We have shown previously that fibrin(ogen) binding potentiates the capacity of fibroblast growth factor 2 (FGF-2) to stimulate endothelial cell (EC) proliferation. We have now investigated the receptor requirement for EC proliferation by fibrinogen-bound FGF-2. ECs were cultured with 25 ng/mL FGF-2 with or without 10 μg/mL fibrinogen, and proliferation was measured as 3H-thymidine incorporation. Proliferation was increased 2.4 ± 0.5-fold over medium alone with FGF-2 and increased significantly more to 4.0 ± 0.7-fold with fibrinogen and FGF-2 (P < .005). Addition of 7E3 or LM609, antibodies to αvβ3, inhibited EC proliferation with fibrinogen-bound FGF-2 by 80% ± 8% (P < .001) or 67% ± 14% (P < .002), respectively, to levels significantly less than that observed with FGF-2 alone (P < .001). Neither LM609 nor 7E3 exhibited any inhibition of activity with FGF-2 alone. Peptide GRGDS caused dose-dependent inhibition of proliferation by fibrinogen-bound FGF-2 of 31% ± 8%, 45% ± 9%, and 68% ± 11% at 0.25, 0.5, and 1 mM, respectively. Coimmunoprecipitation and immunofluorescence studies demonstrated a direct specific association between αvβ3 and FGF receptor 1 (FGFR1) in ECs and fibroblasts when exposed to both FGF-2 and fibrinogen but not with vitronectin. We conclude that fibrinogen binding of FGF-2 enhances EC proliferation through the coordinated effects of colocalized αvβ3 and FGFR1.
Introduction
Endothelial cells (ECs) normally have a low rate of proliferation in the adult, but the endothelium retains its capacity for proliferation, which occurs physiologically in the corpus luteum, uterus, and adipose tissue and also during wound healing.1,2 EC proliferation, differentiation, and migration are also needed for angiogenesis, an important process in many pathologic conditions including tumor growth, diabetic retinopathy, inflammation, and ischemic thrombotic diseases.2 Polypeptide growth factors play an important regulatory role in angiogenesis, and several stimulatory and inhibitory molecules have been identified,3,4 including fibroblast growth factor-2 (FGF-2, basic fibroblast growth factor), an 18-kDa polypeptide of the FGF family, which exerts a variety of effects on many cells and organ systems.5,6
ECs in culture require FGF to support proliferation and prevent apoptosis.7 In addition, FGFs promote EC migration8 and increase synthesis of several proteins that are important in degradation of the extracellular matrix during cell migration or angiogenesis, including collagenase,9,10 urokinase plasminogen activator, urokinase plasminogen activator receptor,10-12 and plasminogen activator inhibitor 1.13,14 FGFs also regulate EC adhesion by modulating expression of integrin receptors,15 and they can increase expression of vascular endothelial growth factor (VEGF), another angiogenic peptide.16 Although FGF-1 and FGF-2 lack signal peptides, they are both active in the pericellular environment and bind with high affinity to specific receptor tyrosine kinases.17 FGFs are released from vascular cells following injury, and FGF-2 mRNA is up-regulated in atherosclerotic arteries18 and following vessel injury.19
ECs are physiologically exposed to a high concentration of fibrinogen in blood, and their response to fibrinogen depends on the activation of cell surface integrin receptors. Fibrinogen binds to ECs through αvβ3 and α5β1 by interacting with the RGD sequence near the carboxyl terminus of the Aα chain at residues 572 to 574.20-22 ECs adhere, spread, and proliferate on both fibrinogen and fibrin in vitro through binding to integrins αvβ3 and α5 β1,20,21 and fibrinogen supports leukocyte tethering to ECs at sites of inflammation through intercellular adhesion molecule 1 (ICAM-1).23
We have previously reported that fibrinogen and fibrin bind to FGF-2 and potentiate its ability to stimulate proliferation of ECs and that binding of FGF-2 to fibrin(ogen) provides protection from proteolysis.24-27 In the present report we have investigated the involvement of specific receptors for fibrinogen and FGF-2 in EC proliferation by fibrinogen-bound FGF-2. The results indicate that FGF-2 in the presence of fibrinogen requires αVβ3 binding to stimulate EC proliferation. Also, fibrinogen-bound FGF-2 promotes the specific association of the fibrinogen receptor, αVβ3, with FGF-2 receptor 1 (FGFR1). This study demonstrates that fibrinogen binding of FGF-2 enhances EC proliferation through the coordinated effects of αVβ3 and FGFR1.
Materials and methods
Fibrinogen preparation
Human fibrinogen was obtained from Enzyme Research Laboratories (South Bend, IN) and copurifying fibronectin was removed by gelatin-Sepharose chromatography. Residual fibronectin remaining after gelatin-Sepharose chromatography was further depleted by immunoaffinity chromatography as described elsewhere.28 The fibronectin concentration was determined by enzyme-linked immunosorbent assay (ELISA; American Diagnostica, Greenwich, CT) and represented less than 0.02% of the total protein.
Cell culture
Primary ECs were obtained from human umbilical veins seeded on 0.2% wt/vol gelatin-coated 25-cm2 tissue culture flasks and cultured in McCoy 5A medium (Flow Laboratories, McLean, VA) containing 20% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 50 μg/mL endothelial cell growth supplement (ECGS; Collaborative Research, Bedford, MA), and 100 μg/mL heparin (Sigma Chemical, St Louis, MO) until they reached confluence, typically within 4 or 5 days. Fibroblasts were isolated from human foreskins (HFFs) and cultured in McCoy 5A medium supplemented with 10% FBS, 100 U/mL penicillin, and 0.1 mg/mL streptomycin until they reached confluence. The cells were passaged up to 2 times before use and then placed in suspension by rinsing in Hanks balanced salt solution followed by brief incubation with trypsin-EDTA (ethylenediaminetetraacetic acid; Gibco Invitrogen, Carlsbad, CA). The cells were pelleted by centrifugation for 10 minutes at 500g and resuspended in McCoy 5A medium in the absence of serum. This wash procedure was repeated twice prior to use in experimental protocols.
3H-Thymidine incorporation
Approximately 2 × 104 ECs suspended in McCoy 5A medium supplemented with 20% FBS, 50 μg/mL ECGS, and 100 μg/mL heparin were plated in gelatin-coated 12-well plates (Becton Dickinson, Franklin Lakes, NJ) and allowed to adhere for 6 hours. The medium was then removed, and the cells were washed twice with McCoy 5A medium. Serum-free medium was then added containing 1% Nutridoma (Boehringer Mannheim, Indianapolis, IN), 5 μg/mL of either LM609 (Chemicon International, Temecula, CA), or 7E3 antibody or various concentrations of GRGDS or γ 400-411 peptide for 2 hours. Then, 25 ng/mL FGF-2 and 1 μCi/mL (0.037 MBq) 3H-thymidine (New England Nuclear, Boston, MA) were added to the medium in the presence or absence of 10 μg/mL fibrinogen. After incubation at 37°C for 24 hours, cells were washed with ice-cold phosphate-buffered saline (PBS). Then, 500 μL 10% ice-cold trichloroacetic acid (TCA) was added to each well, and precipitates were collected on a filter using a filtration manifold. Filters were washed twice with ice-cold 5% TCA, followed by 95% ethanol, allowed to air dry, and then suspended in scintillation fluid and were quantitated using a scintillation counter.
Immunoprecipitation and Western blotting
ECs or HFFs were grown to confluence, and then medium containing 100 ng/mL of either FGF-1 or FGF-2 or 10 ng/mL interleukin-1 (IL-1α; Peprotech, Rocky Hill, NJ), was added in the presence or absence of 10 μg/mL fibrinogen. In some experiments 10 μg/mL vitronectin (Sigma Chemical) was also used. After 1 hour, the cells were washed 3 times with PBS and then lysed with lysis buffer (Promega, Madison, WI) containing protease inhibitors and immunoprecipitated by the addition of antibodies to αVβ3 (LM609), αVβ5 (P1F6), α5β1 (JBS5), or αvβ1 integrins, (JB1A and LM142; Chemicon), at 25°C for 2 hours, following which protein A–Sepharose beads (Pierce, Rockford, IL) were added. The beads were centrifuged at 3000g for 10 minutes and washed twice with 0.1 M sodium phosphate buffer, pH 7.4, containing 0.25M NaCl. Beads were then boiled in electrophoresis diluent for 5 minutes. The eluates and supernatants were electrophoresed using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After protein transfer, membranes were immunoblotted with antibody to FGFR1 (Abgent, San Diego, CA). Bands were detected by chemiluminescence. Similar experiments were performed using anti-FGFR1 for immunoprecipitation and anti-αVβ3 (LM609) to probe the blot. In some experiments, anti-αVβ3, anti-α5β1, anti-αVβ5, or anti-αVβ1 was used to immunoprecipitate various integrins, and the blots were probed using anti-FGFR1.
Immunofluorescent detection
ECs and HFFs were seeded on round glass coverslips, grown to confluence, and treated with 100 ng/mL FGF-2 added to the medium in the presence or absence of 10 μg/mL fibrinogen. After 1 hour cells were washed twice with cold PBS and fixed with 3.7% formaldehyde in PBS and stained using 10 μg/mL polyclonal FGFR1 and 10 μg/mL monoclonal 7E3 antibodies. Secondary antibodies were Alexa Fluor 568 (red) Alexa Fluor 488 (green) from Molecular Probes (Eugene, OR). Cells were viewed using a Nikon Eclipse E-800 fluorescence microscope equipped with a dual-wavelength filter cube. A color digital camera (Spot II CCD; Diagnostic Instruments, Sterling Heights, MI) and a computer with color monitor were used to capture images. FGFR was visualized as red fluorescence, αVβ3 as green fluorescence, and colocalization of FGF-2 and fibrinogen receptors as yellow fluorescence.
Propidium iodide staining
ECs were cultured on Thermanox coverslips coated with gelatin. After the cells reached confluence, 5 μg/mL of either LM609 or 7E3 antibody or 1 mM GRGDS or anti-α5β1 (AB1950) was added for 2 hours. Then, 25 ng/mL FGF-2 was added to the medium in the presence or absence of 10 μg/mL fibrinogen. After incubation at 37°C for 24 hours, nonadherent cells were removed by washing twice with ice-cold PBS. Cells were then fixed with 3.7% formaldehyde in PBS for 20 minutes, washed 3 times with PBS, permeabilized with 0.5% Triton X-100 for 20 minutes, and then washed again 3 times with PBS. They were then mounted on glass microscope slides using gel mount (Gel/Mount; Birmedia, Foster City, CA). Propidium iodide counterstain (3 μg/mL in PBS) was applied, and the slides were covered with a glass coverslip, the edges were sealed using rubber cement, and the slides were stored at -20°C. Cells were viewed using a Nikon (Garden City, NY) Eclipse E-800 fluorescence microscope equipped with a dual wavelength filter cube.
Data analysis
Unless indicated otherwise data are expressed as mean ± SD. Each experiment was performed at least 3 times, and either triplicate or quadruplicate wells were used in each experiment. The significance of differences in means was determined using a 2-tailed Student t test.
Results
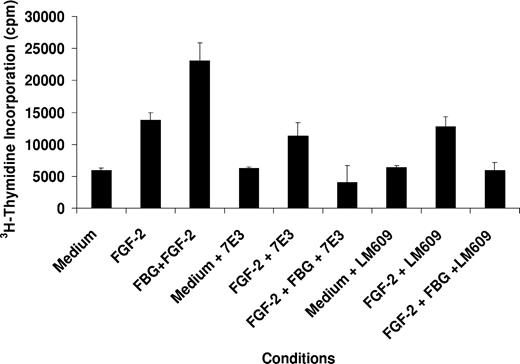
The involvement of specific receptors for fibrinogen and FGF-2 in EC proliferation by fibrinogen-bound FGF-2 was determined using inhibitory antibodies. ECs were cultured on gelatin-coated wells in the presence of 25 ng/mL FGF-2 with or without 10 μg/mL fibrinogen in the medium, and proliferation was measured as 3H-thymidine incorporation. Cell proliferation was increased 2.4 ± 0.5-fold over medium alone with FGF-2 and increased significantly more to 4.0 ± 0.7-fold with fibrinogen-bound FGF-2 (P < .005; Figure 1). Addition of 5 μg/mL LM609, a monoclonal antibody to αVβ3, inhibited proliferation induced by fibrinogen-bound FGF-2 by 67% ± 14% (P < .002). Similar results were observed with 7E3, also reactive with αVβ3, which inhibited EC proliferation with fibrinogen-bound FGF-2 by 80% ± 8% (P < .001). The proliferation stimulated by fibrinogen and FGF-2 in the presence of LM609 or 7E3 was comparable to that with medium alone and significantly less than that with FGF-2 alone in the absence of fibrinogen (P < .05 for each). Neither LM609 nor 7E3 inhibited the activity of FGF-2 alone (Figure 1). These findings indicate that FGF-2 has no proliferative activity when fibrinogen is present if αVβ3 is blocked.
Effect of LM609 and 7E3 on EC proliferation. ECs were plated on gelatin-coated wells in McCoy 5A medium supplemented with 20% FBS, 50 μg/mL ECGS, and 100 μg/mL heparin and allowed to adhere for 6 hours. The cells were then washed twice with McCoy medium and incubated in serum-free medium containing 1% Nutridoma and 5 μg/mL of either LM609 or 7E3. After 2 hours, 25 ng/mL FGF-2, with or without 10 μg/mL fibrinogen, and 1 μCi/mL (0.037 MBq) 3H-thymidine were added to the medium. After 24 hours, cells were washed with PBS and isotope incorporated into DNA was precipitated with TCA, collected by vacuum filtration, and measured by scintillation counting. The results are the mean ± SD of 3 separate experiments.
Effect of LM609 and 7E3 on EC proliferation. ECs were plated on gelatin-coated wells in McCoy 5A medium supplemented with 20% FBS, 50 μg/mL ECGS, and 100 μg/mL heparin and allowed to adhere for 6 hours. The cells were then washed twice with McCoy medium and incubated in serum-free medium containing 1% Nutridoma and 5 μg/mL of either LM609 or 7E3. After 2 hours, 25 ng/mL FGF-2, with or without 10 μg/mL fibrinogen, and 1 μCi/mL (0.037 MBq) 3H-thymidine were added to the medium. After 24 hours, cells were washed with PBS and isotope incorporated into DNA was precipitated with TCA, collected by vacuum filtration, and measured by scintillation counting. The results are the mean ± SD of 3 separate experiments.
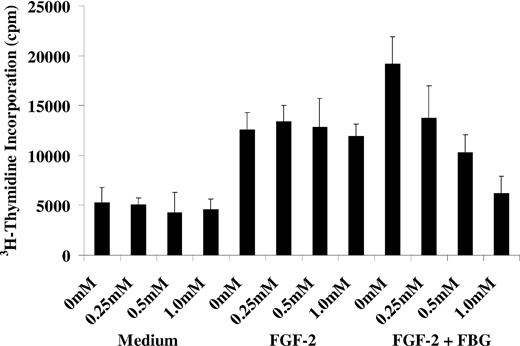
To confirm that the inhibition of FGF-2 activity by LM609 and 7E3 was mediated through fibrinogen we used peptide GRGDS, corresponding to the binding site for αVβ3 on fibrinogen, to inhibit proliferation. The peptide did not inhibit proliferation with FGF-2 alone at concentrations up to 1 mM. However, in the presence of fibrinogen it caused dose-dependent inhibition of proliferation by 38% ± 8%, 45 ± 9%, and 68% ± 11% at concentrations of 0.25, 0.5, and 1 mM, respectively (P < .05 for all; Figure 2). Another fibrinogen-derived peptide that interacts with αIIbβ3, γ 400-411, showed no inhibitory activity on cell proliferation at the same concentrations (data not shown).
Effect of GRGDS peptide on EC proliferation. ECs were plated on gelatin-coated wells and allowed to adhere for 6 hours. The cells were then washed twice with McCoy medium and incubated in serum-free medium containing 1% Nutridoma and GRGDS at concentrations of 0.25 mM, 0.5 mM, or 1 mM. After 60 minutes, 25 ng/mL FGF-2, with or without 10 μg/mL fibrinogen, and 1 μCi/mL (0.037 MBq) 3H-thymidine was added to the medium. After 24 hours, cells were washed with PBS and isotope incorporated in DNA was precipitated with TCA, collected by vacuum filtration, and measured by scintillation counting. Results are the mean ± SD of 3 different experiments.
Effect of GRGDS peptide on EC proliferation. ECs were plated on gelatin-coated wells and allowed to adhere for 6 hours. The cells were then washed twice with McCoy medium and incubated in serum-free medium containing 1% Nutridoma and GRGDS at concentrations of 0.25 mM, 0.5 mM, or 1 mM. After 60 minutes, 25 ng/mL FGF-2, with or without 10 μg/mL fibrinogen, and 1 μCi/mL (0.037 MBq) 3H-thymidine was added to the medium. After 24 hours, cells were washed with PBS and isotope incorporated in DNA was precipitated with TCA, collected by vacuum filtration, and measured by scintillation counting. Results are the mean ± SD of 3 different experiments.
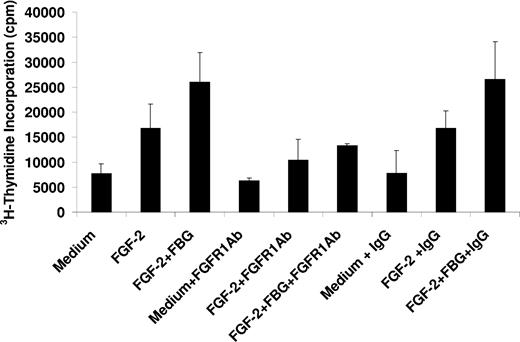
FGF-2 stimulates EC proliferation through FGFR1. To characterize the involvement of this receptor in the presence of fibrinogen, cell proliferation was measured in the presence of an antibody to FGFR1. The antibody at 5 μg/mL inhibited proliferation with FGF-2 alone by 42% ± 6% and by 51% ± 7% with both FGF-2 and fibrinogen (P < .05 for both). The inhibition observed with FGF-2 alone was not significantly different from that observed in the presence of FGF-2 and fibrinogen (Figure 3). In control experiments, nonimmune IgG had no effect on cell proliferation with either free or fibrinogen-bound FGF-2 (Figure 3). We conclude that inhibition of the interaction of fibrinogen and αVβ3 blocks the proliferative effect of fibrinogen-bound FGF-2 on ECs. When bound to fibrinogen, both αVβ3 and FGFR1 are involved in stimulating EC proliferation.
Effect of an FGFR1 antibody on EC proliferation. ECs were plated on gelatin-coated wells in McCoy 5A medium supplemented with 20% FBS, 50 μg/mL ECGS, and 100 μg/mL heparin and allowed to adhere for 6 hours. The cells were then washed twice with McCoy medium and incubated in serum-free medium containing 1% Nutridoma and 5 μg/mL of either FGFR1 or control mouse IgG. After 60 minutes, 25 ng/mL FGF-2,with or without 10 μg/mL fibrinogen, and 1 μCi/mL (0.037 MBq) 3H-thymidine were added to the medium. After 24 hours, nonadherent cells were removed, and isotope incorporated into DNA was precipitated with TCA, collected by vacuum filtration, and measured by scintillation counting. The results are the mean ± SD of 3 separate experiments.
Effect of an FGFR1 antibody on EC proliferation. ECs were plated on gelatin-coated wells in McCoy 5A medium supplemented with 20% FBS, 50 μg/mL ECGS, and 100 μg/mL heparin and allowed to adhere for 6 hours. The cells were then washed twice with McCoy medium and incubated in serum-free medium containing 1% Nutridoma and 5 μg/mL of either FGFR1 or control mouse IgG. After 60 minutes, 25 ng/mL FGF-2,with or without 10 μg/mL fibrinogen, and 1 μCi/mL (0.037 MBq) 3H-thymidine were added to the medium. After 24 hours, nonadherent cells were removed, and isotope incorporated into DNA was precipitated with TCA, collected by vacuum filtration, and measured by scintillation counting. The results are the mean ± SD of 3 separate experiments.
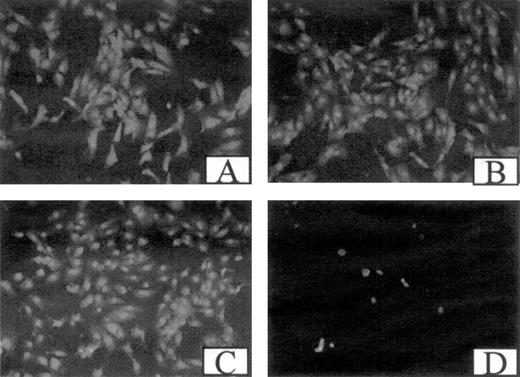
Although cells were plated on gelatin, antibodies to αVβ3 or the RGDS peptide could affect adhesion because fibrinogen is an adhesive substrate for ECs. To evaluate effects on adhesion, ECs were examined microscopically after exposure to 5 μg/mL 7E3 or LM609, anti-α5β1, or with 1 mM GRGDS (Figure 4). At baseline, cells were adhered and fully spread, and no change was observed during incubation with fibrinogen or FGF-2. Also, cell morphology was unaffected by antibodies to αVβ3 or GRGDS. However, incubation with anti-α5β1 caused marked detachment of the cells, which was expected because they were plated on gelatin to which they adhere through β1 integrins. This indicates that the antiproliferative effect of GRGDS or antibodies to αVβ3 was not primarily dependent on alterations in cell adhesion.
Effect of antibodies and peptides on cell adhesion. Confluent ECs plated on gelatin-coated coverslips were incubated in McCoy 5A medium (A), with 5 μg/mL 7E3 (B), 1 mM GRGDS (C), or 5 μg/mL α5β1 antibody (D). After 24 hours, the cells were fixed, washed with PBS twice, stained with propidium iodide, and the coverslips were viewed under a fluorescence microscope.
Effect of antibodies and peptides on cell adhesion. Confluent ECs plated on gelatin-coated coverslips were incubated in McCoy 5A medium (A), with 5 μg/mL 7E3 (B), 1 mM GRGDS (C), or 5 μg/mL α5β1 antibody (D). After 24 hours, the cells were fixed, washed with PBS twice, stained with propidium iodide, and the coverslips were viewed under a fluorescence microscope.
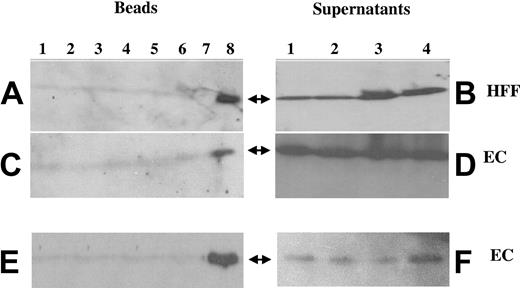
The ability of antibodies to αVβ3 to inhibit the activity of FGF-2 suggested that FGFR1 and αVβ3 may colocalize on the membrane in the presence of both fibrinogen and FGF-2. Immunoprecipitation studies were performed to investigate this possibility. ECs or HFFs were grown to confluence and exposed to FGF-2 (100 ng/mL), fibrinogen (10 μg/mL), or the combination of both in the medium. After washing, cells were lysed after 1 hour, following which 7E3 (Figure 5A-D) or FGFR1 antibody (Figure 5E-F) was added, and immune complexes were isolated by incubation with protein A–coupled Sepharose beads. After centrifugation, beads were boiled in diluent and subjected to SDS-PAGE, and Western blots were prepared and probed with anti-FGFR1 or 7E3 (Figure 5). Only cells exposed to the combination of fibrinogen and FGF-2 demonstrated positive Western blotting for FGFR1 following immunopurification with 7E3. After incubation with either FGF-2 alone or with fibrinogen alone, Western blotting was negative for FGFR1 (Figure 5A,C). Results were similar in HFFs, with colocalization of αVβ3 and FGFR1 only with exposure to the combination of fibrinogen and FGF-2. Notably, there was no colocalization with FGF-1 or IL-1α, neither of which binds to fibrinogen (Figure 5A lanes 2 and 5-7). After αVβ3 was immunoprecipitated the supernatants were positive for FGFR1 in all samples including medium, fibrinogen alone, FGF-2 alone, and the combination of both (Figure 5B,D). Findings were similar when FGFR1 was immunoprecipitated and the blots were probed with 7E3 (Figure 5E-F). Only cells exposed to the combination of both fibrinogen and FGF-2 demonstrated positive Western blotting for αVβ3 following immunopurification with anti-FGFR1 (Figure 5E). The supernatants were positive for αVβ3 in all samples after FGFR1 was immunoprecipitated (Figure 5F). These results indicate that exposure of ECs or HFFs to the combination of FGF-2 and fibrinogen promoted the association of αVβ3 with FGFR1.
Colocalization of αvβ3 and FGFR1 using coimmunoprecipitation. ECs or HFFs were exposed to various conditions, as described. After 1 hour, cells were washed with PBS, lysed with lysis buffer, and incubated with 5 μg/mL of either 7E3 (A-D) or anti-FGFR1 (E-F). Protein A–Sepharose beads were then added. Following washing, the beads were incubated with diluent and electrophoresed on 10% gels. Western blotting was performed with anti-FGFR1 (A-D) or with 7E3 (E-F). In panels A, C, and E: lane 1, medium alone; lane 2, 100 ng/mL FGF-1; lane 3, 100 ng/mL FGF-2; lane 4, 10 μg/mL fibrinogen; lane 5, 10 ng/mL IL-1β; lane 6, 100 ng/mL FGF-1 plus 10 μg/mL fibrinogen; lane 7, 10 ng/mL IL-1α plus 10 μg/mL fibrinogen; lane 8, 100 ng/mL FGF-2 plus 10 μg/mL fibrinogen. Panels B, D, and F are supernatants after centrifugation of protein A–Sepharose: lane 1, medium alone; lane 2, 10 μg/mL fibrinogen; lane 3, 100 ng/mL FGF-2; lane 4, 100 ng/mL FGF-2 plus 10 μg/mL fibrinogen. Arrows indicate the location of FGFR1 (Mr ∼100 kDa) in panels A-D; the arrows indicate the location of αvβ3 in panels E-F (Mr ∼110 kDa).
Colocalization of αvβ3 and FGFR1 using coimmunoprecipitation. ECs or HFFs were exposed to various conditions, as described. After 1 hour, cells were washed with PBS, lysed with lysis buffer, and incubated with 5 μg/mL of either 7E3 (A-D) or anti-FGFR1 (E-F). Protein A–Sepharose beads were then added. Following washing, the beads were incubated with diluent and electrophoresed on 10% gels. Western blotting was performed with anti-FGFR1 (A-D) or with 7E3 (E-F). In panels A, C, and E: lane 1, medium alone; lane 2, 100 ng/mL FGF-1; lane 3, 100 ng/mL FGF-2; lane 4, 10 μg/mL fibrinogen; lane 5, 10 ng/mL IL-1β; lane 6, 100 ng/mL FGF-1 plus 10 μg/mL fibrinogen; lane 7, 10 ng/mL IL-1α plus 10 μg/mL fibrinogen; lane 8, 100 ng/mL FGF-2 plus 10 μg/mL fibrinogen. Panels B, D, and F are supernatants after centrifugation of protein A–Sepharose: lane 1, medium alone; lane 2, 10 μg/mL fibrinogen; lane 3, 100 ng/mL FGF-2; lane 4, 100 ng/mL FGF-2 plus 10 μg/mL fibrinogen. Arrows indicate the location of FGFR1 (Mr ∼100 kDa) in panels A-D; the arrows indicate the location of αvβ3 in panels E-F (Mr ∼110 kDa).
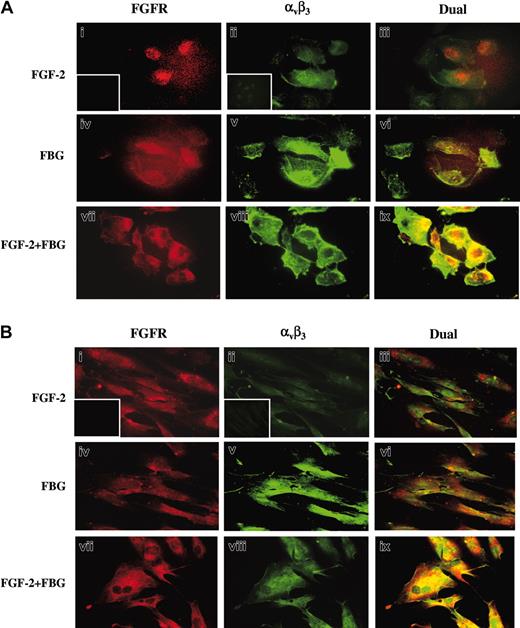
Immunofluorescence studies were carried out to further confirm the colocalization of the fibrinogen (FBG) and FGF receptors (Figure 6). Confluent ECs (A) or HFFs (B) were exposed to 100 ng/mL FGF-2 (i-iii), 10 μg/mL FBG (iv-vi), or both (vii-ix) in the medium for 1 hour. Cells were then washed, fixed, and immunostained with anti-FGFR1 or 7E3. In both ECs and HFFs, dual immunofluorescent detection revealed colocalization of αvβ3 and FGFR1 only when the cells were exposed to both fibrinogen and FGF-2 (vii-ix) as shown by yellow fluorescence. Background staining for red (i) and green (ii) fluorescence is shown in the insets.
Colocalization of αvβ3 and FGFR1 using immunofluorescence. Confluent ECs (A) or HFFs (B) were treated with or without 100 ng/mL FGF-2 in the presence or absence of 10 μg/mL fibrinogen. After 1 hour, cells were washed and fixed with 3.7% formaldehyde and stained using 10 μg/mL FGFR1 and 7E3 antibody. FGFR is visualized as red fluorescence (i,iv,vii), αvβ3 is visualized as green fluorescence (ii,v,viii), and colocalization of FGF-2 and fibrinogen receptors is shown as yellow fluorescence (iii,vi,ix). Insets represent the background staining for red (i) and green (ii) fluorescence. Bars represent 25 μm.
Colocalization of αvβ3 and FGFR1 using immunofluorescence. Confluent ECs (A) or HFFs (B) were treated with or without 100 ng/mL FGF-2 in the presence or absence of 10 μg/mL fibrinogen. After 1 hour, cells were washed and fixed with 3.7% formaldehyde and stained using 10 μg/mL FGFR1 and 7E3 antibody. FGFR is visualized as red fluorescence (i,iv,vii), αvβ3 is visualized as green fluorescence (ii,v,viii), and colocalization of FGF-2 and fibrinogen receptors is shown as yellow fluorescence (iii,vi,ix). Insets represent the background staining for red (i) and green (ii) fluorescence. Bars represent 25 μm.
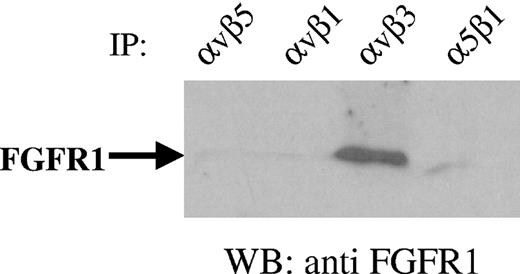
Although αVβ3 is an important receptor for fibrinogen, other integrins such as α5β1 also bind to fibrinogen under certain conditions.20 To determine the specificity and to identify the receptor responsible for enhanced proliferation by fibrinogen-bound FGF-2, ECs were incubated with the combination of FGF-2 and fibrinogen. Lysates were then incubated with anti-αVβ3, anti-α5β1, anti-αVβ5, or anti-αVβ1 to immunoprecipitate various integrins, and Western blots were prepared and probed with anti-FGFR1. Positive Western blotting for FGFR1 was observed only with immunoprecipitates using anti-αVβ3 (Figure 7). FGFR1 did not coprecipitate with integrins αVβ5, αVβ1, or α5β1 indicating the specificity of colocalization of FGFR1 with αVβ3. This further confirms that combination of FGF-2 and fibrinogen promotes the specific association of FGFR1 with αVβ3.
Specificity of association of FGFR1 with αvβ3. ECs were incubated with 100 ng/mL FGF-2 and 10 μg/mL fibrinogen for 1 hour. Cells were then lysed and incubated with 10 μg/mL integrin antibodies as indicated. Protein A–Sepharose beads were then added and incubated further for 30 minutes. Following washing, beads were boiled in electrophoresis diluent for 5 minutes and samples were electrophoresed on 10% gels. Western blotting (WB) was performed with anti-FGFR1. The result of a representative experiment is shown.
Specificity of association of FGFR1 with αvβ3. ECs were incubated with 100 ng/mL FGF-2 and 10 μg/mL fibrinogen for 1 hour. Cells were then lysed and incubated with 10 μg/mL integrin antibodies as indicated. Protein A–Sepharose beads were then added and incubated further for 30 minutes. Following washing, beads were boiled in electrophoresis diluent for 5 minutes and samples were electrophoresed on 10% gels. Western blotting (WB) was performed with anti-FGFR1. The result of a representative experiment is shown.
To determine if FGFR1 colocalizes with αVβ3 activated by another ligand, ECs were incubated with vitronectin (10 μg/mL) or fibrinogen (10 μg/mL) with or without 100 ng/mL FGF-2. Lysates were then incubated with anti-αVβ3 and Western blots were prepared and probed with anti-FGFR1 (Figure 8). Only cells exposed to the combination of fibrinogen and FGF-2 demonstrated positive Western blotting for FGFR1 following immunopurification with anti-αVβ3. No colocalization was observed with vitronectin and FGF-2, indicating the specificity of fibrinogen in promoting the association of the 2 receptors in the presence of FGF-2.
Specificity of fibrinogen and FGF-2 in promoting the association of FGFR1 with αvβ3. ECs were incubated with 10 μg/mL fibrinogen or 10 μg/mL vitronectin in the presence or absence of 100 ng/mL FGF-2 for 1 hour. Cells were then lysed and incubated with 5 μg/mL anti-αvβ3. Protein A–Sepharose beads were then added and incubated further for 30 minutes. Following washing, beads were boiled in electrophoresis diluent for 5 minutes and samples were electrophoresed on 10% gels. Western blotting (WB) was performed with anti-FGFR1. Lane 1, medium alone; lane 2, FGF-2; lane 3, fibrinogen; lane 4, FGF-2 plus fibrinogen; lane 5, vitronectin; lane 6, FGF-2 plus vitronectin; arrow indicates the location of FGFR1 (Mr ∼ 100 kDa). IP indicates immunopurification.
Specificity of fibrinogen and FGF-2 in promoting the association of FGFR1 with αvβ3. ECs were incubated with 10 μg/mL fibrinogen or 10 μg/mL vitronectin in the presence or absence of 100 ng/mL FGF-2 for 1 hour. Cells were then lysed and incubated with 5 μg/mL anti-αvβ3. Protein A–Sepharose beads were then added and incubated further for 30 minutes. Following washing, beads were boiled in electrophoresis diluent for 5 minutes and samples were electrophoresed on 10% gels. Western blotting (WB) was performed with anti-FGFR1. Lane 1, medium alone; lane 2, FGF-2; lane 3, fibrinogen; lane 4, FGF-2 plus fibrinogen; lane 5, vitronectin; lane 6, FGF-2 plus vitronectin; arrow indicates the location of FGFR1 (Mr ∼ 100 kDa). IP indicates immunopurification.
Discussion
The results presented demonstrate that inhibition of the interaction of fibrinogen with αVβ3 blocks the proliferative effect of FGF-2 on ECs in the presence of fibrinogen and that αVβ3 and FGFR1 colocalize when both fibrinogen and FGF-2 are present. Our previous studies have shown that FGF-2 binds with high affinity to fibrinogen, indicating that FGF-2 would be bound to fibrinogen under these experimental conditions. Monoclonal antibodies to αVβ3 inhibited EC proliferation induced by fibrinogen-bound FGF-2 but showed no inhibition of activity with FGF-2 alone. This implies that either FGFR1 is not available for binding FGF-2 in the presence of fibrinogen or that it can bind but cannot activate the receptor. This may be due to shielding of the binding site for FGFR when FGF-2 is bound to fibrinogen. The amino acid residues involved in the interaction with fibrinogen are close to those previously shown to be necessary for FGFR binding. Crystallographic studies demonstrate that Glu99 to Tyr103 are involved in the interaction between FGFR and FGF-2.29 Our recent data indicate that binding of FGF-2 to fibrinogen is mediated by residues near these including Phe95, Ser100, Asn102, Arg107, and Arg109.30 Additional evidence supporting the importance of αvβ3 includes a dose-dependent inhibition of proliferation by fibrinogen-bound FGF-2 with RGD peptides. The effect of GRGDS peptide or 7E3 antibody on proliferation was independent of cell adhesion. In the presence of fibrinogen-bound FGF-2, αVβ3 was coimmunoprecipitated with FGFR1, indicating that αVβ3 and FGFR associate in initiating the signaling pathway leading to proliferation. This colocalization did not occur with the αVβ3 ligand vitronectin, indicating specificity for fibrinogen. These findings link the hemostatic and matrix functions of fibrinogen with the endothelial cell regulatory activities of these growth factors and are consistent with our previous reports that both VEGF and FGF-2 bind to fibrin(ogen) with high affinity and retain activity when bound. The interaction potentiates the capacity of FGF-2 to stimulate endothelial cell proliferation,25 increases its ability to support prolonged endothelial cell growth,31 and provides protection from proteolysis.26
There are several other examples of interactions between growth factors and matrix proteins with their receptors that result in altered activity, which suggests that this is a common theme in cell regulation. Wijelath et al32 found that VEGF bound to fibronectin and that this interaction enhanced its capacity to stimulate EC migration due to the association of FLK-1 and α5β1. Tsou and Isik33 demonstrated that vitronectin altered the expression of FGF and VEGF receptors and that matrix-integrin interactions regulated EC responsiveness to growth factors. Similarly, Moro et al34 showed that the interaction of fibroblasts with specific matrix proteins activated the epidermal growth factor receptor. Thus, specific interactions between growth factors and matrix proteins are critical in regulation of cell properties.
Our finding that αVβ3 and FGFR1 colocalize is consistent with prior reports regarding other receptors and suggests that this is a common mechanism in mediating cellular events such as proliferation, migration, and differentiation.35 Ligand-mediated integrin clustering in ECs induces aggregation of FGFR36 and stimulates phosphorylation of platelet-derived growth factor-β (PDGF-β) receptors.37 Tyrosine phosphorylated PDGF-β and insulin receptors coimmunoprecipitate with αvβ3, and this requires growth factor stimulation of the receptor.38 In fibroblasts, EGF, PDGF-BB, and FGF-2 activate ERK synergistically with integrin activation and receptor aggregation.39 After activation, αVβ3 and PDGF-β receptor coimmunoprecipitate in ECs and demonstrate synergistic stimulation.40 A direct interaction of surface-immobilized FGF-2 with αvβ3 was demonstrated that influenced EC adhesion, mitogenesis, and urokinase plasminogen activator up-regulation.41 This was confirmed by Tanghetti et al,42 who found that FGFR and αvβ3 cooperated in EC signaling, and both growth factor and integrin activation may be required for sustaining prolonged cell activation. Together, this evidence indicates that the coordinated effects of matrix proteins and growth factors in regulating cell function can be mediated by physical association of receptors.
ECs have receptors for fibrinogen on their luminal surface that are involved in mediating interactions with blood cells and also on their abluminal surface that support adhesion. Conforti et al43 have previously demonstrated that fibrinogen binds specifically to ECs in culture, which express αVβ3 on both their adherent and nonadherent surfaces in vitro and also in vivo.43,44 Little information is available, however, regarding the effects of exposure of adherent ECs to soluble fibrinogen on their nonadherent surface. Although fibrinogen is an adhesive substrate for ECs, it is unlikely that the enhanced proliferation observed is due to an effect of fibrinogen on adhesive properties, because the effects of the GRGDS peptide or an antibody to αVβ3 were independent of cell adhesion. Cells were fully adhered prior to FGF-2 or fibrinogen exposure, and no change in cell morphology was observed during incubation with fibrinogen. Although effects at the abluminal cell surface cannot be excluded, the evidence suggests that the effects of FGF-2 and fibrinogen in the medium were mediated at the luminal cell surface.
The activity and distribution of FGFs are affected by binding to other molecules on the cell surface or in the matrix or blood. At normal plasma concentrations of fibrinogen (approximately 7 μM) and FGF-2 (up to 6 pM), and a dissociation constant (Kd) in the low nanomolar range, nearly all FGF-2 should be bound to fibrinogen, if no other high-affinity FGF-2–binding proteins are present.24 Binding of soluble heparin also potentiates the activity of FGF-2 when used pharmacologically or in vitro, but heparin is not present physiologically. Other FGF-2–binding proteins have also been identified in blood including α2-macroglobulin45 and soluble forms of FGFR.46 However, binding affinities have not been described.
Binding to matrix molecules also affects FGF-2 activity. In addition to being present in blood, fibrinogen may also serve as a matrix binding site for FGF-2 because it is found in both normal and atherosclerotic arterial walls.47,48 FGF-2 also binds to fibrin, which forms following hemostatic activation at sites of vessel injury or inflammation. Fibrin contributes to the hemostatic plug formation and also provides a provisional matrix to support local cell responses. Heparan sulfate proteoglycans (HSPGs) are present in extracellular matrix where they bind FGFs and possibly serve as a local reservoir of growth factor that can be released by enzymes that degrade proteoglycans.49 However, in distinction to the potentiation caused by fibrinogen, binding to HSPGs in extracellular matrix results in inhibition of FGF activity that can be recovered by solubilization.49 Cell surface HSPGs also modulate the activities of FGFs50 and represent abundant low-affinity (Kd 470 nM) receptors for FGF-2.51 HSPGs function as cofactors to facilitate binding and activation, and crystal structures of receptor/ligand/heparin complexes are available that identify the interactive sites.52 Thus, the activity of FGF-2 is determined by its association with specific binding partners and EC receptors. The findings presented here demonstrate the importance of the interaction between fibrinogen and αVβ3 in determining the activity of FGF-2 and indicate further that this is related to direct receptor interactions.
Prepublished online as Blood First Edition Paper, August 5, 2004;DOI 10.1182/blood-2004-04-1358.
Supported in part by grant HL-30616 from the National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank LiHua Rong for expert technical assistance in culturing primary endothelial cells and Jennifer Jodeksnis for excellent technical assistance. The assistance of Dawn Goetze in preparing this manuscript is acknowledged gratefully. The authors thank Dr P. J. Simpson-Haidairis for critically reading the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal