Abstract
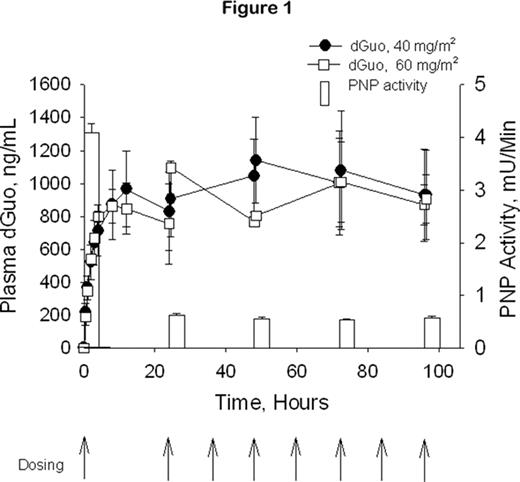
Forodesine is a potent, specific transition-state analog inhibitor of PNP with clinical activity in T-cell malignancies. Pharmacodynamic studies support that this anti-leukemic effect is mediated through the accumulation of plasma 2′-deoxyguanosine (dGuo) and intracellular Dgtp. In vitro studies indicated that B-cell acute lymphoblastic leukemia (B-ALL) cells can also accumulate Dgtp. These preclinical data led to a phase I/II multicenter dose-escalation study to evaluate forodesine in pts with various hematologic malignancies. 15 pts were treated with forodesine, including 6 with B-ALL. Forodesine was given via a 30-min IV infusion followed 24 hrs later by doses every 12 hrs for a total of 9 doses. A second course could be repeated after a 2-week break. Doses were escalated by 50% to pts grouped in cohorts of 3 (starting dose: 40 mg/m2). There was a rapid rise in plasma dGuo and a maximum PNP inhibition was achieved at 40 mg/m2 (Fig 1). 7/15 treated pts (2 T-cell malignancies and 5 B-ALL), including 5/6 treated B-ALL pts, demonstrated a hematologic benefit, defined as a decrease in tumor burden. 3 of the responding B-ALL pts were further treated with 6 courses of forodesine on a compassionate use protocol. One pt treated at 135 mg/m2 demonstrated a complete response with a decrease in bone marrow blast cells from 22% to 5% at the end of therapy. The other 2 pts, treated with 90 mg/m2 and 135 mg/m2 forodesine respectively, showed dramatic improvement in their CBC despite the persistence of bone marrow blasts. The dramatic fall in WBC count was accompanied in each pt by a rise in plasma dGuo (Cmax: 1.9–7.1 μM) and intracellular Dgtp (380–800 pmoles/107 cells vs 25–40 pmoles/107 cells pre-treatment), The response of the pt treated with 90 mg/m2 forodesine is shown in Fig 2. The absolute neutrophil count (ANC) improved from ~0 at pre-treatment to 1800 cells/mm3 at Day 18.
To-date forodesine has been safe and well-tolerated at all dose levels tested with no dose-limiting toxicities. Clinical activity has been seen in T- and B-ALL. It is interesting to note the restoration of normal hematopoiesis, indicating the specificity of forodesine for leukemic cell populations and supporting that forodesine represents an important breakthrough in the development of less toxic ALL therapy. This encouraging clinical activity has led to the initiation of a phase II trial in pts with B-ALL. Additional clinical and pharmacodynamic results will be presented.
Author notes
Corresponding author


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal