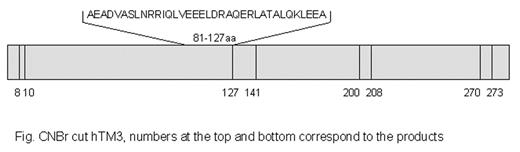
We previously reported that two chain human high molecular weight kininogen (HKa) inhibited angiogenesis by selectively inducing apoptosis of proliferating endothelial cells (Zhang et al. FASEB J., 2000). This activity appears to depend upon binding of HKa to endothelial cell tropomyosin, as it was completely inhibited by anti-tropomyosin monoclonal antibody (mAb) TM-311. mAb TM-311 also blocked the high-affinity Zn2+-dependent binding of HKa to both purified tropomyosin (TM) and proliferating endothelial cells (Zhang et al., PNAS 2003). However, endothelial cells express several different isoforms of TM, and the isoform(s) expressed on the endothelial cell surface, and the regions within it that bind HKa are unknown. To identify which isoform of TM is expressed on the surface of endothelial cells, human umbilical vein endothelial cells (HUVEC) were briefly exposed to a buffer containing 0.05 M glycine, 0.1 M NaCl, pH 3.0, and the resultant eluate was probed with isoform specific anti-tropomyosin antibodies that recognize TMs 1–5. Only TM5 was detected, with approximately 2.5-fold more TM5 eluted from proliferating versus confluent cells. Northern blot analysis also showed that the expression of TM5 mRNA was higher in the proliferating versus confluent cells. To locate the binding site for HKa in human TM, hTM3 was digested by CNBr, producing three fragments containing amino acids 10–127, 141–200 and 208–270. These were incubated with HKa and the mixture then passed through a Superose 6 gel filtration column. Analysis of the first peak that eluted from the column by tricine gel electrophoresis revealed two bands, the smaller of which contained the N-terminal CNBr-derived TM fragment (aa 10–127), and the larger of which contained HKa. Taken together with our previous observation that HKa binds with similar affinity to TM isoforms 1–5, suggesting that it binds to a homologous region among these proteins, these findings suggest that the binding site for HKa resides within the homologous region within the N-terminal TM fragment, and is likely contained within amino acids 81 to127aa of TM3 (Figure). Since these results suggest that at least hTM5 is non-covalently associated with the endothelial cell surface, we have begun to explore the nature of this interaction. Endothelial cell surface proteins were cross linked using the membrane-impermeable cross-linker, BS3, and cell extracts were then immunoblotted using the anti-hTM5 antibody LC-1. These result revealed native hTM5 (~30 kD), as well as a new band of ~60 kD, suggesting an association of hTM5 with a cell surface protein of approximately equal size. In conclusion, our results suggest that the anti-endothelial cell activity of HKa is mediated through binding to cell surface hTM5, via a homologous region of this protein shared with other non-muscle tropomyosins.
Skip Nav Destination
Abstracts Not Selected for Presentation|
November 16, 2004
Characterization of the Interaction between Cleaved High Molecular Weight Kininogen (HKa) and Tropomyosin (TM) on Endothelial Cells. Free
Xiaoping Qi, M.D., Ph.D.,
Xiaoping Qi, M.D., Ph.D.
1Medicine, Case Western Reserve University School of Medicine, Cleveland, OH, USA.
Search for other works by this author on:
Keith R. McCrae, M.D.
Keith R. McCrae, M.D.
1Medicine, Case Western Reserve University School of Medicine, Cleveland, OH, USA.
Search for other works by this author on:
Blood (2004) 104 (11): 3918.
Citation
Xiaoping Qi, Keith R. McCrae; Characterization of the Interaction between Cleaved High Molecular Weight Kininogen (HKa) and Tropomyosin (TM) on Endothelial Cells.. Blood 2004; 104 (11): 3918. doi: https://doi.org/10.1182/blood.V104.11.3918.3918
Download citation file:
November 16 2004
Advertisement intended for health care professionals
Cited By
Advertisement intended for health care professionals

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal