Abstract
Recombinant human activated protein C (rhAPC) is a natural anticoagulant with potentially important anti-inflammatory properties. In humans with severe sepsis, rhAPC treatment reduces mortality, but mechanisms responsible have not been well characterized. Accumulation of activated neutrophils in the lungs and other organs during severe infection contributes to sepsis-induced organ dysfunction, including acute inflammatory lung injury. Because neutrophils express an APC receptor, we hypothesized that immunomodulatory effects of rhAPC occur, in part, via modulation of neutrophil responses. To examine this issue, we performed a double-blinded, placebo-controlled study of rhAPC in a human model of endotoxin-induced pulmonary inflammation. Administration of rhAPC significantly reduced leukocyte accumulation to the airspaces, independent of pulmonary cytokine or chemokine release. Neutrophils recovered from bronchoalveolar lavage fluid of volunteers receiving rhAPC demonstrated decreased chemotaxis ex vivo. Decreased neutrophil chemotaxis following exposure to rhAPC was confirmed in vitro. No differences were detected in gene expression, kinase activation, cytokine release, cell survival, or apoptosis of neutrophils recovered in the presence or absence of rhAPC. These studies demonstrate that rhAPC reduces both endotoxin-induced accumulation of leukocytes in the airspaces and neutrophil chemotaxis. These rhAPC-induced effects on neutrophil function may represent a mechanism by which rhAPC improves survival in patients with sepsis. (Blood. 2004;104:3878-3885)
Introduction
Activated protein C (APC) is a natural anticoagulant that plays an important role in coagulation homeostasis by inactivating the procoagulant factors Va and VIIIa. Recently, recombinant human APC (rhAPC) has been demonstrated to have potentially important anti-inflammatory properties. rhAPC is able to inhibit leukocyte adhesion to vascular endothelial cells and to reduce activated neutrophil accumulation in rat lungs.1 In monocytes, which express the endothelial protein C receptor (EPCR), exposure to rhAPC inhibits lipopolysaccharide (LPS)-induced release of tumor necrosis factor α (TNFα)2 and nuclear translocation of nuclear factor (NF)-κB.3 In a lethal baboon model of E coli-induced sepsis, infusion of rhAPC was demonstrated to be protective,4 and in humans with multiple organ system dysfunction due to severe infection, treatment with rhAPC reduces mortality.5,6
Despite the ability of rhAPC to improve survival in primate models of severe infection and in humans with sepsis, the mechanisms responsible for such effects have not been fully characterized. Although initial hypotheses focused on interruption of the coagulopathic and fibrinolytic cascades activated in sepsis, other agents that also have potent effects on such pathways, such as tissue factor pathway inhibitor and antithrombin, did not demonstrate the same clinical benefit in severe sepsis as was seen with rhAPC.7,8 In human models of endotoxemia, infusion of rhAPC did not affect proinflammatory responses, including elevations in circulating levels of proinflammatory cytokines, nor did it limit thrombin generation.9,10
Recent data have shown that neutrophils express receptors for APC and also that neutrophil chemotaxis is inhibited by exposure to protein C, APC, or rhAPC.11 Activated neutrophils accumulate in the lungs and other organs during severe infection, and contribute to organ system dysfunction and mortality in this setting.12-14 Thus, the ability of rhAPC to affect neutrophil functions, particularly those associated with neutrophil migration, provides an additional potential mechanism for its beneficial effects in sepsis. In order to test this hypothesis, we used a human model of endotoxin-induced pulmonary inflammation to examine the effects of rhAPC in vivo on inflammatory responses in which neutrophils play a major role.
Patients, materials, and methods
Materials
Model of endotoxin-induced pulmonary inflammation
Subjects eligible for this study had to meet the following criteria: (1) 18 to 40 years of age, male or female; (2) nonsmoker; (3) no active medical problems; and (4) no concurrent medications, including aspirin or nonsteroidal anti-inflammatory drugs. Women taking oral contraceptives were not excluded. Exclusions included pregnancy; lactation; history of recent clinically significant asthma; allergies to both trimethoprim/sulfamethoxazole and penicillin (or amoxicillin); allergy to lidocaine or related compounds; allergy to opiates or benzodiazepines, used for sedation during bronchoscopy; history of asthma or history of exercise-induced wheezing; signs of any acute illness on the day of endotoxin instillation; abnormalities on screening laboratory tests, electrocardiogram, chest radiograph, or pulmonary function tests; and any personal or family history of bleeding disorders.
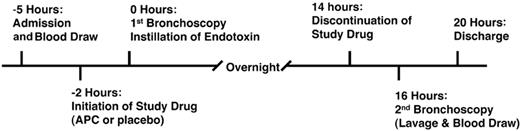
All subjects were admitted to the General Clinical Research Center (GCRC) at the University of Colorado Hospital. Approval for this study was obtained from the Colorado Multiple Institutional Review Board, and informed consent was provided according to the Declaration of Helsinki. The timeline for the study is shown in Figure 1. Using a double-blinded placebo-controlled design, subjects (n = 16) were randomized to receive either rhAPC (drotrecogin alfa [activated]; 24 mcg/kg per hour) or normal saline (the solution for drotrecogin alfa [activated]) starting 2 hours before the initial bronchoscopy and continuing for 16 hours. The infusion of rhAPC or placebo was discontinued 2 hours prior to the second bronchoscopy to lessen the risk of hemorrhage resulting from anticoagulant properties of rhAPC. The volunteers were premedicated with one double-strength trimethoprim/sulfamethoxazole tablet 12 hours and 1 hour before bronchoscopy. Any subject with a history of sulfa allergy was given 2 doses of oral amoxicillin (500 mg), separated by 8 hours.
Experimental design of the endotoxin-induced pulmonary inflammation study.
At the time of the first bronchoscopy, 10 mL saline was instilled into a lung subsegment (either the right middle lobe or lingula) followed by instillation of the test dose of reference endotoxin (4 ng/kg in 10 mL saline) into the contralateral lung. The subjects were randomized to left or right lungs for endotoxin or saline instillation. Following reconstitution of the endotoxin, a quantitative Limulus amebocyte lysate test was done to verify the proper reconstitution and dosage of endotoxin. At the time of the first bronchoscopy, 30 mL peripheral blood was obtained for isolation of neutrophils.
A second bronchoscopy was performed 16 hours after the initial bronchoscopy. At the time of the second bronchoscopy, both the endotoxinand placebo-instilled subsegments were lavaged with 150 mL normal saline, and 60 mL peripheral blood was obtained for isolation of neutrophils.
A pulmonary symptom scale (dyspnea, cough, wheezing, chest pain), as previously used,17 was obtained every 8 hours during the admission period, and every 12 hours for 72 hours following the second bronchoscopy. All subjects were observed for at least 4 hours after the second bronchoscopy. All subjects were contacted by phone 1 week after completing the study to further assess safety. If subjects had any ongoing medical complaints, they were asked to return to the GCRC where they were examined by one of the investigators.
Isolation of neutrophils recovered by bronchoalveolar lavage (BAL)
The cells in bronchoalveolar lavage fluid (BALF) were pelleted, resuspended in 10 mL phosphate-buffered saline (PBS) with 1 mM EDTA (ethylenediaminetetraacetic acid) and 2% fetal calf serum (FCS), then passed over a siliconized glass wool column. After washing, the cells were again pelleted by centrifugation, then resuspended in PBS with 1 mM EDTA and 2% FCS at 50 × 106 cells/mL. Anti-HLA-DR antibody and anti-human Glycophorin A tetramer (StemCell Technologies, Vancouver, BC, Canada) were added to a final concentration of 1 μg/mL. After incubation at 4°C for 20 minutess, 60 μL/mL colloidal magnetic dextran iron particles were added to the suspension and incubated for 20 minutes at 4°C. The entire cell suspension was then placed into a column surrounded by a magnet, followed by washing of the column with at least 12 sample volumes of PBS with 1 mM EDTA and 2% FCS. The cells in the eluate were pelleted by centrifugation and resuspended in 2 mL RPMI 1640 with 2% heat-inactivated platelet-poor plasma (HIPPP). Neutrophil purity was determined for each sample, and was consistently more than 98%. Priority for neutrophil distribution was given to microarray analysis, with the remaining cells used for studies of intracellular signal transduction, protein expression, migration, and cell survival.
Microarray analysis of BALF neutrophil gene expression
Total RNA was stabilized in freshly isolated neutrophils by resuspension of 2 × 107 cells in 1 mL RNAlater (Ambion, Austin TX), then stored at -20°C. Subsequent isolation with Trizol (Life Technologies, CA) and purification with RNEasy MinElute columns (Qiagen, CA) was performed following the manufacturer's protocol. From 1 μg to 5 μg total RNA was used for microarray target labeling using standard methods for reverse transcription and one round of in vitro transcription.18 HG-U133A microarrays were hybridized with 20 μg cRNA and processed per the manufacturer's protocol (Affymetrix, Foster City, CA).
Hybridization signals were quantified using the statistical algorithms implemented in the Affymetrix Gene Chip Operating System. Individual arrays were determined to be of high quality by visual inspection, comparison of the overall fluorescence intensity (scaling factor) to other arrays in the group, and low 3′/5′ ratios for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and Β-actin (ratio < 3). This insures that all of the arrays in the group can be directly compared, and that the input mRNA was intact.
Microarray data were analyzed using BRB ArrayTools v3.1. Tabular intensity data were imported, log2 transformed, and each array was normalized (mean centered) to the median intensity array. Class comparison using the univariate 2-sample t test was performed using the set of 11 853 genes that were reliably detected on 2 or more arrays. Exact multivariate permutation testing was conducted using all 126 possible permutations.
Neutrophil functional assays
Human neutrophils were isolated from blood by the plasma Percoll method19 and suspended in RPMI 1640 culture medium (Bio-Whittaker, Walkersville, MD). Neutrophils isolated from peripheral blood or BALF were resuspended in RPMI 1640 and human HIPPP (2%) containing 10 × 106 cells/mL. One mL of the cell suspension combined with LPS or left unstimulated was placed in a 1.5 mL microcentrifuge tube (Eppendorf, Germany) and rotated continuously for up to 4 hours at 37°C. Release of cytokines and various growth factors was screened for by enzyme-linked immunosorbent assay (ELISA) and by an antibody-based protein microarray (RayBiotech, Norcross, GA).20 Selected cytokines (TNF-α, interleukin 8 [IL-8], macrophage inflammatory protein 1 β [MIP-1β], monocyte chemoattractant protein 1 [MCP-1]) were quantified by immunoassay (Elisa Tech, Denver, CO). Quantification of neutrophil apoptosis was performed by immunoassay for cytoplasmic histone-associated DNA fragments (Roche, Mannheim, Germany). Cell death via necrosis was assayed by percent of total lactate dehydrogenase (LDH) release (Cytotoxicity Detection Kit; Roche).
Neutrophil chemotaxis assay
Migration of neutrophils through a microporous polyethylene terephthalate membrane to gradients of IL-8 was measured using a 96-Multiwell Insert System (HTS FluoroBlok; BD Falcon, Bedford, MA) in which a 3-μm pore filter separated the upper from the lower chamber as a modified Boyden system.21 Cells were labeled in situ with 10 μM Calcein AM in Hanks balanced salt solution (HBSS) for 15 minutes at 37°C and washed once with HBSS, then resuspended in Krebs-Ringer phosphate buffer with 2% dextrose (KRPD) with 2.5% HIPPP. For in vitro studies of rhAPC deactivation, neutrophils were incubated following labeling for 20 minutes at a range of concentrations of rhAPC (10-4 g/mL to 10-10 g/mL) as previously described.11 In all experiments neutrophil chemotaxis was tested toward IL-8 (1 nM) in the lower chamber, and nondirectional migration was tested toward KRPD with 2.5% HIPPP. Migration was assayed by fluorescence of cells passing through the microporous membrane on a FLX 800 Microplate Fluorescence Reader with KC Junior Software (Bio-Tek Instruments, Winooski, VT) using excitation/emission wavelengths of 485 nm/530 nm from 2 minutes to 120 minutes at 5-minute intervals at 37°C with constant rotation of the plate. Migration was reported as a chemotaxis index, which is the ratio between the fluorescence intensity of directed and undirected migration through the filters for each sample.
Protein expression and kinase phosphorylation
Whole cell extracts from neutrophils were collected from cells denatured in ice-cold lysis buffer (50 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA [ethylene glycol-bis(beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid], 1 mM Na3 vanadate, 10 mM Na pyrophosphate, 10 mM NaF, 300 μM p-nitrophenyl phosphate, 1 mM phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, 10 μg/mL aprotinin, pH 7.3) for 15 minutes. The protein concentration of each sample was assayed using the BCA protein assay kit (Pierce, Rockford, IL) standardized to bovine serum albumin.
For Western blots, 70 μg protein from whole cell extracts was loaded on a 10% Tris-HCl sodium dodecyl sulfate (SDS) polyacrylamide gel. Protein was electrotransferred to a nitrocellulose membrane and then blocked with 5% nonfat dry milk, 20 mM Tris-buffered saline, with 0.1% Tween. After blocking, the membrane was incubated overnight at 4°C with antibodies to phos-p38 MAPK, total p38 MAPK, phos-p42/44 extracellular signal-regulated kinase (ERK) MAPK (1/2), total ERK 1/2 MAPK, phos-Akt, total Akt, or total 5-lipoxygenase (5-LO) using a dilution of 1:1000, followed by horseradish peroxidase-coupled secondary antibody at a dilution of 1:2000. After washing 5 times, bands were detected using chemiluminescence Western blotting detection reagents (ECL Detection System, Amersham-Pharmacia, NJ). Densitometry was performed using chemiluminescence system and analysis software (BioRad, Hercules, CA). Kinase phosphorylation was quantified as a normalized ratio of phosphorylated to total kinase, while total 5-LO was quantified as a normalized ratio to total p38 MAPk, and expressed in arbitrary units.
Statistical analysis
Data were analyzed using JMP statistical software (SAS Institute, Cary, NC). Studies of neutrophil chemotaxis were expressed as mean index value plus or minus standard error of the mean (SEM). Significance of the effect of rhAPC administration on neutrophil chemotaxis over time (Figure 7) was determined by 2-way analysis of variance (ANOVA). Log values of leukocyte recovery by BAL (Figure 2) were determined to have a normal distribution, and the significance of the effect of rhAPC administration was determined by Student t test. Significance of the effect of rhAPC administration on cytokine and receptor recovery (Figure 4) in BALF, and functional responses of BALF and blood-derived neutrophils (Figures 5 and 6), where a normal distribution was not present, were determined by Wilcoxon unpaired exact test. For all tests, P less than .05 was considered significant.
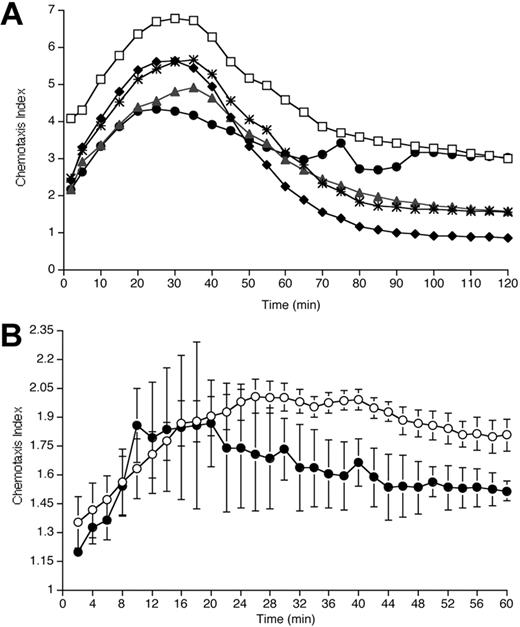
Blockade of IL-8-induced migration by exposure to rhAPC. (A) Effect of rhAPC on neutrophil chemotaxis in vitro. Neutrophils isolated from blood of healthy volunteers were exposed to rhAPC (♦, 10-4 g/mL; *, 10-6 g/mL; ▴, 10-8 g/mL; •, 10-10 g/mL) or left untreated (□) for 20 minutes. Plot depicts mean chemotaxis index (n = 5) of IL-8-induced migration (1 nM gradient) over a 2-hour period in a modified Boyden chamber system. Pretreatment with rhAPC resulted in a significant reduction in IL-8-induced migration over time for all concentrations tested when compared with untreated cells (10-4 g/mL, P < .0001; 10-6 g/mL, P < .0001; 10-8 g/mL, P < .0001; 10-10 g/mL, P = .01; by 2-way ANOVA). (B) Effect of rhAPC on neutrophil chemotaxis ex vivo. Neutrophils isolated 16 hours after endobronchial LPS-instillation from BALF of volunteers who received rhAPC (•) demonstrate a significant decrease in IL-8-induced migration over a 1-hour period compared with placebo-treated volunteers (○). Mean index ± SEM (n = 3 for rhAPC-treated group, n = 4 for untreated group), P = .012 by 2-way ANOVA.
Blockade of IL-8-induced migration by exposure to rhAPC. (A) Effect of rhAPC on neutrophil chemotaxis in vitro. Neutrophils isolated from blood of healthy volunteers were exposed to rhAPC (♦, 10-4 g/mL; *, 10-6 g/mL; ▴, 10-8 g/mL; •, 10-10 g/mL) or left untreated (□) for 20 minutes. Plot depicts mean chemotaxis index (n = 5) of IL-8-induced migration (1 nM gradient) over a 2-hour period in a modified Boyden chamber system. Pretreatment with rhAPC resulted in a significant reduction in IL-8-induced migration over time for all concentrations tested when compared with untreated cells (10-4 g/mL, P < .0001; 10-6 g/mL, P < .0001; 10-8 g/mL, P < .0001; 10-10 g/mL, P = .01; by 2-way ANOVA). (B) Effect of rhAPC on neutrophil chemotaxis ex vivo. Neutrophils isolated 16 hours after endobronchial LPS-instillation from BALF of volunteers who received rhAPC (•) demonstrate a significant decrease in IL-8-induced migration over a 1-hour period compared with placebo-treated volunteers (○). Mean index ± SEM (n = 3 for rhAPC-treated group, n = 4 for untreated group), P = .012 by 2-way ANOVA.
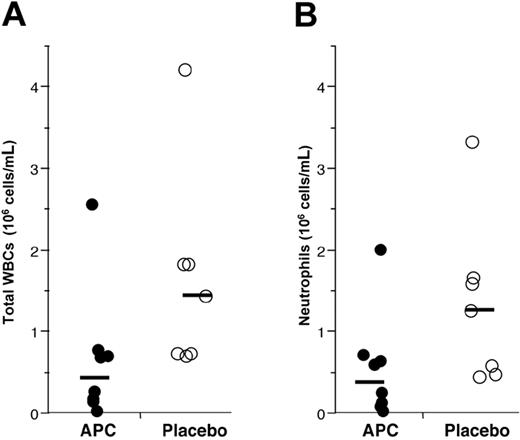
Administration of rhAPC reduces endotoxin-induced leukocyte accumulation to the airspaces. Leukocytes present in BALF recovered 16 hours after endobronchial LPS-instillation from volunteers who received rhAPC (•) or placebo (○). Solid bar represents the mean of log values for each group; n = 8 for rhAPC and n = 7 for placebo. (A) Total WBCs recovered per mL of lavage fluid. Administration of rhAPC significantly reduced recovery of all leukocytes, P = .037 by Student t test. (B) Total neutrophils recovered per mL of lavage fluid. Administration of rhAPC reduced recovery of neutrophils, P = .048 by Student t test.
Administration of rhAPC reduces endotoxin-induced leukocyte accumulation to the airspaces. Leukocytes present in BALF recovered 16 hours after endobronchial LPS-instillation from volunteers who received rhAPC (•) or placebo (○). Solid bar represents the mean of log values for each group; n = 8 for rhAPC and n = 7 for placebo. (A) Total WBCs recovered per mL of lavage fluid. Administration of rhAPC significantly reduced recovery of all leukocytes, P = .037 by Student t test. (B) Total neutrophils recovered per mL of lavage fluid. Administration of rhAPC reduced recovery of neutrophils, P = .048 by Student t test.
Administration of rhAPC does not reduce endotoxin-induced cytokine, endotoxin-induced receptor, or receptor-antagonist release in the airways. (A) Cytokines and chemokines present in BALF recovered 16 hours after endobronchial LPS-instillation from volunteers who received rhAPC (•) or placebo (○). Concentrations of IL-8, IL-6, TNF-α, and MCP-1 were quantified by ELISA. (B) Receptor and receptor-antagonist present in BALF recovered 16 hours after endobronchial LPS-instillation from volunteers who received rhAPC (•) or placebo (○). Concentrations of IL-1ra, TNF-RI, and TNF-RII were quantified by ELISA. For panels A and B, solid bars represent the median value for each condition, n = 8 for rhAPC, and n = 7 for placebo. Administration of rhAPC did not significantly modify the release of these proteins, as analyzed by the Wilcoxon unpaired exact test.
Administration of rhAPC does not reduce endotoxin-induced cytokine, endotoxin-induced receptor, or receptor-antagonist release in the airways. (A) Cytokines and chemokines present in BALF recovered 16 hours after endobronchial LPS-instillation from volunteers who received rhAPC (•) or placebo (○). Concentrations of IL-8, IL-6, TNF-α, and MCP-1 were quantified by ELISA. (B) Receptor and receptor-antagonist present in BALF recovered 16 hours after endobronchial LPS-instillation from volunteers who received rhAPC (•) or placebo (○). Concentrations of IL-1ra, TNF-RI, and TNF-RII were quantified by ELISA. For panels A and B, solid bars represent the median value for each condition, n = 8 for rhAPC, and n = 7 for placebo. Administration of rhAPC did not significantly modify the release of these proteins, as analyzed by the Wilcoxon unpaired exact test.
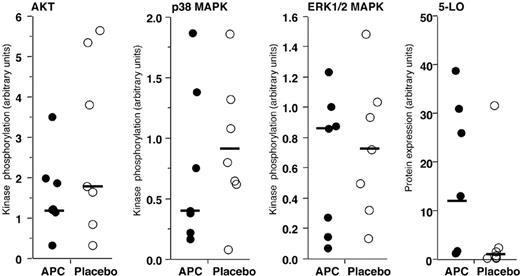
Lack of modification of key neutrophil intracellular signaling mechanisms by in vivo administration of rhAPC. Relative phosphorylation of kinases and expression of 5-LO in neutrophils isolated 16 hours after endobronchial LPS-instillation from volunteers who received rhAPC (•) or placebo (○). The ratio of phosphorylated kinase to the total quantity of kinase recovered is plotted. The quantity of 5-LO is relative to the total quantity of p38 MAPK. Solid bars represent the median value for each condition; n = 7 for rhAPC and n = 7 for placebo. Phosphorylation of AKT, p38 MAPK, or ERK (1/2) MAPK, and expression of 5-LO were not significantly modified by the presence or absence of rhAPC, as analyzed by the Wilcoxon unpaired exact test.
Lack of modification of key neutrophil intracellular signaling mechanisms by in vivo administration of rhAPC. Relative phosphorylation of kinases and expression of 5-LO in neutrophils isolated 16 hours after endobronchial LPS-instillation from volunteers who received rhAPC (•) or placebo (○). The ratio of phosphorylated kinase to the total quantity of kinase recovered is plotted. The quantity of 5-LO is relative to the total quantity of p38 MAPK. Solid bars represent the median value for each condition; n = 7 for rhAPC and n = 7 for placebo. Phosphorylation of AKT, p38 MAPK, or ERK (1/2) MAPK, and expression of 5-LO were not significantly modified by the presence or absence of rhAPC, as analyzed by the Wilcoxon unpaired exact test.
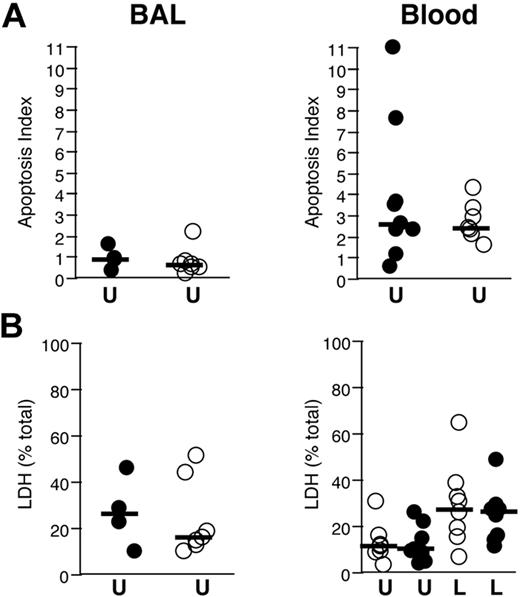
Lack of modification of neutrophil survival by in vivo administration of rhAPC. Analysis of apoptosis and necrosis in neutrophils isolated 16 hours after endobronchial LPS-instillation from volunteers who received rhAPC (•) or placebo (○). Solid bars represents the median value for each condition. (A) Effect of rhAPC on spontaneous apoptosis. Histone-bound DNA isolated from unstimulated BAL (left) and blood (right) neutrophils in volunteers who received rhAPC, expressed as a ratio to histone-bound DNA isolated from LPS-stimulated blood neutrophils (apoptosis index), n = 4 for rhAPC and n = 7 for placebo. (B) Effect of rhAPC on neutrophil necrosis. The percent of unstimulated BAL neutrophils (left) and unstimulated and LPS-stimulated blood neutrophils (right) that underwent necrosis after 4 hours of culture was measured by release of LDH, expressed as a percent of the total LDH content of an equal number of neutrophils; n = 8 for rhAPC and n = 8 for placebo. Exposure to rhAPC did not significantly modify spontaneous apoptosis or necrosis in BAL or blood neutrophils, as analyzed by the Wilcoxon unpaired exact test. U indicates unstimulated; L, LPS-stimulated.
Lack of modification of neutrophil survival by in vivo administration of rhAPC. Analysis of apoptosis and necrosis in neutrophils isolated 16 hours after endobronchial LPS-instillation from volunteers who received rhAPC (•) or placebo (○). Solid bars represents the median value for each condition. (A) Effect of rhAPC on spontaneous apoptosis. Histone-bound DNA isolated from unstimulated BAL (left) and blood (right) neutrophils in volunteers who received rhAPC, expressed as a ratio to histone-bound DNA isolated from LPS-stimulated blood neutrophils (apoptosis index), n = 4 for rhAPC and n = 7 for placebo. (B) Effect of rhAPC on neutrophil necrosis. The percent of unstimulated BAL neutrophils (left) and unstimulated and LPS-stimulated blood neutrophils (right) that underwent necrosis after 4 hours of culture was measured by release of LDH, expressed as a percent of the total LDH content of an equal number of neutrophils; n = 8 for rhAPC and n = 8 for placebo. Exposure to rhAPC did not significantly modify spontaneous apoptosis or necrosis in BAL or blood neutrophils, as analyzed by the Wilcoxon unpaired exact test. U indicates unstimulated; L, LPS-stimulated.
Results
There were no differences in baseline parameters, including age or sex, between the groups randomized to receive rhAPC or placebo. No severe or unexpected adverse events occurred in either group, and there were no episodes of bleeding complications associated with rhAPC administration.
Effects of rhAPC on bronchoalveolar lavage cell and neutrophil counts
Accumulation of inflammatory cell populations was found in BALF from the pulmonary subsegment where endotoxin was instilled, but not BALF from the contralateral lung. Significantly fewer leukocytes were found in BALF from volunteers treated with rhAPC, as compared with those randomized to placebo (Figure 2A, P = .037). The majority of leukocytes recovered by BALF were neutrophils, thus fewer neutrophils were present in BALF from rhAPC-treated volunteers compared with those receiving placebo (Figure 2B, P = .048). As expected, fewer leukocytes were recovered from the countralateral lobe receiving saline instillation. For the saline-exposed control lobe, 178 146 ± 95 598 WBCs (mean ± SEM) and 18 470 ± 9710 neutrophils per mL were recovered in subjects administered placebo, while 113 375 ± 45 437 WBCs and 23 150 ± 13 960 neutrophils per mL were recovered from subjects receiving APC (not significantly different). There were no differences in peripheral total white blood cell or neutrophil counts between rhAPC- and placebo-treated subjects (data not shown).
Lack of effect of rhAPC on pulmonary indices of inflammation
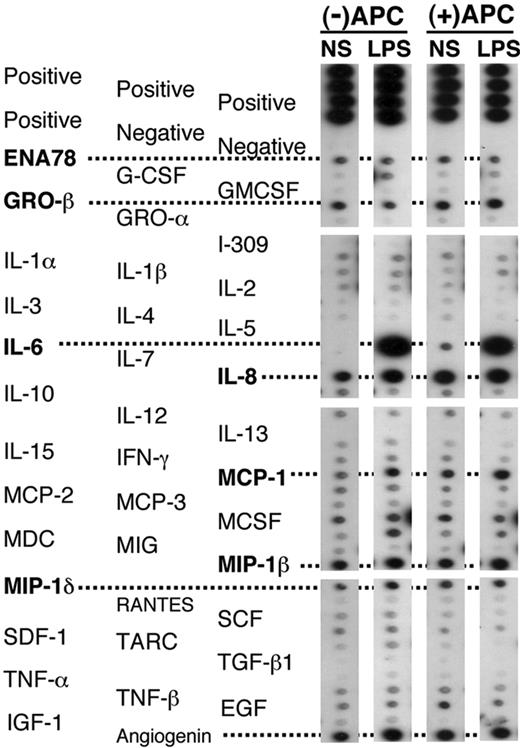
To assess inflammatory responses, we measured protein concentration in the BALF and screened for the presence of major cytokines, chemokines, and growth factors with an antibody-based protein array. There were no differences in BALF protein concentrations from the endotoxin-exposed lungs between rhAPC- and placebo-treated subjects: 234 ± 40 μg/mL in those given rhAPC and 238 ± 54 μg/mL in the placebo group. We screened BALF samples for the presence of 79 cytokines, chemokines, and growth factors by protein array. Although a number of peptides were present in an equivalent manner in both the saline- and the LPS-treated lobes, only IL-6, IL-8, and MCP-1 were consistently elevated in response to LPS 16 hours following its administration (Figure 3). The presence of rhAPC did not consistently modify the pattern of cytokines and chemokines released into either the LPS- or the saline-treated lung lobes.
Protein array screening of cytokine release in BALF 16 hours after endobronchial administration of LPS or normal saline (NS) in volunteers receiving rhAPC, compared with untreated volunteers. Representative arrays from BALF of volunteers receiving rhAPC or placebo. ENA indicates epithelial neutrophil-activating peptide; GRO, growth regulated gene; MCP, monocyte chemoattractant protein; MDC, monocyte-derived DC; MIG, monokine induced by IFNγ; MIP, macrophage inflammatory protein; SCF, stem cell factor; SDF, stromal cell-derived factor; TARC, thymus and activation-regulated chemokine; EGF, epidermal growth factor; IGF, insulin-like growth factor. An additional 41 cytokines, chemokines, and growth factors were screened, without evidence of modification by rhAPC administration (data not shown). These peptides include oncostatin M (OSM); thrombopoietin (TPO); vascular endothelial growth factor (VEGF); platelet-derived growth factor (PDGF); leptin, brain-derived neurotrophic factor (BDNF); B-lymphocyte chemoattractant (BLC); CKβ8-1; eotaxin-1, -2, and -3; fibroblast growth factor 4 (FGF-4), -6, -7, and -9; Fit-3 ligand; fractalkine; granulocyte chemotactic protein 2 (GCP-2); glial cell line-derived neurotrophic factor (GDNF); hepatocyte growth factor (HGF); insulin-like growth factor binding proteins (IGFBP)-1, -2, -3, and -4; IL-16; IFNγ-inducible protein 10 (IP-10); leukemia inhibitory factor (LIF); LIGHT; MCP-4; macrophage migration inhibitory factor (MIF); MIP-3α; neutrophil-activating protein 2 (NAP-2); neurotrophin (NT)-3 and -4; osteopontin (OPN); pulmonary and activation-regulated chemokine (PARC); placental growth factor (PIGF); TGF-β2 and -β3; and tissue inhibitor of metalloproteinase (TIMP)-1 and -2.
Protein array screening of cytokine release in BALF 16 hours after endobronchial administration of LPS or normal saline (NS) in volunteers receiving rhAPC, compared with untreated volunteers. Representative arrays from BALF of volunteers receiving rhAPC or placebo. ENA indicates epithelial neutrophil-activating peptide; GRO, growth regulated gene; MCP, monocyte chemoattractant protein; MDC, monocyte-derived DC; MIG, monokine induced by IFNγ; MIP, macrophage inflammatory protein; SCF, stem cell factor; SDF, stromal cell-derived factor; TARC, thymus and activation-regulated chemokine; EGF, epidermal growth factor; IGF, insulin-like growth factor. An additional 41 cytokines, chemokines, and growth factors were screened, without evidence of modification by rhAPC administration (data not shown). These peptides include oncostatin M (OSM); thrombopoietin (TPO); vascular endothelial growth factor (VEGF); platelet-derived growth factor (PDGF); leptin, brain-derived neurotrophic factor (BDNF); B-lymphocyte chemoattractant (BLC); CKβ8-1; eotaxin-1, -2, and -3; fibroblast growth factor 4 (FGF-4), -6, -7, and -9; Fit-3 ligand; fractalkine; granulocyte chemotactic protein 2 (GCP-2); glial cell line-derived neurotrophic factor (GDNF); hepatocyte growth factor (HGF); insulin-like growth factor binding proteins (IGFBP)-1, -2, -3, and -4; IL-16; IFNγ-inducible protein 10 (IP-10); leukemia inhibitory factor (LIF); LIGHT; MCP-4; macrophage migration inhibitory factor (MIF); MIP-3α; neutrophil-activating protein 2 (NAP-2); neurotrophin (NT)-3 and -4; osteopontin (OPN); pulmonary and activation-regulated chemokine (PARC); placental growth factor (PIGF); TGF-β2 and -β3; and tissue inhibitor of metalloproteinase (TIMP)-1 and -2.
To quantify the effects of rhAPC administration on pulmonary inflammatory responses, levels of the cytokines IL-1β, IL-6, IL-8, IL-10, TNF-α, and MCP-1, as well as of the soluble cytokine receptors and receptor-blocking agents TNF receptor type 1 (TNF RI), TNF receptor type 2 (TNF RII), and the interleukin-1 receptor antagonist (IL-1ra), were assayed in BALF from the pulmonary subsegment into which LPS had been placed and from the contralateral lung of the volunteers treated with rhAPC or saline. No detectible quantity of any of these inflammatory mediators was found in the BALF from the unexposed lung, and no levels of IL-1β or IL-10 were detectible in the endotoxin-exposed lung.
Increased concentrations of IL-6, IL-8, TNF-α, and MCP-1 were found in BALF from pulmonary subsegments exposed to LPS, but no significant differences were present when rhAPC- and saline-treated volunteer groups were compared (Figure 4A). Similarly, elevated levels of TNF RI, TNF RII, and IL-1ra were present in BALF from endotoxin-treated lungs, but did not differ between the groups given rhAPC or saline (Figure 4B).
Effects of rhAPC on kinase phosphorylation, protein expression, and cytokine release by pulmonary neutrophils
Although no differences in inflammatory mediators were found in the BALF or released by neutrophils from volunteers treated with rhAPC or saline, the decreased numbers of total cells and neutrophils in the BALF of the subjects receiving rhAPC suggested that exposure to rhAPC might alter neutrophil intracellular signaling. The kinases p38 MAPK and Akt, as well as 5-LO, have been identified as important regulators of neutrophil accumulation in murine models of acute lung inflammation.22-25 To examine this issue, we determined phosphorylation of p38 MAPK, Akt, and ERK1/2 MAPK relative to the total quantity of each kinase in pulmonary neutrophils between subjects treated with rhAPC or placebo (Figure 5). Potential effects of APC on 5-LO expression were analyzed by comparing the detected quantity of 5-LO with the quantity of p38 MAPK, which varies little between treatment conditions. No significant effect of APC treatment on kinase phosphorylation or 5-LO expression was detected in pulmonary neutrophils (Figure 5).
Neutrophils are capable of releasing a limited number of cytokines and chemokines, which are synthesized de novo in response to proinflammatory stimuli.26-28 In vivo exposure of neutrophils to rhAPC did not significantly modify spontaneous release of TNF-α, MCP-1, IL-8, or MIP-1β by BALF neutrophils, or spontaneous and LPS-induced release of these cytokines by peripheral neutrophils (data not shown).
Effect of in vivo rhAPC administration on survival of neutrophils isolated from BALF and peripheral blood
The effects of systemic rhAPC administration on parameters of neutrophil survival and synthetic response were assayed 2 hours following discontinuation of rhAPC in neutrophils recovered from BALF and peripheral blood. Neutrophils isolated from BALF did not receive additional stimulation in vitro, while neutrophils recovered from blood were either left unstimulated or stimulated with LPS.
In the absence of exogenous stimulation, neutrophils undergo “spontaneous” apoptosis within hours,29,30 while exposure to LPS typically prevents such apoptosis.31,32 In vivo exposure to rhAPC did not significantly inhibit spontaneous apoptosis in either circulating or BALF neutrophils (Figure 6A). Neutrophils that do not undergo apoptosis die by necrosis, which is typified by disruption of the cell membrane and release of the cytosolic content. In vivo exposure of neutrophils to rhAPC did not significantly inhibit spontaneous or LPS-induced necrosis in peripheral neutrophils or spontaneous necrosis in BALF neutrophils (Figure 6B).
Gene expression in pulmonary neutrophils after rhAPC or placebo treatment
High-quality gene expression microarray data were obtained from 9 BAL neutrophil samples in which adequate numbers of cells were obtained for such analysis: 4 samples were from individuals treated with rhAPC, and 5 were from individuals treated with placebo. Comparison of these 2 groups did not reveal a statistically significant gene expression signature attributable to rhAPC administration. Some individual genes did attain a nominal significance in this class comparison: 2 genes are significant at the α = 0.001 level, 32 are significant at the α = 0.01 level (Table 1). However, both sets of nominally significant genes failed to sustain significance upon permutation testing, resulting in an 80% probability that there is no difference between the rhAPC- and placebo-treated groups.
Gene expression of pulmonary neutrophils from rhAPC-or placebo-treated volunteers
. | . | Mean signal . | . | . | . | |
|---|---|---|---|---|---|---|
| Gene symbol . | Gene title . | APC . | Placebo . | Fold increase, APC relative to placebo . | Probeset ID . | |
| TLK1 | Tousled-like kinase 1 | 15.7 | 70.3 | 0.22 | 210379_s_at | |
| LRAP | Leukocyte-derived arginine aminopeptidase | 199.5 | 862.5 | 0.23 | 219759_at | |
| ARTS-1 | Type 1 tumor necrosis factor receptor shedding aminopeptidase regulator | 43.9 | 138.6 | 0.32 | 214012_at | |
| ATP6V1A | ATPase, H+ transporting, lysosomal 70 kDa, V1 subunit A | 382.3 | 964.1 | 0.40 | 201971_s_at | |
| DNCLI1 | Dynein, cytoplasmic, light intermediate polypeptide 1 | 493.4 | 1088.5 | 0.45 | 217976_s_at | |
| HDGFRP3 | Likely ortholog of mouse hepatoma-derived growth factor, related protein 3 | 87.2 | 182.3 | 0.48 | 209526_s_at | |
| AHCYL1 | S-adenosylhomocysteine hydrolase-like 1 | 253.2 | 516.1 | 0.49 | 200848_at | |
| FLOT1 | Flotillin 1 | 756.4 | 1510.8 | 0.50 | 208748_s_at | |
| MAFF | v-maf musculoaponeurotic fibrosarcoma oncogene homolog F (avian) | 352.1 | 656.0 | 0.54 | 205193_at | |
| FLJ21439 | Hypothetical protein FLJ21439 | 869.5 | 1614.0 | 0.54 | 203513_at | |
| SSFA2 | Sperm specific antigen 2 | 455.8 | 811.5 | 0.56 | 202506_at | |
| ZFX | Zinc finger protein, X-linked | 178.0 | 310.8 | 0.57 | 207920_x_at | |
| EIF2S3 | Eukaryotic translation initiation factor 2, subunit 3 gamma, 52 kDa | 490.1 | 853.0 | 0.58 | 205321_at | |
| ALOX5 | Arachidonate 5-lipoxygenase | 3467.4 | 6004.8 | 0.58 | 214366_s_at | |
| PRKAB1 | Protein kinase, AMP-activated, beta 1 noncatalytic subunit | 220.7 | 379.5 | 0.58 | 201834_at | |
| C19orf6 | Chromosome 19 open reading frame 6 | 247.7 | 425.8 | 0.58 | 212574_x_at | |
| TIPR | Homo sapiens TIPR mRNA for inositol 1,4,5-trisphosphate receptor type 2 | 272.6 | 448.8 | 0.61 | 202662_s_at | |
| KIAA0252 | KIAA0252 protein | 265.5 | 436.1 | 0.61 | 212301_at | |
| ICAM1 | Intercellular adhesion molecule 1 (CD54), human rhinovirus receptor | 621.8 | 1007.6 | 0.62 | 215485_s_at | |
| RRM2B | Ribonucleotide reductase M2 B (TP53 inducible) | 199.9 | 320.9 | 0.62 | 208883_at | |
| FLJ14345 | Hypothetical protein FLJ14345 | 224.9 | 356.1 | 0.63 | 220760_x_at | |
| NFYC | Nuclear transcription factor Y, gamma | 706.7 | 1073.4 | 0.66 | 211251_x_at | |
| BRD1 | Bromodomain containing 1 | 339.4 | 506.8 | 0.67 | 215460_x_at | |
| EKI1 | Ethanolamine kinase | 147.7 | 217.9 | 0.68 | 222262_s_at | |
| CASP9 | Caspase 9, apoptosis-related cysteine protease | 829.9 | 1129.7 | 0.74 | 210775_x_at | |
| nexilin | Likely ortholog of rat F-actin binding protein nexilin | 187.8 | 254.1 | 0.74 | 212847_at | |
| ZNF434 | Zinc finger protein 434 | 498.9 | 667.0 | 0.75 | 218937_at | |
| FLJ10948 | Hypothetical protein FLJ10948 | 303.3 | 244.2 | 1.24 | 218552_at | |
| MGC11256 | Hypothetical protein MGC11256 | 828.4 | 581.7 | 1.42 | 218358_at | |
| SPEC1 | Small protein effector 1 of Cdc42 | 1623.4 | 1121.4 | 1.45 | 218157_x_at | |
| RPL38 | Ribosomal protein L38 | 1164.4 | 733.7 | 1.59 | 202028_s_at | |
| RPS20 | Ribosomal protein S20 | 213.4 | 116.4 | 1.83 | 216246_at | |
| ZPBP | Zona pellucida binding protein | 198.1 | 84.6 | 2.34 | 207021_at | |
. | . | Mean signal . | . | . | . | |
|---|---|---|---|---|---|---|
| Gene symbol . | Gene title . | APC . | Placebo . | Fold increase, APC relative to placebo . | Probeset ID . | |
| TLK1 | Tousled-like kinase 1 | 15.7 | 70.3 | 0.22 | 210379_s_at | |
| LRAP | Leukocyte-derived arginine aminopeptidase | 199.5 | 862.5 | 0.23 | 219759_at | |
| ARTS-1 | Type 1 tumor necrosis factor receptor shedding aminopeptidase regulator | 43.9 | 138.6 | 0.32 | 214012_at | |
| ATP6V1A | ATPase, H+ transporting, lysosomal 70 kDa, V1 subunit A | 382.3 | 964.1 | 0.40 | 201971_s_at | |
| DNCLI1 | Dynein, cytoplasmic, light intermediate polypeptide 1 | 493.4 | 1088.5 | 0.45 | 217976_s_at | |
| HDGFRP3 | Likely ortholog of mouse hepatoma-derived growth factor, related protein 3 | 87.2 | 182.3 | 0.48 | 209526_s_at | |
| AHCYL1 | S-adenosylhomocysteine hydrolase-like 1 | 253.2 | 516.1 | 0.49 | 200848_at | |
| FLOT1 | Flotillin 1 | 756.4 | 1510.8 | 0.50 | 208748_s_at | |
| MAFF | v-maf musculoaponeurotic fibrosarcoma oncogene homolog F (avian) | 352.1 | 656.0 | 0.54 | 205193_at | |
| FLJ21439 | Hypothetical protein FLJ21439 | 869.5 | 1614.0 | 0.54 | 203513_at | |
| SSFA2 | Sperm specific antigen 2 | 455.8 | 811.5 | 0.56 | 202506_at | |
| ZFX | Zinc finger protein, X-linked | 178.0 | 310.8 | 0.57 | 207920_x_at | |
| EIF2S3 | Eukaryotic translation initiation factor 2, subunit 3 gamma, 52 kDa | 490.1 | 853.0 | 0.58 | 205321_at | |
| ALOX5 | Arachidonate 5-lipoxygenase | 3467.4 | 6004.8 | 0.58 | 214366_s_at | |
| PRKAB1 | Protein kinase, AMP-activated, beta 1 noncatalytic subunit | 220.7 | 379.5 | 0.58 | 201834_at | |
| C19orf6 | Chromosome 19 open reading frame 6 | 247.7 | 425.8 | 0.58 | 212574_x_at | |
| TIPR | Homo sapiens TIPR mRNA for inositol 1,4,5-trisphosphate receptor type 2 | 272.6 | 448.8 | 0.61 | 202662_s_at | |
| KIAA0252 | KIAA0252 protein | 265.5 | 436.1 | 0.61 | 212301_at | |
| ICAM1 | Intercellular adhesion molecule 1 (CD54), human rhinovirus receptor | 621.8 | 1007.6 | 0.62 | 215485_s_at | |
| RRM2B | Ribonucleotide reductase M2 B (TP53 inducible) | 199.9 | 320.9 | 0.62 | 208883_at | |
| FLJ14345 | Hypothetical protein FLJ14345 | 224.9 | 356.1 | 0.63 | 220760_x_at | |
| NFYC | Nuclear transcription factor Y, gamma | 706.7 | 1073.4 | 0.66 | 211251_x_at | |
| BRD1 | Bromodomain containing 1 | 339.4 | 506.8 | 0.67 | 215460_x_at | |
| EKI1 | Ethanolamine kinase | 147.7 | 217.9 | 0.68 | 222262_s_at | |
| CASP9 | Caspase 9, apoptosis-related cysteine protease | 829.9 | 1129.7 | 0.74 | 210775_x_at | |
| nexilin | Likely ortholog of rat F-actin binding protein nexilin | 187.8 | 254.1 | 0.74 | 212847_at | |
| ZNF434 | Zinc finger protein 434 | 498.9 | 667.0 | 0.75 | 218937_at | |
| FLJ10948 | Hypothetical protein FLJ10948 | 303.3 | 244.2 | 1.24 | 218552_at | |
| MGC11256 | Hypothetical protein MGC11256 | 828.4 | 581.7 | 1.42 | 218358_at | |
| SPEC1 | Small protein effector 1 of Cdc42 | 1623.4 | 1121.4 | 1.45 | 218157_x_at | |
| RPL38 | Ribosomal protein L38 | 1164.4 | 733.7 | 1.59 | 202028_s_at | |
| RPS20 | Ribosomal protein S20 | 213.4 | 116.4 | 1.83 | 216246_at | |
| ZPBP | Zona pellucida binding protein | 198.1 | 84.6 | 2.34 | 207021_at | |
All genes shown are transcripts with a parametric P < .001.
Activated protein C blocks neutrophil migration in vitro
Previous studies had shown that rhAPC inhibited in vitro neutrophil chemotaxis.11 However, in those experiments, neutrophil migration was measured at a single time point, 30 minutes after establishment of the chemotactic gradient. To more completely characterize the time course for the effects of rhAPC on neutrophil chemotaxis, IL-8-induced migration was monitored over a 2-hour period using neutrophils from healthy humans that had been pretreated with a range of concentrations of rhAPC.
Pretreatment of neutrophils with rhAPC resulted in a significant reduction in IL-8-induced migration in a concentration-dependent manner, while simultaneous analysis of nondirectional migration (NDM) of the neutrophils demonstrated no effect of APC (data not shown). IL-8-induced migration relative to NDM was also reduced by APC in a concentration-dependent manner (Figure 7A). Although decreased chemotaxis was noted in rhAPC-exposed neutrophils 30 minutes after the initiation of the chemotactic gradient, the greatest effect of rhAPC on neutrophil migration was observed from 60 minutes to 120 minutes.
Effect of in vivo rhAPC administration on pulmonary neutrophil migration
BALF neutrophils recovered from individuals treated with rhAPC demonstrated a significant decrease in IL-8-induced chemotaxis relative to NDM when assayed ex vivo (Figure 7B). As with the in vitro studies (Figure 7A), the greatest differences in relative migration among pulmonary neutrophils from the rhAPC- and placebo-treated groups was found at later time points following the initiation of chemotaxis.
Discussion
RhAPC administered in vivo reduced neutrophil accumulation into the airways in response to endobronchial endotoxin administration, a model for localized pulmonary inflammation induced by bacterial pneumonia. Neutrophils recovered from the airspace of volunteers who received rhAPC demonstrated a significantly decreased chemotaxic response to IL-8 when analyzed ex vivo. Using a modified Boyden chamber method of neutrophil migration analysis, we were able to appreciate that the greatest effect of rhAPC in reducing chemotaxis occurred after 30 minutes. A similar pattern of reduced chemotaxis, particularly at late time points, was found among neutrophils exposed to rhAPC in vitro.
No other effect of in vivo rhAPC administration was seen in a broad range of neutrophil responses or in measures of systemic inflammation. Neutrophils that migrated to the airspaces during rhAPC administration demonstrated no differences in gene expression, intracellular signaling, cytokine release, survival, or apoptosis when compared with neutrophils isolated from the BALF of volunteers who received placebo. Likewise, no differences in cytokine release, survival, or apoptosis were detected in circulating neutrophils 2 hours after discontinuation of the APC infusion when compared with circulating neutrophils from the placebo-treated volunteers. Finally, there was no evidence of decreased inflammatory response of the lung, as measured by total protein concentrations in BALF or the recovery of cytokines, chemokines, and soluble cytokine receptors and receptor-antagonists in BALF.
The results of this coordinated functional and genomic analysis detected only decreased neutrophil migration as a mechanism by which rhAPC administration reduced leukocyte accumulation in response to endobronchial administration endotoxin. This conclusion is supported by recent findings that rhAPC blocks neutrophil migration to a number of chemokines in vitro, but does not reduce responses such as bacterial phagocytosis, respiratory burst, or apoptosis.11
The mechanism by which rhAPC reduces neutrophil migration is unknown. However, 30 minutes after establishment of a chemotactic gradient, migration induced by formyl-Met-Leu-Phe (fMLP), C5a, antithrombin (AT), as well as IL-8, was significantly reduced in neutrophils pretreated with rhAPC.11 It was speculated that since rhAPC inhibits migration toward a range of substances with a variety of receptors and postreceptor signaling pathways, then rhAPC must work at a more central point in the regulation of neutrophil chemotaxis.11 Thus, in the present study, all studies of neutrophil migration were performed only toward IL-8, as it has a clearly defined role in sepsis-related neutrophil accumulation.33 Based on the recent report by Sturn et al,11 it is likely that equivalent responses would be observed in the setting of chemotaxis toward other agents, but confirmation of the effect of rhAPC in blocking migration induced by fMLP, C5a, or AT could not be performed in the present studies due to the limited numbers of neutrophils isolated from BALF in rhAPC-treated volunteers.
Microarray studies of pulmonary neutrophils failed to show a statistically significant pattern of expression differences between rhAPC- and placebo-treated individuals. Furthermore, the functional makeup of the nominally significant genes found in these analyses (Table 1) does not support a consistent effect of rhAPC on neutrophil gene expression. In particular, none of the approximately 80 chemotaxis-related genes (as assigned by Gene Ontology categorization) exhibit differential expression between these groups. However, the relatively small list of significant genes found in these analyses (Table 1) contains at least one gene implicated in lung inflammation. The gene encoding 5-LO had the greatest expression in placebo-treated BAL neutrophils and was reduced 42% in the APC-treated volunteers (P = .009). 5-LO is the enzyme responsible for the conversion of arachidonic acid (AA) to LTA4, thereby mediating the synthesis of all leukotrienes (LTs). The LTs, especially LTB4, are potent inflammatory mediators released by leukocytes in response to inflammatory stimuli. Effects of LTs include increased permeability of postcapillary venules, bronchoconstriction, chemotaxis of neutrophils, and increased adhesion of leukocytes to endothelial cells,34-36 and 5-LO knockout mice demonstrate decreased neutrophil migration in a model of acute lung injury.24 However, we were unable to detect a significant decrease in 5-LO protein expression in APC-treated BAL neutrophils, as predicted by decreased transcription of the gene detected by oliogonucleotide array analysis. It is possible that other genes identified to be affected by rhAPC administration may have roles in neutrophil migration that are presently not appreciated.
The present results, showing that rhAPC decreases neutrophil chemotaxis and accumulation in a relevant in vivo human model of acute pulmonary inflammation, provide important insights into a potential mechanism by which rhAPC decreases organ injury and mortality in sepsis. Although inflammation induced by endobronchial instillation of LPS is less severe, of shorter duration, and less “complex” then the proinflammatory stimuli encountered in the setting of sepsis or pneumonia, no anti-inflammatory effects of rhAPC were found in this study. A similar lack of anti-inflammatory properties was demonstrated when rhAPC was administered to endotoxemic humans.9,10 Minimal effects of rhAPC on circulating cytokine levels were found in the PROWESS (Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis) study, in which rhAPC reduced mortality from severe sepsis.37 Such findings suggest that beneficial effects of rhAPC in severe infection may result from directly inhibiting neutrophil migration, thereby reducing the injurious effects associated with excessive accumulation of activated neutrophils into the lungs and other organs. The specificity of this action, independent of detectable modulation of other neutrophil functions or inflammatory responses, has the potential to be a very selective intervention to reduce overly exuberant neutrophil recruitment without inducing immunosuppression.
Prepublished online as Blood First Edition Paper, August 31, 2004; DOI 10.1182/blood-2004-06-2140.
Supported by grants HL068743 and M01 RR0051 from the National Institutes of Health, and Eli Lilly and Company.
E.A. has served as a paid consultant for Eli Lilly and Company.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal