Abstract
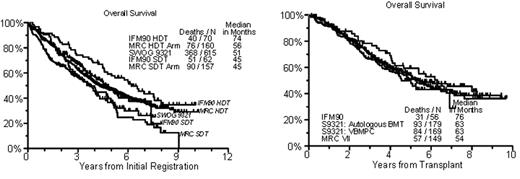
ECOG, CALGB and SWOG (coordinating group) enrolled 899 patients with newly diagnosed MM to receive VAD x 4 (813 were considered eligible), followed by randomization to PBSC-supported MEL-TBI (n=261) versus VBMCP (n=255), using CTX 4.5g/m2 + G-CSF for PBSC mobilization in all patients. Responders to VBMCP or MEL-TBI were randomized to IFN (n=121) or no maintenance (n=121). With a median follow-up of alive patients of 60 mos, median EFS and OS from 1st randomization were 25 and 62 mos for MEL-TBI and 21 and 53 mos for VBMCP (p=.14 for EFS, p=.87 for OS). EFS and OS from IFN randomization were also identical at 23/59 mos for IFN versus 18/74 mos for observation (p=.2/.6). 87 VBMCP failures received salvage autotransplants, with a post-relapse median survival of 24 mos which was similar to the 27 mos observed among the remaining 83 VBMCP failures managed with standard salvage therapies. When examined in the context of 2 other randomized trials (IFM 90, MRC VII), OS from baseline of S932, limited to patients <60 yr (as in IFM 90 + MRC VII), is intermediate between the high dose arms of IFM 90/MRC VII and the standard dose arms of these two trials (randomization at baseline) (Figure 1). OS from the time of randomization to high or standard dose therapy on S9321 (again restricted to age <60 yr) was similar to OS timed from transplant for the high dose arms of IFM 90 and MRC VII. (Figure 2).
The comparable outcome after high- versus standard-dose therapies on S9321, interpreted in the context of 2 other published randomized clinical trials, may relate to (1) higher dose intensity with VBMCP than with other standard regimens or (2) a detrimental effect of TBI in S9321. We conclude that with the current follow-up MEL-TBI is no better than VAD/VBMCP with the option of salvage transplant and that IFN maintenance is of no benefit in this maintenance setting. Data from this trial will be provided to MRC to be included in their on-going meta-analyses of high-dose therapies and IFN maintenance.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal