Abstract
Graft failure (GF), either primary or after transient engraftment, following matched, related allogeneic hematopoetic stem cell transplantation (HSCT) without T-cell depletion is usually less than 3% for hematological diseases. Graft failure using non-T-cell depleted unrelated donors in chronic myelogenous leukemia (CML) as reported by the national marrow registry is approximately 9.7%. For aplastic anemia, the incidence of graft failure is estimated to be 10%. In this report, we present 3 patients (Pts) with GF following unrelated ablative allogeneic HSCT (two with peripheral blood stem cells and one with bone marrow). Pt. 1 had transient engraftment and Pts 2 & 3 had primary GF. Their relevant demographics are as follows:
TABLE 1
| . | Disease . | Unrelated Donor . | Age/Sex . | GVHD: BMT1 . | GVHD: BMT2 . | PTLD . |
|---|---|---|---|---|---|---|
| Pt1 | CML, 1st Chronic Phase | Matched | 29/F | None | Grade II | Yes |
| Pt2 | MDS | Matched | 57/M | None | None | No |
| Pt3 | MDS/AML | 1ag Mismatched | 55/M | None | None | No |
| . | Disease . | Unrelated Donor . | Age/Sex . | GVHD: BMT1 . | GVHD: BMT2 . | PTLD . |
|---|---|---|---|---|---|---|
| Pt1 | CML, 1st Chronic Phase | Matched | 29/F | None | Grade II | Yes |
| Pt2 | MDS | Matched | 57/M | None | None | No |
| Pt3 | MDS/AML | 1ag Mismatched | 55/M | None | None | No |
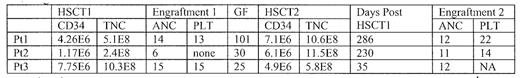
All patients were initially conditioned with alemtuzumab (A) 30mg/day on days −8 to −6, fludarabine (F) 30mg/m2/day on days −7 to −3, and busulfan .8mg/kg x 16 doses (two patients) or x 8 doses (one patient) on days −5 to −2. Conditioning for HSCT #2 included cyclophosphamide 50mg/kg/day, Mesna 50mg/kg/day, and thymoglobulin 2.5mg/kg/day on days −5 to −2. Graft versus host disease (GVHD) prophylaxis included tacrolimus for both HSCT transplants, and low dose methotrexate (3 doses) combined with prednisone (2mg/kg) on days 0–18 followed by a two week taper for HSCT #2. The stem cell product and engraftment kinetics are shown below.
Significant toxicities after HSCT#2 have included successful resolution of PTLD in patient #1 (who also experienced infection with pulmonary respiratory syncitial virus, BK viruria, and fatty infiltration of the liver). BK viruria was noted in patient #2, as well as hypertension and a modest pericardial effusion with global hypokinesis (but with a normal ejection fraction). Hepatomegaly was documented, but no vaso-occlusive disease was established. Patient #3 developed a cardiac arrhythmia, hypertension, and BK viruria and remains Plt transfusion dependent. Patient #1 had grade II GVHD of the skin and gut, but patients 2 and 3 had none. GVHD has been <grade II. GF occurred in 3/17 Pts with MUD transplants that included alemtuzamab in the conditioning regimen. While GF is unacceptably high, it is encouraging that all 3 Pts. successfully completed 2nd MUD transplant with transfusion and cytokine independence using same donor preceded in Pts. 1 & 2 by depletion of anti-ABO incompatible antibodies in the recipient.[table2]
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal