Chemokine stromal derived factor 1 (SDF-1) is involved in trafficking of hematopoietic stem cells (HSCs) from the bone marrow (BM) to peripheral blood (PB) and has been found to enhance postischemia angiogenesis. This study was aimed at investigating whether SDF-1 plays a role in differentiation of BM-derived c-kit+ stem cells into endothelial progenitor cells (EPCs) and in ischemia-induced trafficking of stem cells from PB to ischemic tissues. We found that SDF-1 enhanced EPC number by promoting α2, α4, and α5 integrin–mediated adhesion to fibronectin and collagen I. EPC differentiation was reduced in mitogen-stimulated c-kit+ cells, while cytokine withdrawal or the overexpression of the cyclin–dependent kinase (CDK) inhibitor p16INK4 restored such differentiation, suggesting a link between control of cell cycle and EPC differentiation. We also analyzed the time course of SDF-1 expression in a mouse model of hind-limb ischemia. Shortly after femoral artery dissection, plasma SDF-1 levels were up-regulated, while SDF-1 expression in the bone marrow was down-regulated in a timely fashion with the increase in the percentage of PB progenitor cells. An increase in ischemic tissue expression of SDF-1 at RNA and protein level was also observed. Finally, using an in vivo assay such as injection of matrigel plugs, we found that SDF-1 improves formation of tubulelike structures by coinjected c-kit+ cells. Our findings unravel a function for SDF-1 in increase of EPC number and formation of vascular structures by bone marrow progenitor cells.
Introduction
It has been shown that endothelial progenitor cells (EPCs) play a role in vascular repair following ischemic injury.1 EPCs give rise to endothelial-like cells in culture, growing as spindle-shaped cells attaching to culture dishes coated with extracellular matrix (ECM) components.2 However, the mechanisms driving EPC differentiation are largely unknown. Stromal-derived factor 1 (SDF-1) regulates adhesion/chemotaxis of bone marrow hematopoietic progenitor cells through activation/regulation of specific integrin molecules.3-5 This factor is, therefore, suggested to play a major role in successful hematopoietic stem cell (HSC) engraftment in the bone marrow.6 In vivo gene inactivation of SDF-1 and its receptor C-X-C chemokine receptor 4 in mice led to early embryonic lethality due to abnormal cerebellar, gastrointestinal vasculature, and hematopoiesis development.7-9 A role for SDF-1 in HSC/EPC recruitment from bone marrow (BM) to peripheral blood (PB) has been proposed, based on the evidence that granulocyte colony stimulating factor (G-CSF)–mediated HSC/EPC mobilization causes an imbalance between the expression of BM SDF-1 and CXCR4 in HSCs,10 and that SDF-1α adenovirus gene transfer enhances the number of circulating HSCs/EPCs.11-13 Recently, overexpression of SDF-1 in ischemic tissues has been found to enhance EPC recruitment from PB and to induce neoangiogenesis.14,15
In this paper, we show that SDF-1 increases EPC number through enhancement of (BM) c-kit+ stem cell adhesion onto extracellular matrix components by integrin receptors. Further, we show that treatment of c-kit+ cells with mitogenic cytokines abolished SDF-1–mediated EPC differentiation, and that inhibition of cell cycle progression, obtained by overexpressing p16INK4, restored the ability to differentiate in the presence of cytokines. We also found that SDF-1 expression following hind-limb ischemia in mice was up-regulated in plasma and down-regulated in bone marrow, thus providing a novel potential mechanism for mobilization of c-kit+ cells in peripheral blood. Moreover, SDF-1 expression was up-regulated in ischemic tissues at comparable time points, suggesting that following hind-limb ischemia, an SDF-1 gradient is established favoring stem cell mobilization in PB and homing into ischemic tissues. Taken together, these results provide a novel insight on the mechanisms driving EPC differentiation and recruitment into ischemic tissues. Our findings have important implications for stem cell–based therapy of ischemic disorders.
Materials and methods
Cell isolation and culture
C-kit+ cells were isolated from mouse bone marrow by magnetic cell sorting (MACS; Miltenyi Biotech, Bergisch Gladbach, Germany). Bone marrow (BM) cells were obtained by flushing femurs and tibiae of 3-month-old Swiss CD-1 female mice or ubiquitous green fluorescent protein (GFP)–expressing mice16 with phosphate-buffered saline containing 5% fetal calf serum (PBS-FCS). BM mononuclear cells (MNCs) were obtained by gradient separation onto a Ficoll Histopaque gradient. BMMNCs were incubated with antimouse c-kit monoclonal antibody (clone 2B8; BD Pharmingen, San Diego, CA) at 0.8 μg/mL concentration in PBS-FCS and with paramagnetic microbeads coated with anti–rat immunoglobulin G antibody (Miltenyi Biotech). C-kit+ cells were separated using a MINI-MACS (Miltenyi Biotech) device. Separation of c-kit+/lineage marker–negative (lin–) cells was performed by labeling BMMNCs with lin– separation kit (BD Pharmingen), containing biotin-conjugated Mac1, B220, CD3e (ϵ chain), Ter119, Ly6G, and CD45R (B220) antibodies followed by streptavidin-conjugated magnetic beads and MACS separation, before positive c-kit separation as described above. Differentiation assays were performed in glass chamber slides (Invitrogen, Frederick, MD) coated with fibronectin (FN), vitronectin (VN), gelatin, collagens I (Coll I) and IV (Coll IV), and 40- and 120-kDa fragments of FN at the following concentrations: 20 μg/mL (FN, 40-120 kDa FN fragments), 50 μg/mL (Coll I and VN), and 1 mg/mL (gelatin and Coll I). Differentiation tests were performed in RPMI medium supplemented with 5% fetal calf serum (FCS) or in serum-free StemSpan medium (Stem Cell Technologies, Vancouver, BC) ± 100 ng/mL SDF-1 (R&D Systems, Minneapolis, MN) as described.17 Briefly, Ac-LDL-DiI+ cells were fixed and stained with Hoechst 33258 nuclear dye for cell counting. Inhibitory (DGEA, RGD) and control (RGDS) peptides were used at 20-μg/mL concentration, while inhibitory anti-α4 and anti-α5 antibodies (and the corresponding isotypes, all from Research Diagnostics, Flanders, NJ) were added to the culture medium at a 10-μg/mL concentration. Bromodeoxyuridine (BrdU) incorporation assay was performed by incubating EPCs with 0.02 mM BrdU in medium for 4 hours followed by fixation and immunofluorescence of BrdU. Ex vivo expansion and clonogenic assays of c-kit+ cells were performed as described.18 For p16INK4 overexpression, retroviral vectors pBABE wild type (wt; null) and pBABE containing human p16INK4 cDNA (Genebank L27211) were stably transfected into the Phoenix amphotropic packaging cell line as previously described.18 p16INK4- and null viral–containing supernatants were collected, filtered with 0.45-μm filters, and immediately frozen. Infections of c-kit+ cells were performed by mixing in a 1:1 ratio viral supernatants and expansion medium as described.18
FACS analysis and immunohistochemistry
The purity of c-kit+ cells and expression of other markers (CD34, CXCR4, Sca-1, α2/4/5 integrin receptors, CD31, and CD68) was assessed by flow cytometry. Freshly isolated or in vitro–expanded c-kit+ cells were incubated in PBS-FCS for 20 minutes on ice with fluorochrome-conjugated monoclonal antibodies recognizing murine c-kit (clone 2B8); Sca-1 (clone E13-161.7); CD34 (clone RAM34); CXCR4 (clone 2B11); α4 (clone R1-2), α5 (clone 5H10-27), and α2 (clone HMα2) integrins; CD68 (clone FA-11); CD31 (clone 390); and CD144 (11D4), all from BD Pharmingen except for CD68 (Serotec, Raleigh, NC), at a 0.8- to 2-μg/mL concentration and analyzed using FACScalibur Fluorescence activated cell sorting (Becton-Dickinson, San Jose, CA). Immunohistochemistry for kinase domain receptor (KDR), von Willebrand factor (VWF), and CD31 was performed as previously described.18 For cell cycle evaluation by fluorescence-activated cell sorter (FACS) analysis, c-kit+ cells cultured in suspension overnight in RPMI 5% FCS ± SDF-1, were fixed with cold 70% ethanol and further stained with propidium iodide (PI, 0.04 mg/mL).
RNAse protection assay, RT-PCR, and Western blotting
RNA was extracted from c-kit+ cells (approximately 2 × 106) or ischemic adductor muscles. RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer's instruction. Total RNA (10 μg) was hybridized to 32P-labeled m-CR6 or m-CK4 template sets (Multiprobe RPA System; BD Pharmingen) and then run on a 5% acrylamide/bis acrylamide (29:1) urea-containing gel. For reverse-transcription–polymerase chain reaction (RT-PCR), frozen adductor muscle samples (day 0, 6 hours, day 1, day 3, day 7, day 14) were homogenized in Trizol reagent. For both SDF-1 and murine β-actin amplification, we used the following protocol: 1 minute at 95 °C, 45 seconds at 52°C, and 1 minute at 72 °C, for 30 cycles. Primer sequences for SDF-1 α/β mRNA amplification were as follows: sense, 5′-ATGCCCATGCCGATTCTTCG-3′; antisense, 5′-TGTCTGTTGTTGTTCTTCAGCC-3′. Primers for mouse β-actin amplification were as follows: sense, 5′-CACCTTCTACAATGAGCT; antisense, 5′-GAAGGTAGTCTGTCAGGTCCC-3′. Western analysis of p16INK4 was performed using U2OS cell extracts incubated with retro null or retro p16INK4 viral supernatants in radioimmunoprecipitation assay buffer. Total lysate (30 μg) was run into sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by transfer and hybridization with anti-p16INK4 polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) and enhanced chemiluminescence (ECL; Amersham, Arlington Heights, IL) detection system.
ELISA test and stem cell mobilization assays
Peripheral blood (PB) murine samples and BM were obtained from normoperfused mice or from mice at different time points after induction of hind-limb ischemia (day 0, 6 hours, day 1, day 3, day 7, day 14). Blood was centrifuged to collect plasma, while BM was processed as described.10 SDF-1 determination was performed by SDF-1 enzyme-linked immunosorbent assay (ELISA) kit (no. DSA00; R&D Systems) according to the manufacturer's instructions using 100 μL undiluted murine plasma or BM samples. For stem cell mobilization assays, PB was collected at different time points after induction of hind-limb ischemia (day 0, 6 hours, day 3, day 7, day 14). PBMNCs were obtained by Ficoll gradient separation, incubated for 30 minutes at 4°C in PBS containing 5% FCS with phycoerythrin (PE)–conjugated anti–c-kit monoclonal antibody, and then analyzed by flow cytometry.
In vivo procedures and immunohistochemistry
Hind-limb ischemia was produced as previously described.18 Paraffin sections (3 μm) of adductor muscles from ischemic mice were incubated with 2% PBS/bovine serum albumin (BSA) for one hour at room temperature, treated for 30 minutes in 3% H2O2 in PBS/BSA to block endogenous peroxidase and then incubated with 0.2 μg/mL polyclonal antihuman SDF-1 antibody (Immunokontact, Oxon, UK, no. 22140192 APX) in 2% PBS/BSA. After washing, sections were treated with biotinylated antirabbit polyclonal antibody, followed by staining using avidin-biotinperoxidase complex and dibutylamine peroxidase staining kit (Vector Laboratories, Burlingame, CA). Matrigel plug assay was performed as previously described.19 Briefly, c-kit+ cells were obtained from ubiquitous GFP-expressing mice16 by MACS separation and then mixed to cytokine and phenol red–free Matrigel (BD Biosciences, San Jose, CA) in the presence or the absence of SDF-1 at 100 ng/mL concentration. Matrigel-containing cells and/or factor was then injected subcutaneously. After 7 days, matrigel plug was removed, formalin-fixed, and embedded in paraffin for sectioning followed by staining according to trichrome-Masson procedure (Bio-Optica, Milan, Italy). Blood vessel density was determined as described.19 C-kit+/GFP+ cells were also injected in adductor muscles after removing the femoral artery. GFP/α-actin and GFP/VWF staining in paraffin sections of ischemic adductor muscles injected with stem cells was performed as described.18 Tissue sections and isolated cells stained for different antigens were observed and photographed under a Zeiss Axioplan 2 microscope at × 20 and × 40 magnifications.
Statistical analysis
Statistical analysis was performed on at least 3 independent experiments in each experimental set. Student t test was performed as a significance test by Jandel Sigma STAT software (Sigma, St Louis, MO). Results are cited as average ± standard error.
Results
SDF-1 increases EPC number in vitro by a cell adhesion–dependent mechanism
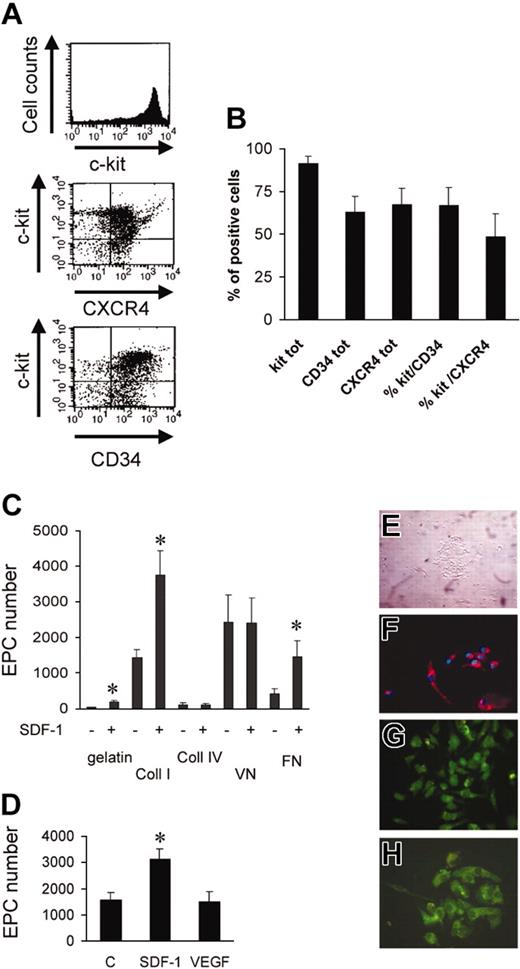
Figure 1A shows the percentage of murine bone marrow c-kit+ cells (91.4% ± 4.2%, n = 3), as assessed by flow cytometry. A high proportion of c-kit+ cells was also CD34+ (Takahashi et al2 ; Wright et al3 ; and Shen et al20 ) and CXCR4+ (Figure 1B), suggesting that the cells respond to SDF-1 stimulation.3,5,6,21,22 It has been shown that adhesion onto extracellular matrix (ECM) components promotes endothelial commitment of circulating and BM-derived progenitors. On the other hand, SDF-1 has been found to enhance the adhesion of stem cells to ECM components, raising the possibility that it enhances differentiation into EPCs. To assess this hypothesis, we plated c-kit+ cells onto gelatin, fibronectin (FN), collagens I and IV (Coll I and Coll IV), and vitronectin (VN). After 3 days in culture, round cell clusters, associated with partially elongated adhering cells, were present17 (Figure 1E). Cells were left in culture for a 7-day period, after which they were incubated with Ac-LDL-DiI as an indicator of EPC differentiation, followed by fixation and Hoechst nuclear staining (Figure 1F). Adherent Ac-LDL-DiI+/Hoechst+-labeled cells were counted in each culture well. Differentiation of c-kit+ cells into EPCs was different between substrates with maximal attachment/differentiation onto Coll I, FN, and VN and minimal onto gelatin and Coll IV (Figure 1C). We also performed immunohistochemical analysis on adherent cells using antibodies against vascular endothelial growth factor receptor 2 (VEGFR-2, KDR; Figure 1G), von Willebrand factor (Figure 1H), and CD31 (not shown). The majority (> 95%) of adherent/differentiated cells were positive for these markers. Several studies have shown that SDF-1 is implicated in adhesion and chemotaxis of primitive hematopoietic stem cells onto extracellular matrix components or onto substrate cells.3,5,21,23,24 To assess whether SDF-1 enhances adhesion and endothelial differentiation of c-kit+ cells under our conditions, we performed differentiation assays using 100 and 300 ng/mL SDF-1. Interestingly, 100 ng/mL SDF-1 significantly enhanced the number of Ac-LDL-DiI+ cells onto Coll I, FN, and gelatin, but not onto Coll IV and VN (Figure 1C), thus suggesting modulation and/or activation of FN and Coll I receptors in c-kit+ cells by the chemokine. Similar results were obtained using 300 ng/mL SDF-1 (not shown). It has been found that VEGF enhances the engraftment of bone marrow–derived EPCs in newborn mice,25 induces endothelial cell differentiation of ES cells,26 and promotes differentiation of EPCs in cooperation with basic fibroblast growth factor (bFGF) and insulinlike growth factor 1 (IGF-1).27 Therefore, we compared the effect of SDF-1 and VEGF onto Coll I ± 100 ng/mL SDF-1 or 50 ng/mL VEGF. In contrast to SDF-1, VEGF did not enhance the number of endothelial cells above control (Figure 1D).
Isolation, characterization, and differentiation of c-kit+ cells in culture. (A) FACS analysis showing the purity of c-kit+ cells obtained by MACS sorting and relative CXCR4 and CD34 expression in these cells. (B) Percentage of c-kit–, CXCR4–, and CD34-expressing cells in MACS-sorted cells (n = 3). tot indicates total. (C) Quantification of differentiated EPC number (Ac-LDL-DiI+ cells) on the indicated substrates in the presence or absence of SDF-1. *P < .05; n = 5. (D) In contrast to SDF-1, VEGF does not act as a differentiation factor for c-kit+ cells onto Coll I. *P < .05; n = 3. C indicates control-untreated cells. (E) Phase contrast image of clusters of c-kit+–derived cells after 3 days of culture. (F) Double staining of Hoechst 33258 and Ac-LDL-DiI+ in differentiated EPCs after 7 days in culture. (G-H) Immunohistochemistry showing VWF (G) and KDR (H) expression in clusters of differentiated EPCs after SDF-1 stimulation in culture for 7 days.
Isolation, characterization, and differentiation of c-kit+ cells in culture. (A) FACS analysis showing the purity of c-kit+ cells obtained by MACS sorting and relative CXCR4 and CD34 expression in these cells. (B) Percentage of c-kit–, CXCR4–, and CD34-expressing cells in MACS-sorted cells (n = 3). tot indicates total. (C) Quantification of differentiated EPC number (Ac-LDL-DiI+ cells) on the indicated substrates in the presence or absence of SDF-1. *P < .05; n = 5. (D) In contrast to SDF-1, VEGF does not act as a differentiation factor for c-kit+ cells onto Coll I. *P < .05; n = 3. C indicates control-untreated cells. (E) Phase contrast image of clusters of c-kit+–derived cells after 3 days of culture. (F) Double staining of Hoechst 33258 and Ac-LDL-DiI+ in differentiated EPCs after 7 days in culture. (G-H) Immunohistochemistry showing VWF (G) and KDR (H) expression in clusters of differentiated EPCs after SDF-1 stimulation in culture for 7 days.
An increase in the number of adherent cells after 7 days in culture may reflect SDF-1–mediated apoptosis suppression or proliferation enhancement in c-kit+ cells.22 We thus analyzed the cell cycle of c-kit+ cells by FACS analysis of PI-stained cells after one day of culture and BrdU uptake in adherent cells at the end of the culture period. PI staining (Figure 2A-B) showed that the majority of c-kit+ cells were in the G1 phase, and that SDF-1 did not significantly affect the percentage of c-kit+ cells in either G1 or sub-G1 phase. Further, BrdU uptake of adherent EPCs was comparable in control and SDF-1–treated cells (Figure 2C-E). This is consistent with the hypothesis that the higher EPC number obtained following SDF-1 treatment (Figure 1C) was not due to an increase in cell proliferation.
SDF-1 does not modify EPC cell cycle. (A-B) FACS analysis of PI-stained one-day–cultured c-kit+ cells ± 100 ng/mL SDF-1. The addition of the chemokine did not result in suppression of apoptosis or in progression into cell cycle as evidenced by the percentage of cells in sub-G1 (brackets in A) and G1 phase, respectively. (B) Average of 3 independent experiments ± SE; filled bars indicate control treatment; open bars, 100 ng/mL SDF-1. (C-E) SDF-1 does not modify the percentage of differentiated EPCs in S-phase after 7 days of culture. BrdU incorporation assays in differentiated EPCs were performed, and BrdU+ cells (arrows in C-D) were counted in randomly selected microscope fields among Hoechst 33258+ cells. (E) Average of 3 independent experiments ± SE. C indicates control-untreated cells.
SDF-1 does not modify EPC cell cycle. (A-B) FACS analysis of PI-stained one-day–cultured c-kit+ cells ± 100 ng/mL SDF-1. The addition of the chemokine did not result in suppression of apoptosis or in progression into cell cycle as evidenced by the percentage of cells in sub-G1 (brackets in A) and G1 phase, respectively. (B) Average of 3 independent experiments ± SE; filled bars indicate control treatment; open bars, 100 ng/mL SDF-1. (C-E) SDF-1 does not modify the percentage of differentiated EPCs in S-phase after 7 days of culture. BrdU incorporation assays in differentiated EPCs were performed, and BrdU+ cells (arrows in C-D) were counted in randomly selected microscope fields among Hoechst 33258+ cells. (E) Average of 3 independent experiments ± SE. C indicates control-untreated cells.
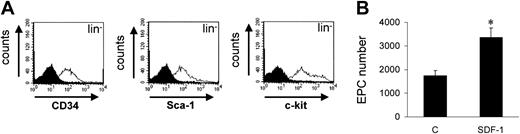
Recent reports have shown that cells bearing monocytic features purified from peripheral blood give rise to EPCs,28,29 thus making unsure the origin (stem cell vs monocytes) of cells taking up AC-LDL-DiI in culture and giving rise to endothelial cells (Figure 1). To this aim, we isolated c-kit+ cells from lineage marker–negative (lin–) cells in the bone marrow by a sequential isolation protocol, which produced highly enriched stem cells (Figure 3A). We then plated these cells onto FN in RPMI containing 5% FCS ± 100 ng/mL SDF-1. The results showed that c-kit+lin– cells differentiated more efficiently than c-kit+ cells onto FN (P < .01) both in the presence or the absence of SDF-1 (compare bars in Figures 1C and Figure 3B).
Effects of SDF-1 on differentiation of c-kit+/lin– cells onto FN. (A) Negative selection for lineage-negative cells was performed prior to isolating c-kit+ cells from mouse BM mononuclear cells. FACS analysis showed that lineage marker selection allowed recovery of cells positive for c-kit, CD34, and Sca-1 markers as shown by histogram overlay of each specific antibody over isotype control antibody. (B) C-kit+/lin– cells were plated into glass chamber slides coated with FN in the presence or the absence of 100 ng/mL SDF-1. Cells taking up Ac-LDL-DiI were counted as in Figure 1. *P < .05; n = 3. Error bars indicate standard errors. C indicates control-untreated cells.
Effects of SDF-1 on differentiation of c-kit+/lin– cells onto FN. (A) Negative selection for lineage-negative cells was performed prior to isolating c-kit+ cells from mouse BM mononuclear cells. FACS analysis showed that lineage marker selection allowed recovery of cells positive for c-kit, CD34, and Sca-1 markers as shown by histogram overlay of each specific antibody over isotype control antibody. (B) C-kit+/lin– cells were plated into glass chamber slides coated with FN in the presence or the absence of 100 ng/mL SDF-1. Cells taking up Ac-LDL-DiI were counted as in Figure 1. *P < .05; n = 3. Error bars indicate standard errors. C indicates control-untreated cells.
To exclude the possibility that our positive c-kit selection may “fish-out” cells displaying monocyte features in the bone marrow, we performed FACS analysis on c-kit–sorted cells using CD68 antibody. The results showed that although almost a half of the cells in the BM (49% ± 7%, average ± SE, n = 3) were CD68low/c-kit–, relatively low numbers of cells were CD68low/c-kit+ (8% ± 4%). In addition, only 16% ± 4% of c-kit+–sorted cells were CD68low, suggesting that c-kit selection allows for enrichment of a majority of cells that are negative or low-expressing monocyte markers. Finally, to assess whether SDF-1 acts on stem cell phenotype reprogramming independent of cell adhesion, we analyzed endothelial and monocyte marker regulation by FACS analysis of CD68 and CD31 in c-kit+ cells cultured for 3 days in the absence of adhesion substrates. The results showed a dramatic increase in CD68 expression that was similar in the presence or absence of SDF-1 (74 ± 8% vs 62% ± 7%, respectively; n = 3; P = .14) and in the presence or absence of SDF-1 at 3 culture days and negligible CD31 up-regulation (not shown). These results were in line with results of methyl cellulose clonogenic assays, showing no enhancement of colony-forming unit endothelial cells (CFU-EC) colonies in the presence of SDF-1 (24% ± 10% vs 32% ± 10% CFU-EC/total colony-forming cells (CFCs) in the absence or the presence of SDF-1, respectively; n = 3; P = .29).
These results suggest that SDF-1 is not sufficient to induce EPC phenotype independently of cell adhesion and that c-kit+ cells, although they express low levels of monocyte marker CD68 prior to being cultured, are likely giving rise to Ac-LDL-DiI+ cells (EPCs) through a monocytelike intermediate CD68+ cell type.28,29
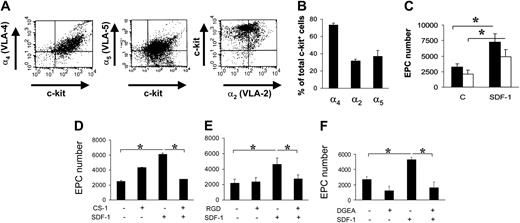
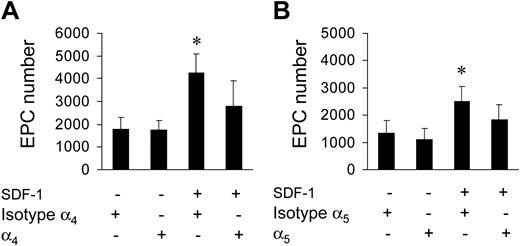
It is well established that SDF-1 acts on migration/adhesion of primitive progenitor cells onto ECM components.21,24 In addition, our results suggested that an increase in Ac-LDL-DiI cell number was mediated by an increase in integrin affinity to FN and Coll I (Figure 1). To assess this, we screened for the expression of FN receptors α4 and α5 integrins, and Coll I receptor α2 integrin, by FACS analysis. A high percentage (> 70%) of c-kit+ cells expressed α4, while a lower number expressed α2 and α5 integrins (Figure 4A-B). To assess the contribution of α4 and α5 integrins to SDF-1–mediated endothelial differentiation of c-kit+ cells, we performed differentiation assays onto 40-kDa FN and the 120-kDa FN fragments that allow discrimination between the contribution of α4 and α5 integrins, respectively, to cell adhesion onto FN.30,31 Differentiation of c-kit+ cells onto both substrates was significantly increased by treatment with SDF-1 (Figure 4C). To further assess the involvement of α4, α5, and α2β1 integrins in EPC differentiation, we used specific antagonists of α4, α5, and α2β1 integrin–mediated cell adhesion, the CS-1,21 the RGD,32 and the DGEA peptides,33 respectively, in culture onto FN (CS-1 and RGD peptides) and Coll I (DGEA peptide). Each peptide reverted the increase of EPC numbers to control levels (Figure 4D-F). Unexpectedly, however, basal differentiation of c-kit+ cells was not reduced by addition of peptides. Specificity of RGD action onto FN was further confirmed by the use of RGDS control peptide that, in contrast to RGD, did not decrease EPC number onto FN (4370 ± 365 vs 4306 ± 250; n = 3; P = .31; and data not shown). Finally, as a further control, we used blocking antibodies raised against α4 and α5 integrins. Both antibodies, but not isotype control antibodies, reverted EPC increase onto FN (Figure 5).
FN and Coll I integrin receptor expression in c-kit+ cells and reversion of EPC differentiation by integrin antagonists in culture. (A-B) FACS analysis and quantification of α4, α5, and α2 integrin expression in c-kit+ cells (n = 3). (C) Plating c-kit+ cells onto different FN subfragments (40-120 kDa) revealed a different contribution of α4 and α5 integrin receptors to EPC differentiation. Onto both fragments, SDF-1 enhanced EPC differentiation compared with controls, although to a different extent. In fact, the amount of differentiated EPCs was significantly higher onto 40-kDa FN fragment, containing the CS-1 epitope for α4 binding (filled bars), compared with the 120-kDa FN fragment, containing the RGD motif, specific for α5 binding (open bars). *P < .05; n = 4. C indicates control-untreated cells. (D-F) Reversion of EPC differentiation using FN or Coll I adhesion antagonists. CS-1 (D), RGD (E), and DGEA (F) peptides antagonizing binding of EPCs to FN via α4 and α5 integrins, or Coll I via α2β1, were added in culture. Cell counts showed that these antagonists reverted SDF-1–enhanced differentiation revealing a cell adhesion–dependent mechanism. *P < .05; n = 4. Error bars indicate standard errors.
FN and Coll I integrin receptor expression in c-kit+ cells and reversion of EPC differentiation by integrin antagonists in culture. (A-B) FACS analysis and quantification of α4, α5, and α2 integrin expression in c-kit+ cells (n = 3). (C) Plating c-kit+ cells onto different FN subfragments (40-120 kDa) revealed a different contribution of α4 and α5 integrin receptors to EPC differentiation. Onto both fragments, SDF-1 enhanced EPC differentiation compared with controls, although to a different extent. In fact, the amount of differentiated EPCs was significantly higher onto 40-kDa FN fragment, containing the CS-1 epitope for α4 binding (filled bars), compared with the 120-kDa FN fragment, containing the RGD motif, specific for α5 binding (open bars). *P < .05; n = 4. C indicates control-untreated cells. (D-F) Reversion of EPC differentiation using FN or Coll I adhesion antagonists. CS-1 (D), RGD (E), and DGEA (F) peptides antagonizing binding of EPCs to FN via α4 and α5 integrins, or Coll I via α2β1, were added in culture. Cell counts showed that these antagonists reverted SDF-1–enhanced differentiation revealing a cell adhesion–dependent mechanism. *P < .05; n = 4. Error bars indicate standard errors.
Reversion of EPC increase by blocking antibodies. Use of anti-α4 (A) and anti-α5 (B) blocking antibodies inhibited SDF-1–mediated EPC increase onto FN. *P < .05; n = 3. Error bars indicate standard errors.
Reversion of EPC increase by blocking antibodies. Use of anti-α4 (A) and anti-α5 (B) blocking antibodies inhibited SDF-1–mediated EPC increase onto FN. *P < .05; n = 3. Error bars indicate standard errors.
Cell-cycle dependence of EPC differentiation
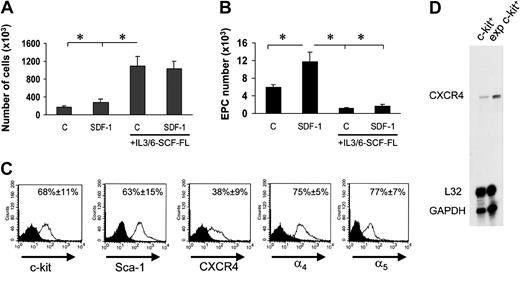
Experiments in Figure 2 suggested that EPC differentiation of c-kit+ cells in culture was possibly linked to a G1 arrest. We therefore cultured c-kit+ cells onto FN in the presence of cytokines (interleukin-3 [IL-3], IL-6, stem cell factor [SCF], and Flt3 ligand [Flt3L]) that provide for an optimal c-kit+ cell proliferation18 into FN-coated dishes in the presence or the absence of SDF-1. Floating stem cell number in the culture wells raised approximately 10 times in 7 days in both conditions (Figure 6A). However, surprisingly, EPC number was significantly lower than by culturing stem cells in the absence of mitogenic stimuli (Figure 6B). In addition, in the presence of mitogens, SDF-1 no longer increased EPC number (Figure 6B). Expansion in culture of hematopoietic progenitor cells may cause CXCR4 down-regulation, with subsequent reduction in SDF-1 adhesion/chemotactic response.5 FACS analysis of in vitro–expanded stem cells showed, however, that these cells were c-kit+ and Sca-1+ and expressed CXCR4, although at a lower level compared with freshly isolated cells. By contrast, ex vivo–expanded cells were highly positive for α4 integrin and α5 integrin (Figure 6C), and expressed higher amounts of CXCR4 at the RNA level (Figure 6D). To assess whether reduction of EPC differentiation is linked to ex vivo mitogen stimulation of BM progenitors, we performed a differentiation assay using cells that were expanded for 7 days in the presence of mitogenic cytokines. Prior to performing differentiation assays onto FN, cytokines were carefully withdrawn from the culture medium prior to seeding cells. Under these conditions, the ability of ex vivo–expanded cells to differentiate was restored (Figure 7A), confirming the hypothesis that cell cycle is important to drive EPC commitment. To further investigate whether EPC cell cycle arrest favors differentiation, we transduced c-kit+ cells with a retroviral vector driving p16INK4 expression34,35 (Figure 7B) and then analyzed for increase in cell number in the presence of cytokines in a clonogenic assay. The number of null retrovirus–infected cells increased over time during 5 days following infection. In contrast, retro-p16INK4 infection abolished cytokine-mediated stem cell proliferation (Figure 7C)34 and significantly reduced the number of CFCs in a methylcellulose clonogenic assay (data not shown). Retro null– or retro p16INK4–infected cells were then cultured for 7 days into FN-coated wells for EPC differentiation in the presence of mitogenic cytokines. Retro null–infected cells differentiated at similar low levels both in the presence and the absence of SDF-1. In contrast, the magnitude of SDF-1 response in p16INK4-overexpressing cells was restored, although not at the levels of freshly isolated cells (compare Figure 7D with Figure 6B). Integrin expression in retrovirus-infected cells was similar to cytokine-expanded cells (Figure 6C), suggesting that failure of SDF-1 to induce endothelial phenotype in retro null–infected c-kit+ cells was not due to a diminished expression of α4 and α5 receptors.
Reversion of SDF-1–enhanced EPC differentiation by mitogenic stimulation of c-kit+ cells. (A) Culturing c-kit+ cells in serum-free medium in the presence of IL-3/IL-6, FL, and SCF dramatically enhanced the number of EPCs either in the presence or absence of SDF-1. *P < .05; n = 3. (B) These cells, however, failed to differentiate both in the presence or absence of SDF-1 when cultured onto FN substrate. *P < .05; n = 3. Error bars indicate standard errors. C indicates untreated cells or cells treated with cytokine cocktail without SDF-1. (C) To assess whether c-kit+ cells cultured in the presence of cytokines express stem cell markers CXCR4 and integrin receptors, we performed a FACS analysis for c-kit, Sca-1, CXCR4, and α4 and α5 integrins. Results are given as average of 3 independent experiments ± SE. (D) RNAse protection assay showing expression of CXCR4 mRNA in freshly isolated c-kit+ cells and its up-regulation following culture in mitogenic condition for 7 days. exp indicates expression.
Reversion of SDF-1–enhanced EPC differentiation by mitogenic stimulation of c-kit+ cells. (A) Culturing c-kit+ cells in serum-free medium in the presence of IL-3/IL-6, FL, and SCF dramatically enhanced the number of EPCs either in the presence or absence of SDF-1. *P < .05; n = 3. (B) These cells, however, failed to differentiate both in the presence or absence of SDF-1 when cultured onto FN substrate. *P < .05; n = 3. Error bars indicate standard errors. C indicates untreated cells or cells treated with cytokine cocktail without SDF-1. (C) To assess whether c-kit+ cells cultured in the presence of cytokines express stem cell markers CXCR4 and integrin receptors, we performed a FACS analysis for c-kit, Sca-1, CXCR4, and α4 and α5 integrins. Results are given as average of 3 independent experiments ± SE. (D) RNAse protection assay showing expression of CXCR4 mRNA in freshly isolated c-kit+ cells and its up-regulation following culture in mitogenic condition for 7 days. exp indicates expression.
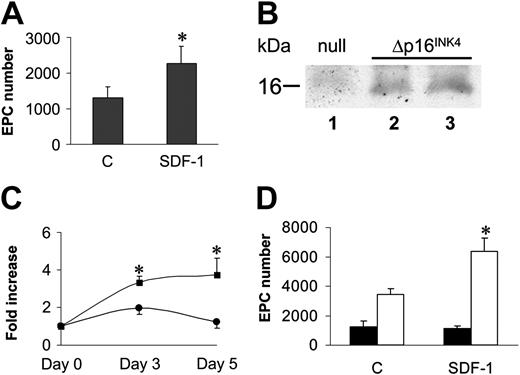
A cell cycle G1 checkpoint likely cooperates with SDF-1–mediated adhesion in EPC differentiation of c-kit+ cells. (A) Stem cells were expanded for 7 days in cytokine containing serum-free medium, after which they were plated onto FN without cytokines ± SDF-1. The results showed that cytokine washing partially restored EPC differentiation of ex vivo–expanded cells. *P < .05; n = 5. Error bars indicate standard errors. (B) Overexpression of p16INK4 was performed by incubating U2OS cells with retrovirus supernatants (null, lane 1 or increasing amounts of p16INK4 retrovirus; Δp16INK4, lanes 2-3) to check transgene expression by Western analysis. (C) C-kit+ cells were transduced with retro null or retro p16INK4 and thereafter analyzed for cell number increase in the presence of mitogens. Both at 3 and 7 days after infection, cell proliferation was blocked in p16INK4-transduced cells. Data are reported as fold increase in cell number compared with number of cells at the beginning of the time course. *P < .05; n = 4, for the comparison between growth of retro null–infected (squares) and retro p16INK4–infected (circles) cells. (D) Differentiation test of retro null– and retro p16INK4–infected stem cells under mitogen stimulation. Although retro null–infected c-kit+ did not significantly differentiate into EPCs (filled bars), cells overexpressing p16INK4 were restored in their ability to differentiate in response to SDF-1 stimulation (open bars). *P < .05; n = 5. Error bars indicate standard errors. C indicates control-untreated cells (A,D).
A cell cycle G1 checkpoint likely cooperates with SDF-1–mediated adhesion in EPC differentiation of c-kit+ cells. (A) Stem cells were expanded for 7 days in cytokine containing serum-free medium, after which they were plated onto FN without cytokines ± SDF-1. The results showed that cytokine washing partially restored EPC differentiation of ex vivo–expanded cells. *P < .05; n = 5. Error bars indicate standard errors. (B) Overexpression of p16INK4 was performed by incubating U2OS cells with retrovirus supernatants (null, lane 1 or increasing amounts of p16INK4 retrovirus; Δp16INK4, lanes 2-3) to check transgene expression by Western analysis. (C) C-kit+ cells were transduced with retro null or retro p16INK4 and thereafter analyzed for cell number increase in the presence of mitogens. Both at 3 and 7 days after infection, cell proliferation was blocked in p16INK4-transduced cells. Data are reported as fold increase in cell number compared with number of cells at the beginning of the time course. *P < .05; n = 4, for the comparison between growth of retro null–infected (squares) and retro p16INK4–infected (circles) cells. (D) Differentiation test of retro null– and retro p16INK4–infected stem cells under mitogen stimulation. Although retro null–infected c-kit+ did not significantly differentiate into EPCs (filled bars), cells overexpressing p16INK4 were restored in their ability to differentiate in response to SDF-1 stimulation (open bars). *P < .05; n = 5. Error bars indicate standard errors. C indicates control-untreated cells (A,D).
Hind-limb ischemia modulates SDF-1 expression and BM progenitors trafficking to peripheral blood
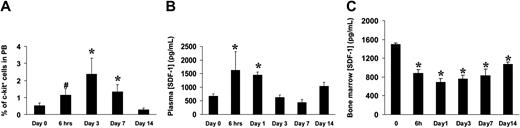
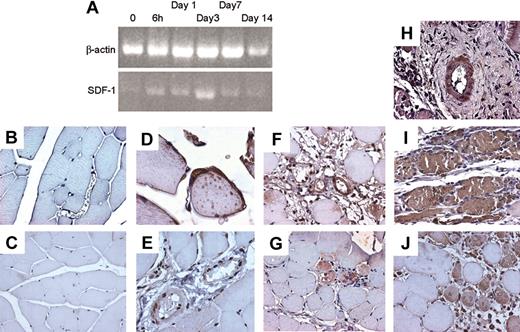
To assess whether the SDF-1/CXCR4 axis is involved in ischemia-induced mobilization and homing of BM progenitor cells to ischemic tissues, we analyzed the levels of c-kit+ cells in PB and SDF-1 plasma and BM concentrations in mice in which ischemia was induced by removal of the femoral artery.18 As previously reported in mice and humans,2,36 the amount of circulating progenitor cells was increased shortly after induction of ischemia and persisted up to 7 days (Figure 8A). Interestingly, a rise in plasmatic concentration of SDF-1 was also noticed at early time points (Figure 8B), while a significant reduction of SDF-1 BM concentration was reported at all time points following ischemia (Figure 8C). To assess whether SDF-1 is regulated in a similar fashion, we analyzed its expression by RT-PCR in the adductor muscles of ischemic mice. The results showed an up-regulation of the SDF-1 mRNA (Figure 9A) peaking at day 3 following ischemia. To identify cells producing SDF-1 in ischemic muscles, we performed immunohistochemistry in histologic sections and found that in normoperfused tissue, the majority of muscle fibers were SDF-1 negative (Figure 9C) except for small fibers expressing SDF-1 in satellite cells (Figure 9D).37 In addition, SDF-1 expression was low in endothelial cells and pericytes in the wall of most arterioles (Figure 9E). However, following ischemia, SDF-1 up-regulation was already evident at 6 hours in arterioles and in some muscle fibers (Figure 9F-G), and peak expression was achieved at 3 days after ischemia (Figure 9H-I). Finally, at day 9, SDF-1 expression was confined to regenerating muscle fibers bearing centrally located nuclei,18 but not in fully differentiated muscle fibers, suggesting that SDF-1 is involved in postischemia muscle regeneration (Figure 9J).
Ischemia-induced mobilization of BM progenitor cells in PB and modulation of plasma and BM levels of SDF-1 following ischemia. (A) C-kit+ cells were evaluated by analyzing 104 peripheral blood mononuclear cells at the indicated time points. *P < .05; #P = .05; n = 6, for each experimental group. (B) ELISA tests revealed a rise in the SDF-1 plasma levels shortly after induction of ischemia. *P < .05; n = 4, for each experimental group. (C) Decrease of SDF-1 BM concentration of SDF-1 as evaluated by ELISA test of bone marrow obtained from the ischemic limbs.10 *P < .05; n = 3, for each experimental group. Error bars indicate standard errors.
Ischemia-induced mobilization of BM progenitor cells in PB and modulation of plasma and BM levels of SDF-1 following ischemia. (A) C-kit+ cells were evaluated by analyzing 104 peripheral blood mononuclear cells at the indicated time points. *P < .05; #P = .05; n = 6, for each experimental group. (B) ELISA tests revealed a rise in the SDF-1 plasma levels shortly after induction of ischemia. *P < .05; n = 4, for each experimental group. (C) Decrease of SDF-1 BM concentration of SDF-1 as evaluated by ELISA test of bone marrow obtained from the ischemic limbs.10 *P < .05; n = 3, for each experimental group. Error bars indicate standard errors.
SDF-1 regulation in adductor muscle following ischemia. (A) Semiquantitative RT-PCR using RNA obtained from an RNA pool of at least 3 animals at each indicated time point showed an up-regulation of the SDF-1 mRNA above control peaking at day 3 following ischemia. Up-regulation of SDF-1 mRNA above control is evident starting 6 hours following induction of ischemia. (B) Negative control for SDF-1 immunohistochemistry in tissue sections of normoperfused mice. (C-E) Immunohistochemistry of SDF-1 in adductor muscles of normoperfused mice. Expression of SDF-1 was generally very low (C), but expressed in satellite cells of some small muscle fibers (D). In nonischemic muscles SDF-1 was also expressed, although at low levels, in arterioles (E). (F-G) At 6 hours after ischemia, SDF-1 was up-regulated in arterioles (F) and muscle fibers associated to infiltrating cells showing strong immunoreactive staining (G). (H-I) At day 3, a strong up-regulation in SDF-1 protein expression was observed in the adductor muscle both at arteriole (H) and muscle fibers levels (I). (J) SDF-1 was expressed in regenerating muscle fibers, showing centrally located nuclei, at 7 days after ischemia.
SDF-1 regulation in adductor muscle following ischemia. (A) Semiquantitative RT-PCR using RNA obtained from an RNA pool of at least 3 animals at each indicated time point showed an up-regulation of the SDF-1 mRNA above control peaking at day 3 following ischemia. Up-regulation of SDF-1 mRNA above control is evident starting 6 hours following induction of ischemia. (B) Negative control for SDF-1 immunohistochemistry in tissue sections of normoperfused mice. (C-E) Immunohistochemistry of SDF-1 in adductor muscles of normoperfused mice. Expression of SDF-1 was generally very low (C), but expressed in satellite cells of some small muscle fibers (D). In nonischemic muscles SDF-1 was also expressed, although at low levels, in arterioles (E). (F-G) At 6 hours after ischemia, SDF-1 was up-regulated in arterioles (F) and muscle fibers associated to infiltrating cells showing strong immunoreactive staining (G). (H-I) At day 3, a strong up-regulation in SDF-1 protein expression was observed in the adductor muscle both at arteriole (H) and muscle fibers levels (I). (J) SDF-1 was expressed in regenerating muscle fibers, showing centrally located nuclei, at 7 days after ischemia.
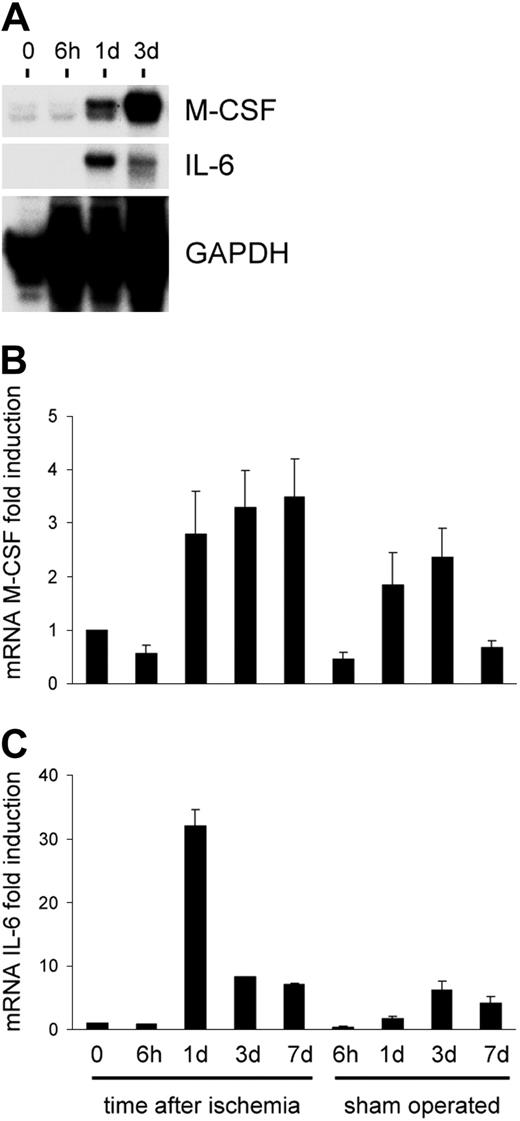
Hypoxia-regulated overexpression of growth factors (ie, VEGF1 ) is associated with ischemia, and is possibly implicated in trafficking and differentiation of circulating precursor cells. To assess whether cytokines associated to EPC growth, differentiation, and mobilization are up-regulated during ischemia, we performed an RNAse protection assay using a panel of probes specific for mouse cytokines and growth factors. The results showed that, among those analyzed (IL-3/IL-6/IL-7/IL-11, G-CSF and macrophage CSF [M-CSF], leukemia inhibitory factor [LIF], and SCF), the factors that are up-regulated by ischemia are IL-6, and, to a lesser extent, M-CSF (Figure 10A-B). This suggests that SDF-1 is one of the major players in c-kit+ cell reallocation from BM to the PB.
Time course of cytokine overexpression following limb ischemia in mice. Although IL-3 was not affected by induction of ischemia by femoral artery dissection, IL-6 and to a lesser extent M-CSF mRNAs were induced over the levels in sham-operated animals starting at day one following ischemia. (A) RNAse protection assay using RNA from nonischemic and ischemic adductor muscles at day 0 and 6 hours, 1 day, and 3 days following ischemia. (B-C) Quantification of M-CSF and IL-6 expression by densitometer analysis. Data are expressed as fold induction compared with nonischemic muscle. Error bars indicate standard errors.
Time course of cytokine overexpression following limb ischemia in mice. Although IL-3 was not affected by induction of ischemia by femoral artery dissection, IL-6 and to a lesser extent M-CSF mRNAs were induced over the levels in sham-operated animals starting at day one following ischemia. (A) RNAse protection assay using RNA from nonischemic and ischemic adductor muscles at day 0 and 6 hours, 1 day, and 3 days following ischemia. (B-C) Quantification of M-CSF and IL-6 expression by densitometer analysis. Data are expressed as fold induction compared with nonischemic muscle. Error bars indicate standard errors.
Angiogenic effects of SDF-1 in vivo
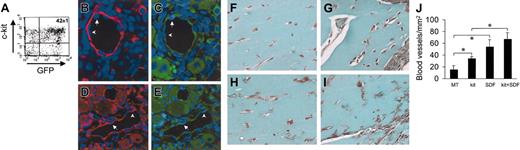
Administration of SDF-1 in a mouse model of hind-limb ischemia has been reported to enhance angiogenesis by coinjected human angioblasts in immunosuppressed mice.14 To assess whether c-kit+ cells take part in formation of new blood vessels in vivo, we injected HSCs obtained from mice ubiquitously expressing GFP16 (Figure 10A) in adductor muscle after removal of the femoral artery. In agreement with previous findings,2,38 we found the presence of GFP+ cells in arterioles (Figure 11B-E). Finally, to test the role of SDF-1 as an angiogenic factor and an in situ EPC differentiation trigger, we performed matrigel (MT) plug assays by mixing c-kit+ cells and MT with or without SDF-1 at the same concentration (100 ng/mL) to that used in our in vitro assays. The ability of SDF-1 and/or c-kit+ cells to enhance angiogenesis in this system was measured by determining the total blood vessels density/mm2 in trichrome-Masson–stained paraffin sections19 (Figure 11F-J). The results showed a clear angiogenic effect in mice injected with MT containing SDF-1 alone or c-kit+ cells alone compared with MT-only injected mice (Figure 11L). However, SDF-1 was not able to significantly enhance the number of blood vessels in mice injected with MT-containing c-kit+ cells. This was probably due to the strong angiogenic effect exerted by addition of recombinant SDF-1 on pre-existing blood vessels that might mask the contribution given to angiogenesis by recruited/differentiated EPCs. Finally, to assess the effects of SDF-1 on EPC differentiation in vivo, we coinjected MT and c-kit+ cells obtained from GFP-expressing mice (Figure 11A). As shown in Figure 12A, in agreement with findings by Tamarat et al39 and Bompais et al,40 in addition to structured vessels, c-kit+ cells obtained from bone marrow of GFP transgenic mice also formed tubulelike structures in MT after 7 days. These structures stained positive for GFP and VWF in immunohistochemistry experiments (Figure 12D-F). Addition of 100 ng/mL SDF-1 to MT containing GFP+ cells increased the dimension of these structures within these 3-dimensional structures in MT plugs (Figure 12B-C,G-I).
Effect of SDF-1 on in vivo differentiation of c-kit+ cells. (A) C-kit+ cells were obtained from mice ubiquitously expressing GFP protein. FACS analysis of GFP+/c-kit+ cells purified from these mice by MACS. (B-E) GFP+/c-kit+ cells were injected into adductor muscles at the time of femoral artery dissection. (B-C) Expression of GFP (green fluorescence) and α-actin (red fluorescence) in 2 consecutive sections. (D-E) Expression of GFP (green fluorescence) and VWF (red fluorescence) in 2 consecutive sections. The presence of GFP+ (arrows) and GFP– (arrowheads) endothelial cells is shown. (F-J) Trichrome-Masson staining of MT plugs containing SDF-1 and/or c-kit+ cells. (F) Background angiogenesis in MT-only injected mice. (H) A relative increase in the number of small vessels by injecting MT-containing c-kit+ cells. (G,I) An enhanced number of blood vessels in MT–SDF-1 (G) and MT–SDF-1/c-kit+ cell injected mice. (J) Quantification of the total blood vessel density (expressed as blood vessels/mm2) in MT plugs in all experimental conditions. *P < .05; n = 4, for each mice group. Error bars indicate standard errors.
Effect of SDF-1 on in vivo differentiation of c-kit+ cells. (A) C-kit+ cells were obtained from mice ubiquitously expressing GFP protein. FACS analysis of GFP+/c-kit+ cells purified from these mice by MACS. (B-E) GFP+/c-kit+ cells were injected into adductor muscles at the time of femoral artery dissection. (B-C) Expression of GFP (green fluorescence) and α-actin (red fluorescence) in 2 consecutive sections. (D-E) Expression of GFP (green fluorescence) and VWF (red fluorescence) in 2 consecutive sections. The presence of GFP+ (arrows) and GFP– (arrowheads) endothelial cells is shown. (F-J) Trichrome-Masson staining of MT plugs containing SDF-1 and/or c-kit+ cells. (F) Background angiogenesis in MT-only injected mice. (H) A relative increase in the number of small vessels by injecting MT-containing c-kit+ cells. (G,I) An enhanced number of blood vessels in MT–SDF-1 (G) and MT–SDF-1/c-kit+ cell injected mice. (J) Quantification of the total blood vessel density (expressed as blood vessels/mm2) in MT plugs in all experimental conditions. *P < .05; n = 4, for each mice group. Error bars indicate standard errors.
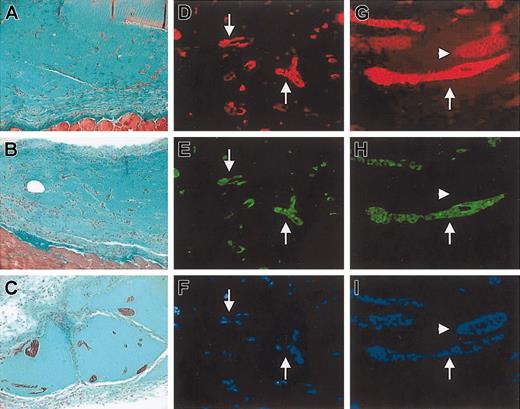
Effect of SDF-1 on formation of tubulelike structures by GFP+/c-kit+ cells in MT plug assay. (A-C) Trichrome-Masson staining of MT plugs containing GFP+/c-kit+ cells (A) and GFP+/c-kit+ cells and 100 ng/mL SDF-1 (B-C). Comparing panel A with panels B-C, it is evident that in SDF-1/c-kit+ cells containing MT the formation of bigger tubulelike structures, which were interconnected by a higher number of branching points, occurred. (D-I) Immunohistochemistry for VWF (D,G), GFP (E,H), and Hoechst staining (F,I) in transverse section of MT plugs containing c-kit+ cells obtained from BM of GFP transgenic mice alone (D-F) or in the presence of 100 ng/mL SDF-1 (G-I). Note that in the presence of stem cells only, sparse and smaller structures were formed (arrows in D-F), while addition of the chemokine induced the formation of more organized cordlike structures that stained positive and negative for GFP (arrows and arrowheads, respectively, in G-I).
Effect of SDF-1 on formation of tubulelike structures by GFP+/c-kit+ cells in MT plug assay. (A-C) Trichrome-Masson staining of MT plugs containing GFP+/c-kit+ cells (A) and GFP+/c-kit+ cells and 100 ng/mL SDF-1 (B-C). Comparing panel A with panels B-C, it is evident that in SDF-1/c-kit+ cells containing MT the formation of bigger tubulelike structures, which were interconnected by a higher number of branching points, occurred. (D-I) Immunohistochemistry for VWF (D,G), GFP (E,H), and Hoechst staining (F,I) in transverse section of MT plugs containing c-kit+ cells obtained from BM of GFP transgenic mice alone (D-F) or in the presence of 100 ng/mL SDF-1 (G-I). Note that in the presence of stem cells only, sparse and smaller structures were formed (arrows in D-F), while addition of the chemokine induced the formation of more organized cordlike structures that stained positive and negative for GFP (arrows and arrowheads, respectively, in G-I).
Discussion
EPC phenotype of BM progenitor cells: stem cell or monocyte cell adhesion–mediated differentiation event?
Our in vitro results show that SDF-1–mediated increase of c-kit+ cell differentiation is due to an enhanced cell adhesion mediated by integrin receptors. Previous studies have reported adhesive and migratory effects of SDF-1 on primitive progenitor cells, leukocytes, and tumor cells mainly due to integrin activation.3,5,21,23,41 Our results showing reversion to basal levels of differentiation by treating cells with DGEA, CS-1, and RGD peptides or blocking antibodies show that SDF-1–enhanced differentiation of c-kit+ cells is likely caused by a similar mechanism. Surprisingly, the basal level of differentiation was not reduced by addition of inhibitors in the absence of SDF-1. This suggests that receptors other than α2, α4, and α2β1 integrins are involved in adhesion and EPC phenotype determination onto FN and Coll I. Several studies reporting EPC differentiation in culture suggest that generation of endothelial cells is a process of “sorting out” of cells clustering from cohorts of immature progenitors.17,27,36 This may reflect heterogeneity in the extent of stem cell plasticity of populations bearing similar surface markers (Sca-1, c-kit, CD34) or may arise from a selection process occurring under activation of intracellular pathways by growth factors and cell adhesion. On the other hand, our results on expression of CD68 marker in cultured c-kit+ cells raise the hypothesis that EPCs derive from an intermediate cell type arising from cultured c-kit+ cells showing monocyte features. This is in agreement with recent reports showing that CD14+ cells in the human PB give rise to Ac-LDL-DiI+ cells bearing an angiogenic potential comparable with that of CD14– after ex vivo expansion28 or that circulating monocyte/macrophages are the main population of cells giving rise to EPCs in PB.29 Interestingly, our analysis of CD68 expression in adhesion-independent cultured cells showed that SDF-1 did not affect the number of cells displaying monocyte features, nor did it induce expression of CD31. Thus, our interpretation of these data is that SDF-1 is not able to induce EPC phenotype in stem cells on its own but it acts downstream of a monocytic default maturation pathway by enhancing adhesion to specific ECM components. It remains to be determined which intracellular activation pathway(s) contributing to EPC phenotype is activated in these cells by SDF-1 and substrate-specific cell adhesion.
Influence of cell cycle in EPC commitment of BM progenitors
Clonal expansion of HSCs is characterized by rapid proliferation accompanied by expression of hematopoietic lineage markers, thereby impairing further clonogenicity. This is in contrast with hematopoietic stem cell self-renewal allowing maintenance of an uncommitted stem cell pool in the bone marrow. To screen for possible cell cycle effects in EPC commitment of BM c-kit+ cells, we assayed EPC differentiation potential under conditions where HSC proliferation is strongly enhanced. To this aim, we cultured c-kit+ cells in the presence of a cytokine cocktail that already proved to be effective in maintaining stem cell activity of human EPCs for endothelial and skeletal muscle in vivo differentiation.18 In vitro culture is generally known to impair in vivo repopulating ability42 and in vitro clonogenicity of human and mouse HSCs (M.P., unpublished observations, 2004). However, under our experimental conditions, maintenance of stem cell markers was tested by analysis of c-kit, Sca-1, CD34, and CXCR4 expression (Figure 6), suggesting that stem cell potential was not dramatically reduced by culture in the presence of mitogenic cytokines. In particular, EPC differentiation potency was not abolished, as we found that washing cells from cytokine-containing medium partially restored the ability to respond to SDF-1 stimulation onto FN and that p16INK4-expressing c-kit+ cells efficiently differentiated into EPCs.
Experiments presented in Figure 2 suggest that a G1 checkpoint is important to promote adhesion-dependent EPC differentiation. Under our experimental conditions, SDF-1 does not appear to act in HSC cell cycle. In fact, no difference was observed between FACS profile of PI-stained SDF-1–treated and untreated c-kit+ cells. Although it is not possible to formally exclude that increased EPC differentiation is due to SDF-1 cell cycle effects on HSC subpopulations, the evidence that CD31 was not up-regulated under identical conditions (“Results”) suggests that the chemokine is not sufficient, per se, to drive EPC phenotype independent of cell adhesion.
Self-renewal versus differentiation implies a complex array of factors having antagonistic effects on cell cycle and regulating maintenance of the uncommitted phenotype versus entry into rapidly expanding stem cell subsets. For example, induction of differentiation in primitive human CD34+ cells by WT-1 causes up-regulation of p21cip1/waf1 and cell cycle arrest.43 In contrast, p21cip1/waf1 is also implicated in maintenance of self-renewal ability of most primitive hematopoietic stem cells and preserves pluripotency and engraftment potential, as loss of p21cip1/waf1 leads to improved stem cell exhaustion under stress conditions.44,45 It has been recently found that EPCs have an extremely low proliferation rate in culture.29 Therefore, to establish a direct link between cell cycle and SDF-1–mediated EPC differentiation, we exploited the ability of p16INK4 protein to induce G1 arrest34 of rapidly cycling cytokine-stimulated c-kit+ cells. Expression of various cyclin–cyclin-dependent kinase (CDK) complex inhibitors such as p27Kip1, p21cip1/waf1, p19ARF, and p16INK4 has been reported to be a hallmark of loss of self-renewal ability and hematopoietic commitment of primitive cells. In particular, it has been proposed that repression of p16INK4 is functional to uncommitted hematopoietic and neuronal stem cell self-renewal in vivo,34,46 while its up-regulation leads to loss of stem cell activity and cellular senescence.34 Our study suggest that, besides loss of self-renewal and induction of senescence, expression of p16INK4 mimics the function of cell cycle regulators establishing a G1 checkpoint that is necessary for EPC differentiation in synergism with adhesion-triggered signals.
SDF-1: a physiologic trigger for ischemia-regulated stem-cell mobilization and differentiation
Ischemia is known to induce mobilization of bone marrow progenitors both in animal models and in humans.2,36,38,47,48 Key molecules involved in mobilization of bone marrow progenitors to the circulation include VEGF,1 placenta growth factor (PlGF),49 SCF,50 G-CSF,10 endothelial nitric oxide synthase (eNOS),51 and several others. SDF-1 has been linked to mobilization of stem cells from the bone marrow. For example, reduction of SDF-1 expression and enhancement of CXCR4 are the mechanisms responsible for G-CSF–induced mobilization of hematopoietic progenitor cells.10 Furthermore, plasma elevation of SDF-1 is involved in BM stem cell mobilization through matrix metalloproteinase-9 (MMP-9)–mediated conversion of kit ligand factor (SCF) from membrane-bound to soluble form.11 Recently, a possible role for SDF-1 in homing of stem cells to damaged sites has been unraveled by studies in animal models of liver, limb, and heart damage.14,52-54 Based on this evidence, we hypothesized that SDF-1 is regulated in limb ischemic tissues and that ischemia-associated hypoxia causes an imbalance between plasma and BM SDF-1 concentration, thereby allowing mobilization of stem cells from the BM. Chemokines (including SDF-1) are well known to act as chemotactic signals for recruitment of inflammatory cells. It is established that SDF-1 gradients promote migration and trans-migration of hematopoietic progenitors.3 In agreement with data obtained in ischemic myocardium,53 our data show that between 6 hours and 1 day after induction of ischemia, a gradient of SDF-1 concentration is established between the PB and the BM. This is likely related to egress of progenitor cells in circulation shortly after induction of hypoxic tissue damage. Additionally, since SDF-1 expression was up-regulated in ischemic tissue at concomitant time points, we surmise that this gradient is generated, at least in part, by overproduction of SDF-1 in hypoxic tissues.54 Even though we do not provide formal demonstration that ischemia-induced up-regulation of SDF-1 in the adductor muscle is responsible for homing of mobilized stem cells, we hypothesize that transient establishment of an SDF-1 gradient favors stem cell translocation into ischemic tissue, thereby enhancing angiogenesis.
To assess EPC differentiation of c-kit+ cells in vivo, we first tested the ability of such cells to participate in blood vessel development following ischemia. According to results by Asahara et al,38 we found that c-kit+ cells from transgenic mice expressing GFP16 were found in newly formed arterioles after induction of ischemia, as assessed by double GFP/α-actin and GFP/VWF immunostaining (Figure 11). We then performed matrigel (MT) plug assays by injecting subcutaneously growth factor–free MT containing SDF-1 and/or c-kit+ cells. In this system, the amount of blood vessels generated in MT–SDF-1 and MT–SDF-1/c-kit plugs was significantly higher compared with the MT-only or MT–c-kit plugs. The evidence that c-kit+ cells alone increase the blood vessel density confirms previous experimental55 and clinical56 evidence that stem cells, per se, may enhance angiogenesis by releasing proangiogenic molecules. Quantification of the blood vessel density revealed, however, a slightly but not significantly higher angiogenic effect by coinjecting SDF-1 and stem cells compared with SDF-1 alone. A possible explanation of this effect is that SDF-1 has a potent angiogenic effect57,58 besides acting through recruitment of recipient's EPCs or invasion of MT plug by recipient's endothelial cells,14 thus masking direct differentiation effects on coinjected stem cells.
Injection of MT plugs containing bone marrow– or peripheral blood–derived stem cells is a feasible way to test for stem cell contribution to vascular structures in vivo.39,40,59 We used this approach to test differentiation of GFP+/c-kit+ cells in the presence or the absence of SDF-1. In this assay, we found that dimension of tubularlike structures was higher in the presence of SDF-1, while smaller tubules and more sparse cells were observed in MT containing c-kit+ cells only (Figure 12). These results suggest that SDF-1 might act in vivo by enhancing the formation of blood vessels by recruited progenitor cells.
Concluding remarks
Several investigations have pointed out that tissue damage (and likely inflammation linked to tissue damage) is a unique way to promote reallocation of stem cell pools from stores in the BM to exogenous tissues. This is also possibly implicated in induction of phenotypical reprogramming of stem cells, or “stem cell plasticity.”60 Our findings provide a mechanism linking tissue damage to recruitment and differentiation of endogenous stem cells from the bone marrow. Being intimately linked to inflammatory responses, however, the extent and the relevance of the SDF-1/CXCR4 axis for tissue regeneration versus scar formation still needs careful evaluation. Specifically, experiments are needed to address the question as to whether SDF-1 gene transfer leads to enhanced regeneration or an exacerbated inflammatory response in nonimmunosuppressed environments such as animal models of ischemia and tissue damage used so far,14,52 and thus may be of clinical relevance. Studies are now under way to analyze possible interplays between SDF-1 and inflammatory mediators in regulating and enhancing stem cell–mediated regeneration versus scar formation in ischemic diseases.
Prepublished online as Blood First Edition Paper, July 29, 2004; DOI 10.1182/blood-2003-12-4423.
Supported by the Italian Ministry of Health, Program Grant “Ricerca Finalizzata Strategica,” “Proliferazione e Transdifferenziamento di cellule Staminali in Terapia Cellulare,” (M.P. and M.C.C.; contract no. 186); Ricerca Corrente, Centro Cardiologico Monzino, IRCCS, Milan, Italy; and Research Program “Biochimica Clinica Sperimentale,” “Analisi dei meccanismi di reclutamento e transdifferenziamento di cellule staminali ematopoietiche in patologie ischemiche” (M.P).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors acknowledge Dr Fabio Martelli at IDI, Laboratorio di Patologia Vascolare, for kindly providing p16INK4 retrovirus. Members of our laboratory are acknowledged for helpful discussions during the preparation of this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal