1 | |
| 2 | p53i | 60.0 | 66.0 | 97.2 | 94.8 | |
| 3 | p53i | 99.4 | 94.2 | 87.7 | 96.3 | |
| 4 | p53i | 97.5 | 60.6 | 99.4 | 94.0 | |
| 5 | p53i | 97.4 | 25.1 | 56.7 | 33.2 | |
| 6 | p53i | 97.6 | 85.9 | 68.3 | 85.8 | |
| 7 | p53i | 98.5 | 29.0 | 98.7 | 53.6 | |
| 8 | Control | 45.2 | 5.2 | 25.9 | 32.4 | |
| 9 | Control | 63.0 | 36.5 | 97.4 | 95.5 | |
| 10 | Control | 99.0 | 99.0 | 95.7 | 76.0 | |
Newborn mice were injected with CD34+ fetal liver cells transduced with p53i siRNA-GFP or control-GFP constructs. Mice were killed, and thymi were analyzed using FACS. The percentages of CD3+ cells in the GFP+ and GFP− gates are given. Percentages of CD45+ cells and the proportion of GFP+ cells in this gate are also indicated.
Inspection of the thymi of mice injected with p53 siRNA and control-transduced cells revealed the presence of GFP+ cells in the CD4CD8 double-negative, double-positive, and single-positive T-cell compartments. However, we did not observe differences in the phenotypes of any of the subsets of T and non-T cells in the thymus (Figure 5A) when gating on p53 siRNA GFP+ cells, untransduced cells (GFP-), or control GFP+ cells (not shown), nor did we observe differences in the presence of T cells in any of the p53 siRNA GFP+ or untransduced cells in the spleen.
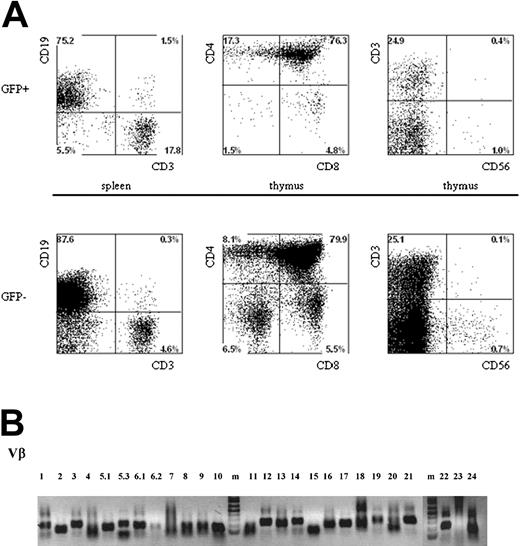
Multilineage presence of GFP+-expressing cells in mice that underwent transplantation with lentivirus-transduced human hematopoietic progenitors. Newborn RAG2-/- γc-/- mice were intraperitoneally injected with human CD34+ hematopoietic stem cells isolated from fetal liver and transduced with the pTRIPΔU3-EF1α p53RNAi lentivirus. (A) Mononuclear cell suspensions from the thymi and spleens of these mice were stained with various human-specific monoclonal antibodies and analyzed by flow cytometry. Cells positive for human CD45 were gated and further analyzed, comparing the GFP+ and GFP- populations. Examples of T-cell development in thymi and spleens are given. Cells were stained with antibodies directed to CD3, CD4, CD8, and CD56 and analyzed by FACS. (B) Vβ family representation from p53i GFP+-expressing T cells in mice that underwent transplantation with lentivirus-transduced human hematopoietic progenitors. RNA was isolated from T cells, and the presence of different Vβ subsets was determined using RT-PCR.
Multilineage presence of GFP+-expressing cells in mice that underwent transplantation with lentivirus-transduced human hematopoietic progenitors. Newborn RAG2-/- γc-/- mice were intraperitoneally injected with human CD34+ hematopoietic stem cells isolated from fetal liver and transduced with the pTRIPΔU3-EF1α p53RNAi lentivirus. (A) Mononuclear cell suspensions from the thymi and spleens of these mice were stained with various human-specific monoclonal antibodies and analyzed by flow cytometry. Cells positive for human CD45 were gated and further analyzed, comparing the GFP+ and GFP- populations. Examples of T-cell development in thymi and spleens are given. Cells were stained with antibodies directed to CD3, CD4, CD8, and CD56 and analyzed by FACS. (B) Vβ family representation from p53i GFP+-expressing T cells in mice that underwent transplantation with lentivirus-transduced human hematopoietic progenitors. RNA was isolated from T cells, and the presence of different Vβ subsets was determined using RT-PCR.
Given the important role of p53 in the control of cell proliferation and the induction of apoptosis in response to DNA damage, we wanted to determine whether the reduced levels of p53 could have an effect on the composition of the TCR repertoire. We investigated TCR diversity in T cells isolated from mice injected with CD34+ cells transduced with the p53i construct. Using reverse transcription-polymerase chain reaction (RT-PCR) analysis, we could establish that all the Vβ families were represented in the repertoire (Figure 5B). Furthermore, detailed analysis of the CDR3 region18 clearly demonstrated that the peripheral T cells present in these mice are polyclonal (data not shown), indicating that the down-regulation of p53 during T-cell development does not result in the prevalence of some clones.
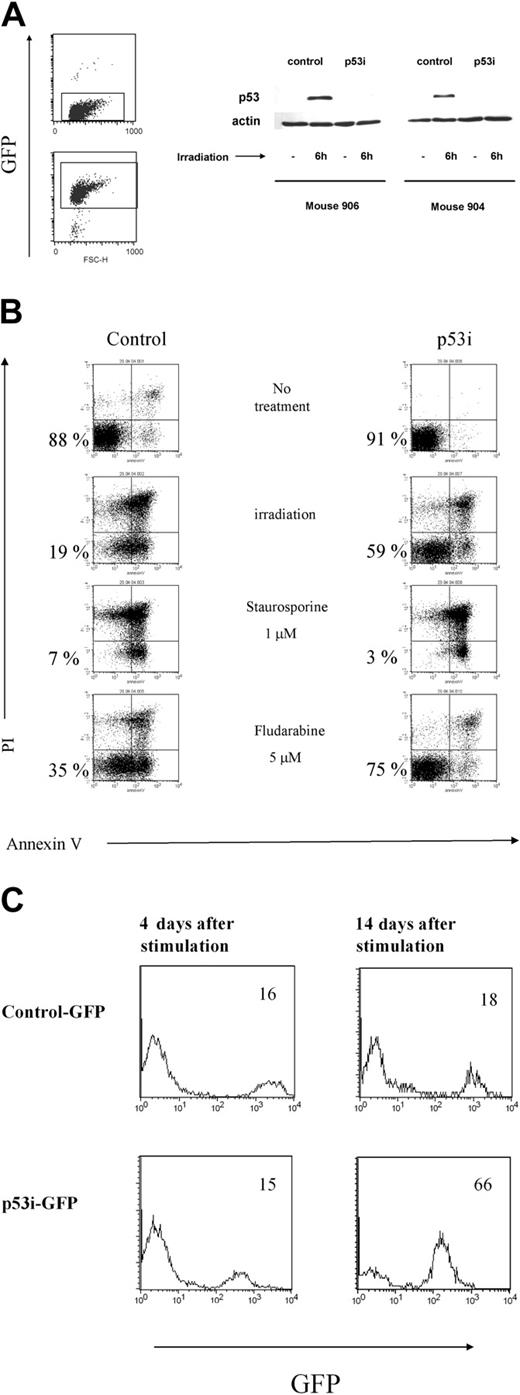
To interpret these results, it was important to verify the stable expression and efficiency of the construct in knocking down the expression of p53 in cells isolated from the mouse periphery. Human mononuclear cells, isolated from the spleens and blood of mice that underwent transplantation 10 weeks earlier with transduced CD34+ cells, were cultured in the presence of human peripheral blood mononuclear cells (PBMCs) from 2 donors, the EBV cell line JY, PHA, and IL-2. Under these conditions, T cells proliferated extensively. Because only mature T cells can be expanded using this protocol,28 our data indicate that the human T cells that developed in the RAG2-/- γc-/- mice were functionally mature and could respond to signals triggered by TCR ligation. In addition, the T cells were capable of responding to alloantigens and could produce a broad array of cytokines on restimulation in vitro, indicating that they were able to recognize and respond to foreign antigens (data not shown). After expanding the T cells derived from the p53 siRNA-transduced CD34+ cells in vivo, we sorted the GFP+ and GFP- T cells and tested p53 levels after γ-irradiation from 2 mice subjected to reconstitution. In both mice, p53 levels increased in the nontransduced population, whereas p53 levels in cells containing the p53 siRNA construct remained almost undetectable (Figure 6A). Consequently, these p53 siRNA+ cells are less susceptible to apoptosis induced by γ-irradiation or to treatment with fludarabine (Figure 6B). In contrast, the loss of p53 did not affect the sensitivity of T cells to apoptosis induced by dexamethasone and staurosporine, which are p53-independent, apoptosis-inducing agents29 (Figure 6 and data not shown).
Lentivirus construct is present and active in mature cells derived from transduced human CD34+ precursors. (A) T cells were isolated from spleens and PB of mice reconstituted with transduced human CD34+ cells and were expanded in vitro and sorted based on the expression of GFP. Cells were then γ-irradiated. Six hours later, whole-cell extracts were prepared, separated on 10% SDS-PAGE, and immunoblotted to detect human p53. A Western blot with antibody against β-actin was used as a control. Results from 2 mice are shown. (B) T cells expressing the p53i or control-GFP constructs were treated with different stimuli and assayed 24 hours later for apoptosis induction using a combination of propidium iodine (PI) and Annexin V double staining and flow cytometry. Double-negative cells represent the viable population (indicated by the percentages). (C) Reduced content of p53 favors the outgrowth of mature T cells derived from human CD34+ precursors. T cells were isolated from spleens and PB of mice reconstituted with transduced human CD34+ cells and were expanded in vitro, as described in “Materials and methods.” The percentage of GFP+ cells was established by flow cytometry at 2 different time points after stimulation.
Lentivirus construct is present and active in mature cells derived from transduced human CD34+ precursors. (A) T cells were isolated from spleens and PB of mice reconstituted with transduced human CD34+ cells and were expanded in vitro and sorted based on the expression of GFP. Cells were then γ-irradiated. Six hours later, whole-cell extracts were prepared, separated on 10% SDS-PAGE, and immunoblotted to detect human p53. A Western blot with antibody against β-actin was used as a control. Results from 2 mice are shown. (B) T cells expressing the p53i or control-GFP constructs were treated with different stimuli and assayed 24 hours later for apoptosis induction using a combination of propidium iodine (PI) and Annexin V double staining and flow cytometry. Double-negative cells represent the viable population (indicated by the percentages). (C) Reduced content of p53 favors the outgrowth of mature T cells derived from human CD34+ precursors. T cells were isolated from spleens and PB of mice reconstituted with transduced human CD34+ cells and were expanded in vitro, as described in “Materials and methods.” The percentage of GFP+ cells was established by flow cytometry at 2 different time points after stimulation.
In addition to its proapoptotic function, p53 is also involved in the induction of growth arrest in response to different stimuli. Moreover, p53 is a negative regulator of hTERT, which regulates the replicative lifespan of T cells.5 We studied the growth of the p53 siRNA-expressing cells after TCR stimulation. The presence of GFP in our constructs allowed us to examine the dynamics of a population of T cells transduced either with p53i or with a control construct in relation to untransduced cells. As can be seen in Figure 6C, we detected a progressive accumulation of cells with reduced p53 content, indicating that p53 siRNA+ T cells have a growth advantage compared with the untransduced or control-transduced cells. Interestingly, these differences were not detected shortly after stimulation but were detected only when the cells were kept in culture for a longer time, indicating that the immediate response to TCR stimulation is similar in p53 siRNA-expressing cells and controls (Figure 6 and data not shown). These results clearly show that the p53 siRNA introduced into the CD34+ cells remained active in down-modulating p53 protein in the T-cell offspring. Our data also indicate that although the down-modulation of p53 protects the T cells against some forms of stress and up-regulates hTERT, it does not affect human T-cell development or homeostasis in this system.
Lack of effect of p53 RNAi on development of B cells, plasmacytoid DCs or monocytes in different organs
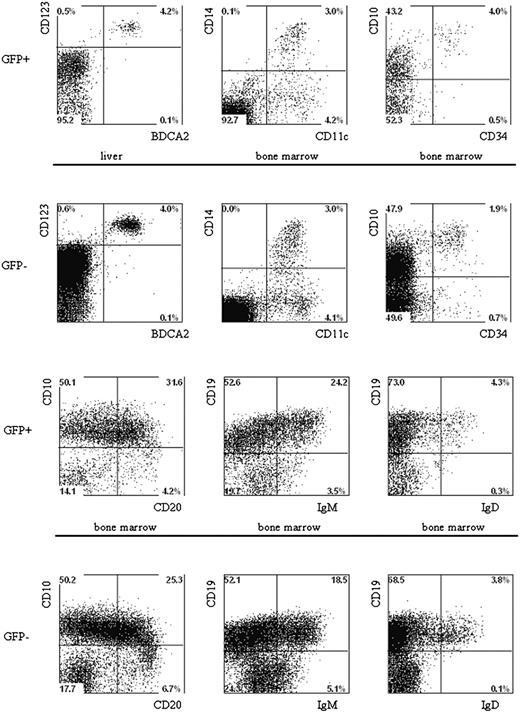
The fact that multiple lineages developed after the injection of CD34+ cells into newborn mice allowed us to analyze the effects of p53 knockdown on the development of B cells, pDCs, and monocytes. The percentages of B cells in the GFP+ and the GFP- populations are similar in cells developed from the control GFP and the p53 siRNA GFP-transduced CD34+ cells (Table 3). In addition, no differences in the expression of B-cell differentiation markers were noted between untransduced control GFP and p53 siRNA GFP cells (Figure 7). Moreover, we could also demonstrate that knocking down p53 had no effect on the development of pDCs and monocytes because the percentages of these cells in the GFP+ populations were similar in untransduced, control-transduced, and p53 siRNA-transduced samples (Figure 7).
B-cell development
. | . | . | . | CD19+, % . | . | |
|---|---|---|---|---|---|---|
| Mouse . | Construct . | CD45+, % . | GFP+in CD45+, % . | GFP+ . | GFP− . | |
| 1 | p53i | 72.3 | 7.3 | 48.7 | 44.2 | |
| 2 | p53i | 78.5 | 47.1 | 83.2 | 81.5 | |
| 3 | p53i | 17.9 | 76.8 | 78.0 | 80.9 | |
| 4 | p53i | 51.2 | 1.0 | 26.7 | 75.8 | |
| 5 | p53i | 66.8 | 12.3 | 80.1 | 76.3 | |
| 6 | p53i | nd | nd | nd | nd | |
| 7 | p53i | 73.9 | 3.7 | 75.9 | 62.2 | |
| 8 | Control | 68.5 | 8.2 | 27.7 | 44.8 | |
| 9 | Control | 73.9 | 12.0 | 69.8 | 73.9 | |
| 10 | Control | 10.0 | 73.1 | 63.6 | 67.6 | |
. | . | . | . | CD19+, % . | . | |
|---|---|---|---|---|---|---|
| Mouse . | Construct . | CD45+, % . | GFP+in CD45+, % . | GFP+ . | GFP− . | |
| 1 | p53i | 72.3 | 7.3 | 48.7 | 44.2 | |
| 2 | p53i | 78.5 | 47.1 | 83.2 | 81.5 | |
| 3 | p53i | 17.9 | 76.8 | 78.0 | 80.9 | |
| 4 | p53i | 51.2 | 1.0 | 26.7 | 75.8 | |
| 5 | p53i | 66.8 | 12.3 | 80.1 | 76.3 | |
| 6 | p53i | nd | nd | nd | nd | |
| 7 | p53i | 73.9 | 3.7 | 75.9 | 62.2 | |
| 8 | Control | 68.5 | 8.2 | 27.7 | 44.8 | |
| 9 | Control | 73.9 | 12.0 | 69.8 | 73.9 | |
| 10 | Control | 10.0 | 73.1 | 63.6 | 67.6 | |
Newborn mice were injected with CD34+ fetal liver cells transduced with p53i siRNA-GFP or control-GFP constructs. Mice were killed, and the BM was analyzed using FACS. Percentages of CD19+ cells in the GFP+ and GFP− gates are given. Percentages of CD45+ cells and the proportion of GFP+ cells in this gate are also indicated.
Multilineage presence of GFP+-expressing cells in the BM and liver of mice that underwent transplantation of lentivirus-transduced human hematopoietic progenitors. Newborn RAG2-/- γc-/- mice were intraperitoneally injected with human CD34+ hematopoietic stem cells isolated from fetal liver and transduced with the pTRIPΔU3-EF1α p53RNAi lentivirus. Mononuclear cell suspensions from the livers and BM of these mice were stained with various human-specific monoclonal antibodies and analyzed by flow cytometry. Cells positive for human CD45 were gated and further analyzed comparing the GFP+ and GFP- populations Examples of plasmacytoid DCs, myeloid cells, and B cells are shown. Liver and BM cells are stained with antibodies directed to CD123, BDCA2, CD11c, CD14, CD10, CD34, CD19, CD20, IgM, and IgD.
Multilineage presence of GFP+-expressing cells in the BM and liver of mice that underwent transplantation of lentivirus-transduced human hematopoietic progenitors. Newborn RAG2-/- γc-/- mice were intraperitoneally injected with human CD34+ hematopoietic stem cells isolated from fetal liver and transduced with the pTRIPΔU3-EF1α p53RNAi lentivirus. Mononuclear cell suspensions from the livers and BM of these mice were stained with various human-specific monoclonal antibodies and analyzed by flow cytometry. Cells positive for human CD45 were gated and further analyzed comparing the GFP+ and GFP- populations Examples of plasmacytoid DCs, myeloid cells, and B cells are shown. Liver and BM cells are stained with antibodies directed to CD123, BDCA2, CD11c, CD14, CD10, CD34, CD19, CD20, IgM, and IgD.
Discussion
We show here that the newborn RAG2-/- γc-/- mouse provides a useful model to study the effects of gene knockdown on the development and function of human hematopoietic cells, particularly of T cells. Robust T-cell development was observed with intraperitoneal injections of CD34+ cells into newborn mice and proceeded at an accelerated pace compared with that observed previously with adult mice (K.W., A.V., and H.S., unpublished observations, September 2003, and Yahata et al26 , Hiramatsu et al27 , and Kerre et al30 ). A similar accelerated T-cell development was observed after the injection of CD34+CD38- cord blood cells (results not shown). There are 2 possible explanations for the acceleration of T-cell development in newborn mice: one is that the thymic rudiment in adult mice is more atrophic than that of 1-day old mice, perhaps as a result of thymic involution.31 The second explanation is that 1-day-old mice have a less active innate immune system. The idea that the murine innate system affects the human T-cell population is based on the observation that preinjecting adult RAG2-/- γc-/- mice with lipid vesicles containing the drug clodronate (which kills phagocytosing cells) strongly favors populating the mice with human peripheral T cells.32 In addition to mainstream CD4+ and CD8+ T cells, all the other subsets of T cells could be observed, including TCR-γδ cells, CD3+CD56+ T cells, and CD25+CD4+ cells that could represent Treg cells.33 We also observed the development of B cells, NK cells, pDCs, and monocytes in the circulatory systems and peripheral organs of mice injected with CD34+ cells. In addition, CD15+CD11c+CD24+ granulocytes and a low but consistent percentage of human glycophorin-positive cells were present (data not shown). Together these data indicate the generation of a complete repertoire of human leukocytes in these mice, raising the possibility that these newborn double-knockout mice can be used for studying “human” immune responses in vivo. After we finished our current studies, another group34 reported that some RAG2-/- γc-/- mice injected with CD34+ neonatal cord blood cells at 1 day of age can develop an adaptive immune response against tetanus toxoid antigen and EBV.
By using siRNA directed against p53 in a lentiviral vector that contained the marker GFP under control of an independent promoter, we demonstrated the feasibility of GFP to track the development of genetically modified CD34+ precursors in vivo. Biochemical analysis revealed that in CD34 precursors, p53 siRNA expression results in more than 95% reduction in γ-irradiation-induced p53 expression. Given that the siRNA construct remained stably and functionally expressed in the T-cell progeny, we can safely assume that siRNA continues to be expressed during the differentiation of CD34+ cells into all mature leukocytes. We did not observe significant quantitative or qualitative differences in leukocyte subsets that developed from p53 siRNA+ or wild-type CD34+ cells, indicating that the down-regulation of p53 does not affect human hematopoietic differentiation. Our data are consistent with those in p53-deficient mice, which did not reveal a gross effect of p53 deficiency on mouse hematopoietic development.35 It has been reported that p53 in the mouse is up-regulated as a consequence of TCR gene rearrangements and that the down-regulation of p53 is required for T-cell differentiation beyond the TCR-β selection checkpoint.6,7,36 Our data indicate that the premature down-modulation of p53 before the TCR-β selection checkpoint does not result in an advantage of differentiation and proliferation of developing T cells. Recently, we observed that p53 knockdown confers an in vitro expansion advantage to human T cells (R.G., E.W., R. Beijersbergen, and H.S., manuscript in preparation). This can be explained, at least in part, by the fact that p53 is a negative regulator of hTERT in T cells, which was previously shown to determine the replicative lifespan of human T cells and, at the same time, confers resistance to apoptotic stimuli.5 We therefore considered the possibility that homeostatic expansion of T cells in vivo would be promoted by p53 knockdown. This turned out not to be the case, though p53 siRNA+ T cells isolated from mice showed the expected in vitro growth advantage compared with control-transduced or untransduced T cells. We observed that down-modulation of p53 renders the human T cells resistant to γ-irradiation, consistent with the effect of p53 deficiency on resistance of mouse thymocytes to γ-irradiation.37,38 The resistance to γ-irradiation and another p53-dependent apoptotic stimulus, fludarabine, conferred by the loss of p53 in human T cells, indicates that p53 regulates the response to certain apoptotic stimuli, which may make the T cells susceptible to transforming events. Indeed p53-deficient mice develop various neoplasms in 6 months. However, thus far we have not observed hematopoietic tumors arising from p53 siRNA+ CD34+ cells for as long as 6 months after injection of the p53 siRNA+ CD34+ cells. This may be attributed to the fact that more oncogenic hits and, thus, longer periods are required for the transformation of human p53-deficient hematopoietic cells than for mouse p53-deficient hematopoietic cells.
Supported by the Netherlands Cancer Foundation grant AMC 2002-2587.
Prepublished online as Blood First Edition Paper, August 19, 2004; DOI 10.1182/blood-2004-02-0656.
R.G and K.W. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Bruno Verhasselt and Veronique Stove (Ghent University) for providing us with the pTRIP construct, the staff of the Bloemenhove Kliniek (Heemstede, The Netherlands) for providing fetal tissues, and Ester B. Remmerswaal for her help analyzing the TCR repertoire. We also thank Henk Grimminck, Dick Grund, Henk Starrevelt, and Louis Tolkamp for their help in the laboratory facility at the Netherlands Cancer Institute/Antoni van Leeuwenhoekhuis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal