Abstract
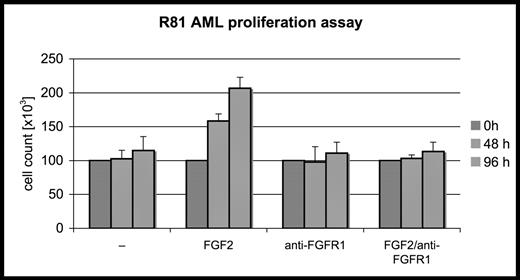
Fibroblast growth factor receptors (FGFRs) are tyrosine kinase receptors affecting cell proliferation, motility and survival. Fibroblast growth factor 2 (FGF2, basic FGF) represents the prototype FGFR ligand. Expression of FGFRs has been demonstrated in a subset of acute myeloid leukemias (AMLs) and FGF2 is overexpressed in the bone marrow of AML patients. The role of FGF/FGFR signaling in AML however is controversial, and downstream signaling cascades activated by FGFR1 are not known. Therefore, we hypothesized that subsets of primary acute leukemic cells may express functional FGFR1 mediating survival and proliferation of leukemic cells. We examined the role of the FGF/FGFR axis in primary AML blasts isolated from a 74 year old male (designated R81). Using RT-PCR we showed that FRFR1 was expressed in leukemic cells. To assess whether FGF2 stimulates FGFR1 signaling, R81 blasts were incubated in serum free medium with and without 20 ng/ml of FGF2 and/or a with 10 μg/ml of neutralizing monoclonal antibody to FGFR1. Viable cells were counted at 48 and 96 hours with trypan blue exclusion (see figure). The results show that FGF2 stimulates growth and/or survival of R81 AML blasts and this effect is abrogated by FGFR1 antibodies. Specifically, FGF2 induced a two-fold increase in the proliferation of leukemic blasts after 48 hours. To identify downstream signaling pathways involved in FGFR1 signaling, proliferation and survival of FGF2-treated R81 cells were examined in the presence or absence of selective PI3 kinase and MAP kinase inhibitors, LY-294002 and PD098059, respectively. The results indicated that FGF2 signaling exerts its proliferative and pro-survival effects primarily through PI3 kinase activation. In summary, we show that FGF/FGFR1 signaling plays a role in a subset of AMLs and may be blocked by a FGFR1 specific antibody. Currently, we are further elucidating the mechanisms involved in FGFR signaling and its effects on cell proliferation, migration, and apoptosis. In addition, animal experiments are underway to assess a possible therapeutic role of anti-FGFR antibodies for the treatment of FGFR-positive AML in murine xenotransplant models.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal