Abstract
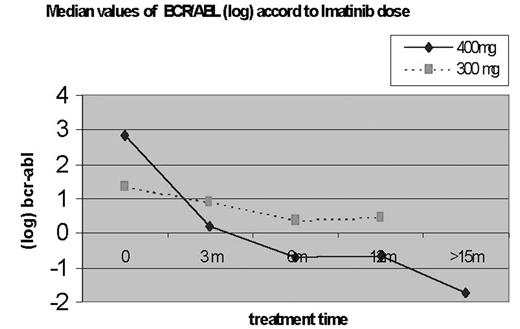
Imatinib, a selective inhibitor of tyrosin-kinasa BCR/ABL fusion protein, produces high response rates in patients with chronic myeloid leukemia (CML) in the chronic phase. The behavior of the levels of the BCR/ABL transcript by qPCR technique was studied in 24 patients (13M/11F; median age: 49.6 years, range: 23–73), diagnosed of CML in chronic phase, before and after the treatment with imatinib (at the 0, 3, 7, 11 and 15 months). Eight patients had previously received interferon alpha or busulphan and 16 hidroxiurea. The amplification of BCR/ABL with the qPCR technique, according to protocol BIOMED-2, was carried out in samples of bone marrow (n = 23) and peripheral blood (n = 48). Results were calculated in relation to a control gene of the glucuronidasa (GUS) and expressed in logarithmic scale (log10). Median time from the diagnosis was of 28 months and median treatment time with imatinib was 14 months. The median reduction of BCR/ABL transcripts was 1,98 log after three months and decreased further (2,71 log) during follow-up. Both of them were significative when compare with base line levels (P <0.001). The reduction was greater in patients who received a dose ³ 400 mg/day (n=16) than those with 300 mg/day (n=8). Three patients reached a major molecular response (ratio BCR-ABL/GUS <0.005) up to 15 months of treatment. In conclusion: the determination of the BCR/ABL transcripts by qPCR contributes with valuable information about the effect of imatinib in patients with CML. The logarithmic reduction is higher in the beginning of the treatment and doses ³ 400 mg/day are associated with a greater reduction of the tumoral load.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal