Abstract
As part of our evaluation of Flu as immunosuppressive (and DNA damage repair inhibiting) agent, and as an alternative to Cy in combination with IV Bu, preparing AML/MDS patients for allogeneic transplant (AlloSCT), we postulated that the IV Bu-Flu regimen would yield improved safety, yet increased antileukemic efficacy. We now compared two consecutive series of patients receiving pretransplant conditioning therapy with either IV Bu-Flu or IV BuCy2.
Methods: Flu 40 mg/m2 IV was given once daily, each dose followed immediately by Bu 130 mg/m2 IV over 3 hr (days −6 till −3) (n=128 patients), and in the IV BuCy2 the Bu was given at 0.8 mg/kg over 2 hr every 6 hr for 16 doses (days −7 till −4), followed by Cy 60 mg/kg on days −3 and −2 (n=73 patients). Patients with an unrelated (MUD) or mismatched donor received ATG on days −3 to −1. Graft-versus-host disease (GVHD) prophylaxis was based on tacrolimus and mini-methotrexate.
Patients: Median age was 46 (19–66) (IV Bu-Flu group) and 39 (13–64) (IV BuCy2 group) years, respectively. At transplant 45% and 46% patients were in CR1–3, the remainder had active disease. Cytogenetic risk categories were equivalent for the two groups, with poor or intermediate risk in 88 % in the IV Bu-Flu, and in 82 % of the BuCy2group. Donors were HLA-identical siblings (SIBS) in 53 %, and 73%, MUDs in 39% and 15 %, others in 8 % and 12% respectively. Median follow-up for patients still alive (August 2004) was 15 mos (3–37; n=78) for the IV Bu-Flu group, and 45 mos (24–76; n=27)) for the IV BuCy2 patients.
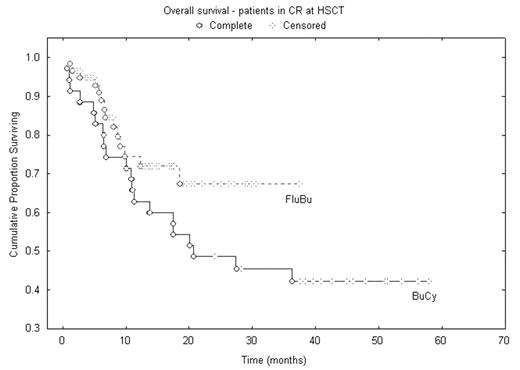
Results: Both regimens were well tolerated; 100-day treatment-related mortality (TRM) was for SIB/MUD 2.7 / 2% after IV Bu-Flu, and 7 / 9% in the IV BuCy2 group, respectively. Overall, one-year TRM was for 7% after IV Bu-Flu and 18% after IV BuCy2. The overall aGvHD rates were 42% and 45%. 2-year overall survival (OS) were 50% for the IV Bu-Flu group, 42% after IV BuCy2. For patients transplanted in CR the 2-yr OS and event-free survival were 69% and 68 % after IV Bu-Flu, and 48% and 41% in the IV BuCy2.
We conclude, that IV Bu-Flu is a safe and highly effective, reduced toxicity myeloablative conditioning regimen for AML/MDS. Its antileukemic efficacy appears to be at least equal to what is experienced with the IV BuCy2 regimen. Thus, our data demonstrate the IV Bu-Flu regimen to be a new standard for myeloablative conditioning therapy for alloSCT in AML/MDS.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal