The antiviral response of CD8 T cells involves the differentiation of naive T cells into distinct types of effector and memory cells, which may be distinguished by the level of CD7 expression. We have investigated CD8 T cells in adults and children infected with HIV-1 to determine the disease relevance of cell subsets defined by CD7. CD8 T cells from patients infected with HIV-1 displayed profound down-modulation of CD7 expression as compared with healthy subjects, with expansion of both CD7low and CD7negative effector subsets. Loss of CD7high cells correlated directly with HIV-1 load and was particularly pronounced in patients with rapid disease progression. CD8 T cells specific for HIV-1, as well as Epstein-Barr virus (EBV) and cytomegalovirus (CMV) were predominantly found in the CD7low effector cell subset. Furthermore, recovery of CD4 counts on antiretroviral therapy was associated with reversion of the skewed CD7 profile in CD8 T cells. Thus, effector CD8 T-cell subsets distinguished by lowered CD7 expression expand in a manner that correlates with the magnitude of HIV-1, EBV, and CMV antigenic challenge and contract in response to successful antiretroviral treatment. The results are discussed in relation to the dual roles of CD7 as a receptor of both costimulation and cell death.
Introduction
CD8 T cells play a key role in suppressing viral replication in HIV-1 infection, and the peak in plasma viremia during primary infection is rapidly suppressed by the HIV-1–specific CD8 T-cell response.1-3 Despite this strong initial antiviral immune response, persistent control of viral replication is not achieved in the vast majority of individuals infected with HIV-1. The inability of the immune system to mount a strong, effective, and persistent immune response in chronic infection is a key element in HIV-1 disease pathogenesis. Although both viral and host factors are likely to contribute to the failure of the immune system to control HIV-1, there is still considerable controversy regarding the mechanisms underlying this failure and the effects of the persistent immune activation that characterizes HIV-1 infection.4-7
There has recently been considerable focus on the differentiation status of CD8 T cells in chronic HIV-1 infection. It has been suggested that HIV-1–specific CD8 T cells may fail to mature into effector cells, and they would therefore be functionally incompetent when compared with CD8 T cells specific for other viruses that cause chronic infections.8,9 Although HIV-1–specific and cytomegalovirus (CMV)–specific CD8 T cells differ in surface phenotype, this may reflect normal differences in the nature of immune response to diverse pathogens rather than a defect in the maturation of HIV-specific T cells.10-16 In addition to changes in antigen-specific cells, it is also clear that HIV-1 has a dramatic effect on the entire T-cell population. For example, several reports have shown close association between the activation status of CD8 T cells and HIV-1 disease progression,17-21 and the level of CD8 T cell activation in early infection is an independent predictor of the rate of CD4 T-cell decline.20 Also, HIV-1 infection is accompanied by phenotypic alterations which include depletion of naive CD4 and CD8 T cells and expansion of antigen-experienced T cells characterized by down-regulation of CD27 and CD28 expression.22,23 The relationship between the capacity of HIV-1 to cause generalized immune activation and its capacity to alter the phenotypic characteristics of antigen-specific cells has not been well studied.
We have recently proposed a model for CD8 T-cell differentiation based on the level of expression of CD7.24 CD7 is a 40-kDa transmembrane glycoprotein of the immunoglobulin superfamily, which is expressed early in T-cell ontogeny and on most T cells in the periphery.25,26 The natural ligand for CD7 has recently been shown to be a secreted molecule called K-12 or SECTM-1.27 CD7 has been recognized as a costimulatory molecule, leading to increased integrin adhesiveness through activation of phosphatidylinositol 3 (PI3) kinase.28-31 Interestingly, CD7 is also necessary for induction of apoptosis in T cells by the β-galactoside–binding lectin galectin-1.32-36 CD7 may thus play contrasting roles during different stages of an immune response.
Three distinct CD8 T-cell populations can be distinguished by the level of CD7 expression.24 The CD7high subset contains cells with characteristics of naive cells and long-term memory cells, whereas the CD7low and CD7negative subsets both contain lytic and cytokine producing effector-like cells.24 In the present study, we examined CD8 T cells from patients infected with HIV-1 to determine the functional relevance of these subsets in the immune response to HIV-1. We show that CD8 T cells in subjects infected with HIV-1 display a profound down-modulation of CD7 expression from early in infection, leading to a skewed CD7 profile in the CD8 T-cell compartment with expansion of CD7low and CD7negative subsets. Loss of CD7high CD8 T cells correlates directly with HIV-1 load, indicating that this is an antigen-driven process. Furthermore, CD8 T cells specific for HIV-1, as well as Epstein-Barr virus (EBV) and CMV are predominantly found in the CD7low subset, indicating that responses to not only HIV-1 but also CMV and EBV contribute to the expansion of this subset. In contrast, CD8 T cells against influenza virus exhibit a CD7high memory phenotype. Recovery of CD7high CD8 T cells correlates with recovery of CD4 T cells in both adult and pediatric subjects infected with HIV-1 on antiretroviral therapy. The results indicate relevance of the CD7-based model for CD8 T-cell differentiation in HIV disease, bearing in mind the dual roles of CD7 as receptor of both costimulation and cell death.
Patients, materials, and methods
Study subjects and samples
Blood samples from 23 healthy blood donors not infected with HIV were obtained after informed consent. Blood samples from subjects infected with HIV-1 were obtained from 17 adult patients from the San Francisco General Hospital (San Francisco, CA) (median age, 47 years; range, 37-56 years; 15 of 17 patients were on antiretroviral treatment [ART]; median CD4 T-cell count = 526 cells/μL; range, 95-1175 cells/μL), 35 adult patients from the Karolinska University Hospital (Stockholm, Sweden) (median age, 41 years; range, 27-62 years; 16 of 35 patients were on ART; median CD4 T-cell count = 443 cells/μL; range, 21-1370 cells/μL), and 16 pediatric patients with vertically acquired HIV-1 infection from the Jacobi Medical Center (Bronx, NY) (median age, 6.5 years; range, 2-11 years; median CD4 T-cell count = 986 cells/μL; range, 84-2090 cells/μL). All 16 pediatric patients were on ART but experienced varying degrees of viral suppression because of adherence problems and drug resistance. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood by Ficoll-Paque PLUS density gradient centrifugation (Amersham Pharmacia Biotech, Uppsala, Sweden) and washed twice in RPMI 1640 (Life Technologies, Gaithersburg, MD) with 15% fetal calf serum. When used, cryopreserved samples were washed and cultured overnight before analysis by flow cytometry. Plasma HIV-1 RNA was measured with the Amplicor HIV-1 Monitor with a lower limit of quantification at 50 copies of RNA/mL (Roche Diagnostic Systems, Branchburg, NJ). The study was based on protocols approved by the local institutional review boards.
Flow cytometry, mAbs, and HLA-A2 tetramers
Anti-CD3 fluorescein isothiocyanate (FITC) and peridinin chlorophyll protein (PerCp), anti-CD4 PerCp, anti-CD8 FITC and allophycocyanin (APC), anti-CD7 phycoerythrin (PE) and PE-Cy5 (cyanine 5), anti-CD27 FITC and PE, anti-CD28 FITC and PE, anti-perforin FITC, anti-granzyme A FITC, anti-CD45RO FITC and APC, and anti-CD62L FITC antibodies were purchased from BD Pharmingen (San Diego, CA). Subjects were evaluated for HLA-A2 expression by staining with anti–HLA-A2 (One Lambda, Canoga Park). CD8 T cells specific for viruses were identified and enumerated using PE- or APC-conjugated HLA-A2 tetrameric complexes refolded with the HIV-1 Gag 77 to 85 peptide epitope SLYNTVATL, the HIV-1 Pol 476 to 484 epitope ILKEPVHGV, the CMV pp65 495 to 503 epitope NLVPMVATV, the EBV BMLF1 280 to 288 epitope GLCTL-VAML, or the influenza virus M1 58 to 66 epitope GILGFVFTL (Coulter Immunotech, Marseilles, France). For surface staining, purified PBMCs were incubated with a panel of fluorochrome-conjugated monoclonal antibodies (mAbs) and HLA-tetramers. For intracellular staining PBMCs were first stained with mAbs to surface molecules, washed once, and permeabilized in fluorescence-activated cell-sorting (FACS) permeabilization buffer (BD Pharmingen) for 10 minutes before staining with mAbs to perforin and granzyme A. Finally, samples were washed 3 times and fixed in phosphate-buffered saline (PBS) with 1% formaldehyde. Samples were analyzed using a FACSCalibur Instrument (BD Pharmingen), and CellQuest (BD Pharmingen) or FlowJo software (Tree Star, Ashland, OR).
Statistical analysis
The flow cytometry data and clinical data obtained were analyzed by descriptive statistics, linear regression, t test, and the Mann-Whitney rank sum test, as appropriate by using Sigma Stat software (SPSS, Chicago, IL).
Results
Decreased CD7 expression in HIV-1 infection is primarily confined to CD8 T cells
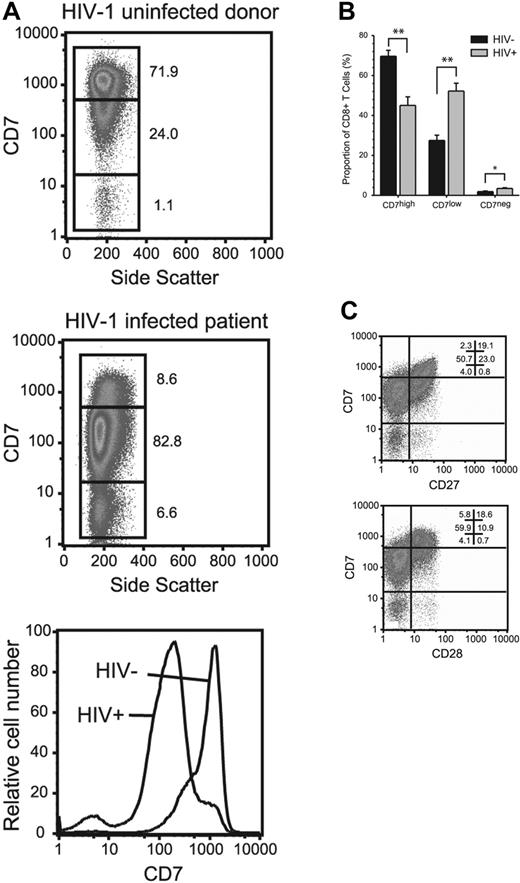
We have previously shown that 3 functionally distinct CD8 T-cell populations can be distinguished by the level of CD7 expression: CD7high, CD7low, and CD7negative.24 The CD7high subset contains cells with characteristics of naive cells and long-term memory cells that express CD27 and CD28 and proliferate well in response to antigen. The CD7low and CD7negative subsets both contain effector-like cells, which are heterogeneous with respect to CD27 and CD28 expression and display poor capacity to proliferate. Here, we measured CD7 expression on lymphocytes in 52 adult subjects with chronic HIV-1 infection and 23 uninfected control subjects. There was a profound decrease in the overall expression of CD7 in the CD8 T-cell compartment of patients infected with HIV-1 as compared with uninfected control subjects, with an expansion of the CD7low and CD7negative subsets (Figure 1A-B). The expanded CD7low and CD7negative CD8 T cells largely lacked the costimulatory receptors CD27 and CD28 (Figure 1C).
Down-modulation of CD7 expression in CD8 T cells in patients infected with HIV-1. (A) PBMCs from healthy adult blood donors and adult patients infected with HIV-1 were stained with monoclonal antibodies, including CD7. Data in plots are gated on CD8 T cells. Numbers in figure indicate percentage of cells within the 3 gates. The bar chart (B) represents accumulative data from uninfected healthy blood donors (n = 23) and subjects infected with HIV-1 (n = 17); **P < .001; *P < .005. Statistical analysis: independent samples t test. Mean and standard error is shown. (C) The expression of CD27 and CD28 splits the CD7low and CD7negative CD8 T-cell subsets into separate subpopulations.
Down-modulation of CD7 expression in CD8 T cells in patients infected with HIV-1. (A) PBMCs from healthy adult blood donors and adult patients infected with HIV-1 were stained with monoclonal antibodies, including CD7. Data in plots are gated on CD8 T cells. Numbers in figure indicate percentage of cells within the 3 gates. The bar chart (B) represents accumulative data from uninfected healthy blood donors (n = 23) and subjects infected with HIV-1 (n = 17); **P < .001; *P < .005. Statistical analysis: independent samples t test. Mean and standard error is shown. (C) The expression of CD27 and CD28 splits the CD7low and CD7negative CD8 T-cell subsets into separate subpopulations.
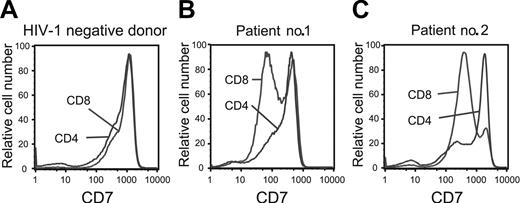
In a comparison of T-cell subsets, we found CD7 to be equally expressed on CD8 and CD4 T cells in healthy control subjects (Figure 2A). In contrast, patients infected with HIV-1 with a CD4 T-cell count above 200 cells/μL showed a largely preserved CD7 expression on CD4 T cells, whereas the CD8 T-cell compartment was skewed toward a CD7low and CD7negative phenotype (Figure 2B). This may highlight differing dynamics of the CD4 and CD8 T-cell compartments in response to HIV-1 infection, because preserved CD7 expression on CD4 T cells and a skewed distribution in the CD8 T-cell compartment were consistent findings in most subjects infected with HIV-1. However, in patients with advanced HIV disease and CD4 T-cell counts below 200 cells/μL we observed the appearance of clear CD7low and CD7negative populations also in the CD4 T-cell compartment (Figure 2C). In summary, there is a clear shift in the expression of the CD7 receptor in subjects infected with HIV-1, and this effect is most prominent in the CD8 T-cell compartment.
Down-modulation of CD7 preferentially occurs in CD8 T cells in HIV-1 infection. The level of CD7 expression on CD4 and CD8 T cells in healthy individuals not infected with HIV (left panel). CD7 expression is preserved on CD4 T cells in patients with chronic HIV-1 infection (patient no. 1), whereas CD7 is partially down-regulated on CD4 T cells in patients with advanced HIV-1 disease (patient no. 2). Representative data are shown.
Down-modulation of CD7 preferentially occurs in CD8 T cells in HIV-1 infection. The level of CD7 expression on CD4 and CD8 T cells in healthy individuals not infected with HIV (left panel). CD7 expression is preserved on CD4 T cells in patients with chronic HIV-1 infection (patient no. 1), whereas CD7 is partially down-regulated on CD4 T cells in patients with advanced HIV-1 disease (patient no. 2). Representative data are shown.
Antigen-driven skewing of CD7 expression in HIV-1 infection
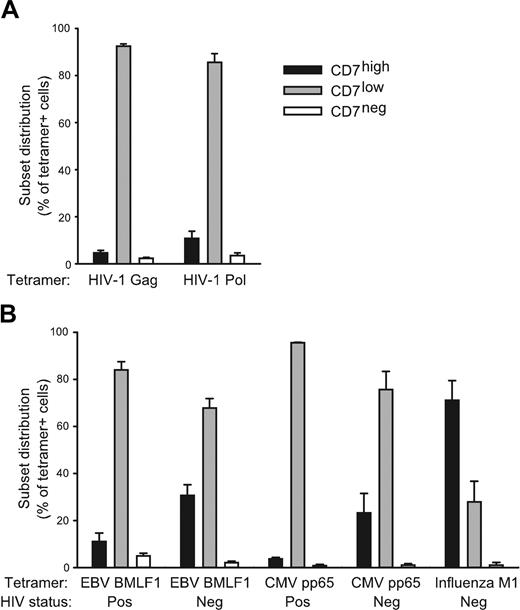
Our previous studies have indicated that CD7 is a differentiation marker that is down-modulated as CD8 T cells differentiate into lytic or cytokine-expressing effector cells.24 We, therefore, hypothesized that the shift from a CD7high to a CD7low profile in the CD8 T-cell compartment occurs as part of the response to HIV-1. To investigate this, we analyzed the CD7 expression in CD8 T cells from 21 subjects with chronic HIV-1 infection who were not on ART. In this patient group there was a significant correlation between loss of CD7high CD8 T cells and higher viral load (R = 0.47, P = .03), suggesting that CD7high CD8 T cells decline in response to HIV antigens (Figure 3). Furthermore, HIV-1–specific CD8 T cells staining positive for HLA-A2 tetramers refolded with the well-defined Gag 77 to 85 and Pol 476 to 484 epitopes were predominantly CD7low with minor CD7high and CD7negative populations (Figure 4A). Thus, HIV-specific effector CD8 T cells likely contribute to the HIV-driven expansion of CD7low CD8 T cells. A predominant CD7low profile was also observed in CD8 T-cell populations specific for the EBV BMLF1 280 to 288 epitope and the CMV pp65 495 to 503 epitope in individuals infected with HIV-1 (Figure 4B). Interestingly, CD8 T cells specific for the EBV epitope in healthy control subjects displayed a different profile with more cells expressing CD7 at high levels (P = .01), and a similar trend was also observed for cells reactive against CMV (Figure 4B). This pattern suggests that effector (CD7low) CD8 T-cell responses to EBV and CMV contribute to the CD7low profile of the CD8 T-cell compartment in subjects infected with HIV-1. Strikingly, influenza virus M1 58 to 66 epitope-specific CD8 T cells were predominantly CD7high, consistent with a long-term memory T-cell phenotype (Figure 4B). Taken together, these data indicate that the level of CD7 expression is down-modulated in the effector phase of CD8 T-cell responses against chronic viral antigens. Furthermore, our results indicate that CD7 can be used to determine the differentiation stage of virus-specific CD8 T cells during HIV-1 infection and to assess the magnitude of such responses.
Skewing of CD7 expression in CD8 T cells is antigen driven. Correlation between the percentage of CD7high cells in the CD8 T-cell compartment and viral load in adult patients with chronic untreated HIV-1 infection. Linear regression: R = 0.47; P = .03; n = 21.
Skewing of CD7 expression in CD8 T cells is antigen driven. Correlation between the percentage of CD7high cells in the CD8 T-cell compartment and viral load in adult patients with chronic untreated HIV-1 infection. Linear regression: R = 0.47; P = .03; n = 21.
Identity of virus-specific CD8 T cells. The CD7 profile of CD8 T cells was determined by 4-color flow cytometry. (A) CD8 T cells specific for HIV-1 were identified in PBMCs for subjects infected with HIV-1 using HLA-A2 tetramers with the HIV-1 Gag 77 to 85 peptide (n = 9) and the HIV-1 Pol 476 to 484 epitope (n = 6). (B) CD8 T cells specific for viruses were identified in PBMCs from patients infected with HIV-1 and healthy subjects as indicated by using HLA-A2 tetramers with the CMV pp65 495 to 503 epitope (n = 3), the EBV BMLF1 280 to 288 epitope (n = 8), and the influenza virus M1 58 to 66 epitope (n = 3). Mean and standard error of CD7high (▪), CD7low (▦), and CD7negative (□) are shown.
Identity of virus-specific CD8 T cells. The CD7 profile of CD8 T cells was determined by 4-color flow cytometry. (A) CD8 T cells specific for HIV-1 were identified in PBMCs for subjects infected with HIV-1 using HLA-A2 tetramers with the HIV-1 Gag 77 to 85 peptide (n = 9) and the HIV-1 Pol 476 to 484 epitope (n = 6). (B) CD8 T cells specific for viruses were identified in PBMCs from patients infected with HIV-1 and healthy subjects as indicated by using HLA-A2 tetramers with the CMV pp65 495 to 503 epitope (n = 3), the EBV BMLF1 280 to 288 epitope (n = 8), and the influenza virus M1 58 to 66 epitope (n = 3). Mean and standard error of CD7high (▪), CD7low (▦), and CD7negative (□) are shown.
Influence of antiretroviral therapy and patient status on CD7 expression in the CD8 T-cell compartment
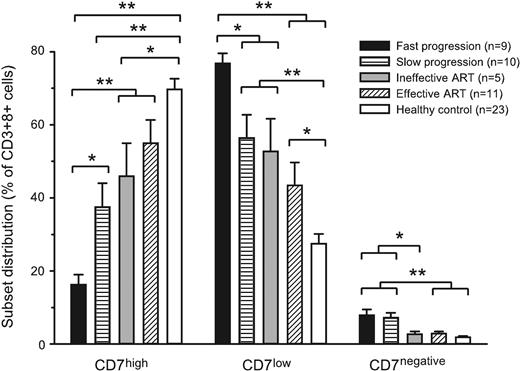
Our data indicate that the level of CD7 expression identifies CD8 T-cell subsets with clear relevance for the immune response to HIV-1. This led us to investigate the effect of ART therapy on the CD7 status of the CD8 T cells, as well as the possible association between rate of disease progression and CD7 status of the CD8 T-cell compartment. We first divided chronically infected patients who were not on ART into 2 groups with relatively fast and slow disease progression, respectively. Patients whose CD4 T-cell counts had not dropped below 300 cells/μL after being infected for more than 10 years (range, 10-18 years) were considered to show slow progression (n = 10). Subjects whose CD4 T-cell counts had reached 400 cells/μL in less than 6 years (range, 1.5-6 years) were considered to have signs of fast disease progression (n = 9). Furthermore, we investigated the CD7 status of the CD8 T-cell compartment in 2 groups of patients on ART therapy; 1 group with complete viral suppression for more than 1 year (n = 11), and 1 group with only partial viral suppression (n = 5). These 4 groups were also compared with a group of healthy laboratory volunteers (n = 23). There were distinct differences in terms of CD7 expression in the CD8 T-cell compartment between the groups, with faster disease progression being associated with a strong skewing toward a CD7low CD8 T-cell phenotype, whereas the healthy control group displayed a predominantly CD7high phenotype (Figure 5). In terms of the expansion of CD7low CD8 T cells, all groups of patients infected with HIV-1 were significantly different from the healthy subjects, and fast disease progression was also significantly different from patients treated with ART as well as patients with slow disease progression (statistical significance indicated in Figure 5). With regard to CD7negative CD8 T cells, the 2 groups of untreated patients displayed expansions of these cells, as compared both with healthy control subjects and with patients on ART (statistical significance indicated in Figure 5). These data suggest that the level of CD7 expression in the CD8 T-cell compartment has disease relevance in HIV-1 infection in that fast progression is associated with a strong skewing toward a CD7low and CD7negative profile, whereas effective antiretroviral therapy is associated with a CD7high profile.
CD7 expression in CD8 T cells versus patient status. CD7 profile of the CD8 T-cell compartment was assessed by 4-color flow cytometry, and the distribution between CD7high, CD7low, and CD7negative subsets was determined in adult subject groups: Fast disease progression (▪, n = 9), slow disease progression (▤, n = 10), subjects on ART with incomplete viral suppression ( , n = 5), subjects on ART with complete viral suppression (▨, n = 11), and healthy control subjects (□, n = 23). Mean and standard error are shown. *P < .05 and **P < .005 as determined by the t test or, if normality test failed, the Mann-Whitney rank sum test.
, n = 5), subjects on ART with complete viral suppression (▨, n = 11), and healthy control subjects (□, n = 23). Mean and standard error are shown. *P < .05 and **P < .005 as determined by the t test or, if normality test failed, the Mann-Whitney rank sum test.
CD7 expression in CD8 T cells versus patient status. CD7 profile of the CD8 T-cell compartment was assessed by 4-color flow cytometry, and the distribution between CD7high, CD7low, and CD7negative subsets was determined in adult subject groups: Fast disease progression (▪, n = 9), slow disease progression (▤, n = 10), subjects on ART with incomplete viral suppression ( , n = 5), subjects on ART with complete viral suppression (▨, n = 11), and healthy control subjects (□, n = 23). Mean and standard error are shown. *P < .05 and **P < .005 as determined by the t test or, if normality test failed, the Mann-Whitney rank sum test.
, n = 5), subjects on ART with complete viral suppression (▨, n = 11), and healthy control subjects (□, n = 23). Mean and standard error are shown. *P < .05 and **P < .005 as determined by the t test or, if normality test failed, the Mann-Whitney rank sum test.
Response to antiretroviral treatment correlates directly with normalization of CD7 status
To further explore the clinical significance of CD7 expression levels in the CD8 T-cell compartment in relation to antiretroviral treatment, we evaluated possible relationships between CD7 expression and CD4 T-cell counts in an expanded cohort of 32 subjects with chronic HIV-1 infection on ART. Twenty of these subjects had undetectable virus, whereas the remaining 12 displayed low-level HIV-1 replication with viral loads in the range of 50 to 3000 copies/mL. The 32 subjects had initiated ART during moderate to advanced HIV-1 infection. There was a positive correlation between the CD4 count and the percentage of cells in the CD7high subset (P = .007, R = 0.48), suggesting that the generation or expansion of the predominant CD7high status of the CD8 T-cell compartment is associated with, or is a part of, the process of CD4 T-cell recovery (Figure 6A). In accordance with this relationship, we observed a corresponding negative correlation between the proportion of CD7low CD8 T cells and CD4 T-cell counts (P = .007, R = 0.47) (Figure 6B). No significant trend was evident between CD4 counts and the proportion of CD7negative CD8 T cells (Figure 6C). Although cross-sectional in nature, these data together suggest that recovery of CD4 T-cell counts in response to ART treatment correlates with gradual restoration of the CD7 subset distribution in the CD8 T-cell compartment.
Correlation between CD7 status and recovery of CD4 T cells on ART. CD7 expression in the CD8 T-cell compartment in PBMCs from adult subjects infected with HIV-1 on ART (n = 32) was assessed by 4-color flow cytometry, and the correlation between (A) CD7high, (B) CD7low, and (C) CD7negative subset frequencies and CD4 T-cell counts was analyzed by linear regression. (D) A similar analysis was performed on PBMCs from 16 pediatric subjects infected with HIV-1 on ART. CD7 expression in CD8 T cells was analyzed in relation to both absolute CD4 T-cell counts (left) and the CD4 T-cell percentage (right). In the pediatric group the CD7low and CD7negative subsets were fused in the analysis because the CD7negative subset frequency was very low.
Correlation between CD7 status and recovery of CD4 T cells on ART. CD7 expression in the CD8 T-cell compartment in PBMCs from adult subjects infected with HIV-1 on ART (n = 32) was assessed by 4-color flow cytometry, and the correlation between (A) CD7high, (B) CD7low, and (C) CD7negative subset frequencies and CD4 T-cell counts was analyzed by linear regression. (D) A similar analysis was performed on PBMCs from 16 pediatric subjects infected with HIV-1 on ART. CD7 expression in CD8 T cells was analyzed in relation to both absolute CD4 T-cell counts (left) and the CD4 T-cell percentage (right). In the pediatric group the CD7low and CD7negative subsets were fused in the analysis because the CD7negative subset frequency was very low.
Strong correlation between CD4 T-cell counts and CD7 status of the CD8 T-cell compartment in pediatric HIV-1 infection
We next assessed the level of CD7 expression on CD8 T cells in 16 children with vertically acquired chronic HIV-1 infection. These patients were diverse with respect to both clinical status and treatment compliance and regimen and, therefore, provided an opportunity to perform a cross-sectional analysis in a heterogeneous population. In this group of infected children, CD7 expression on CD8 T cells correlated directly with both the absolute CD4 T-cell count (P < .001, R = 0.79) and the CD4 T-cell percentage (P < .001, R = 0.81), such that an expansion of the CD7low and CD7negative CD8 T-cell subsets were associated with low CD4 counts as well as low CD4 percentage (Figure 6D). These data indicate a strong correlation between CD7 status in CD8 T cells and loss of CD4 T cells in children infected with HIV-1.
Discussion
The importance of CD8 T cells in the antiviral immune response to HIV-1 infection is well established, and suboptimal CD8 T-cell responses may play an important role in the development of disease. The immunologic determinants of HIV-1 disease progression are only partly understood, although persistent immune activation is associated with, and probably plays an important role in, disease progression.17-21 Immune activation is also a driving force behind T-cell differentiation, a process that may be followed by assessing the expression of several surface markers, including CD45RA, CD62L, CCR7, CD28, CD27, and CD7.8,24,37-39 In the present study, we have examined the CD7 expression on CD8 T cells from patients infected with HIV-1 to determine the functional relevance of effector and memory cell subsets defined by the surface level of CD7 in HIV-1 infection. We observe that early and chronic HIV infection is associated with a profound loss of CD7high CD8 T cells in an antigen-driven manner and that this is associated with the expansion of CD7low and CD7negative effector subsets. This pattern is reversed when HIV-1 replication is effectively suppressed by antiretroviral therapy and CD4 T cells recover. Moreover, we observe that CD8 T cells specific for chronic or latent infections (eg, HIV-1, CMV, and EBV) but not cleared infections (eg, influenza) generally express a CD7low phenotype. Interestingly, the strong effector phenotype bias of EBV- and CMV-specific CD8 T cells is less pronounced in subjects not infected with HIV with significant CD7high memory cell populations. Collectively, these observations indicate that HIV-1 infection drives the differentiation of a diverse number of antigen-specific cells. This is in line with the occurrence of reactivation of these viruses in subjects infected with HIV-1 and/or a direct effect of HIV on some non–HIV-specific T cells. As previously reported by our group, the CD7low and CD7negative subsets can be further subdivided into CD27+ cells that readily express cytokines such as interferon-γ (IFNγ) upon stimulation, and CD27 cells that contain the lytic effector molecule perforin.24,40 Thus, CD7low and CD7negative subsets both contain effector CD8 T cells. However, our previous findings suggest these 2 subsets differ in that CD7low cells may turn over rapidly, whereas the CD7negative cells may be more long lived.24 In patients infected with HIV-1 contraction of the CD7high CD8 T-cell population correlates directly with HIV-1 load, and a CD7low effector phenotype becomes dominant. CD8 T cells specific for HIV-1, as well as cells specific for EBV and CMV, contribute to this CD7low effector population.
Down-modulation of CD7 expression in CD4 T cells has previously been reported in several disease conditions, including HIV-1 infection, with an expansion of a CD7negative subset from less than 5% of CD4 T cells in healthy blood donors to about 20% in subjects infected with HIV-1.41-45 Furthermore, the expansion of this subset adds further prognostic value to the CD4 T-cell count and plasma HIV-1 RNA levels.42 In relation to this it is noteworthy that galectin-1, a β-galectoside–binding lectin, binds CD7 and induces apoptosis in thymocytes and T cells.32-36 Galectin-1 is expressed at sites of immune activation and may, therefore, induce preferential loss of cells that express CD7 at high levels. This may be a mechanism to limit the level of local inflammation and immune activation through inhibition or apoptosis of activated T cells. However, the potential involvement of galectin-1 in HIV-1 pathogenesis remains to be investigated. Nevertheless, the increased frequency of CD7low and CD7negative CD8 T cells may result from the combined effect of apoptosis of CD7high cells at sites of ongoing immune activation, down-modulation of CD7 on CD7high cells, and cellular proliferation and differentiation of such cells into CD7low and CD7negative effector cells. CD7negative effector cells are intriguing from an immunologic perspective because these cells may escape galectin-1–mediated inhibition or apoptosis at sites of immune activation and inflammation.
CD4 T-cell counts in both adult and pediatric subjects infected with HIV-1 on ART were directly correlated to the CD7 status of the CD8 T-cell compartment. Although cross-sectional, these data suggest that recovery of CD4 T-cell counts in response to ART treatment correlates with gradual restoration of the CD7 subset distribution in the CD8 T-cell compartment. To firmly draw this conclusion would require longitudinal data from a cohort followed before and during ART for an extended time period. A likely chain of events is that successful ART removes the main antigenic driver of the CD7low and CD7negative effector cells and those cells die off, whereas CD7high naive and memory cells may develop by de novo production or homeostatic proliferation. Notably, the correlation between CD7 status and state of the CD4 T-cell compartment was stronger in children than in adults, especially when the CD4 T-cell percentage was analyzed (Figure 6D). Young children can have CD4 counts higher than those observed in healthy adults, even in the face of high HIV-1 loads.46 Despite this abundance of both antigen and CD4 T cells, these children rarely mount strong CD8 T-cell responses to HIV-1 before 4 years of age, and responses continue to be weaker that those observed in adults.47-50 In line with this, CD7low effector CD8 T cells were relatively rare in children who maintained high CD4 T-cell counts and percentages (Figure 6D). A plausible explanation for the stronger correlation between CD7 status and CD4 counts in children is that the young immune system has been less exposed to other infections, which may leave an imprint in the CD8 T-cell compartment. Additionally, when compared with adults, an intrinsically more active thymus in childhood may generate larger numbers of naive, CD7high T cells.
HIV-1–specific CD8 T cells identified by HLA class I tetramer staining express low levels of perforin51 (and data not shown), suggesting that HIV-1–specific CD8 T cells have features of an intermediate stage of differentiation with reduced cytolytic capacity.8,9 Subjects with long-term nonprogressive disease have maintained CD8 T-cell proliferative responses to HIV-1 antigens, and this capacity to proliferate may be coupled to the ability to express perforin.52 This suggests that these determinants may be important for preserving the functional integrity of the immune system. However, reduced perforin expression and limited cytotoxic activity of virus-specific CD8 T cells are features of several chronic viral infections.16,53-55 Rather than being a cause of immune failure, reduced perforin expression in HIV-1–specific T cells may be a consequence of the chronicity of viral burden and the associated immune activation. Presence of HIV-specific CD8 T cells primarily in the CD7low subset, together with the perforinlow profile, identifies these cells as cytokine-producing effector cells according to the CD7-based differentiation model.24
In summary, we have found that CD8 T cells from patients infected with HIV-1 display significant down-modulation of CD7 expression as compared with healthy subjects, with expansion of both CD7low and CD7negative effector subsets. This pattern is particularly pronounced in patients with rapid disease progression. Expansion of CD7low CD8 T cells correlates with HIV-1 load, indicating that this change is antigen driven. In support of this, HIV-specific CD8 T cells are predominantly of the CD7low effector phenotype, as are cells specific for EBV and CMV. In contrast, CD8 T cells against influenza virus show a CD7high memory phenotype. Higher CD4 T-cell counts on ART are associated with reversion of the skewed CD7 profile in CD8 T cells. Thus, effector CD8 T-cell subsets distinguished by lowered CD7 expression expand in HIV-1 infection in a manner that correlates with the magnitude of antigenic challenge. Furthermore, contraction of these subsets well describes the immunologic response to successful antiretroviral treatment.
Prepublished online as Blood First Edition Paper, August 12, 2004; DOI 10.1182/blood-2004-07-2540.
This work was supported in part by grants from the National Institutes of Health (NIH; AI44595, AI41531, and AI052745), the Elizabeth Glaser Pediatric AIDS Foundation, the Centers for Disease Control and Prevention (CDC; PA 01158), the California AIDS Research Center (CC99-SF-001), the University of California, San Francisco (UCSF)/Gladstone Institute of Virology & Immunology Center for AIDS Research (P30 MH59037), the General Clinical Research Center at San Francisco General Hospital (5-MO1-RR00083-37), The Swedish Foundation for Strategic Research, the Swedish Research Council, the Harald Jeansson Foundation, the Clas Groschinsky Foundation, and the Karolinska Institutet.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Douglas F. Nixon is an Elizabeth Glaser scientist of the Elizabeth Glaser Pediatric AIDS Foundation. We thank Linda Baum for helpful discussions, and Kimberly Jordan and Margit Halvarsson for technical assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal