Abstract
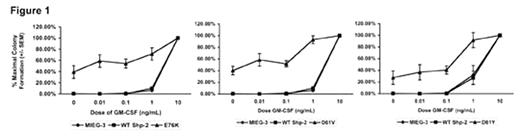
The childhood leukemia, juvenile myelomonocytic leukemia (JMML), is a lethal disease of young children characterized by spontaneous growth of peripheral blood hematopoietic progenitors and hypersensitivity of hematopoietic progenitors to the cytokine GM-CSF. Notably, hematopoietic progenitors from JMML patients do not typically demonstrate hypersensitivity to the cytokine IL-3. Mutations in the RAS and NF1 genes have long been recognized as pathogenic in this disease, and more recently, mutations in the PTPN11 gene, which encodes Shp-2, a non-receptor protein tyrosine phosphatase, have also been found commonly in leukemic cells from children with JMML. We hypothesized that the mutant Shp-2 molecules observed in JMML patients would induce hypersensitivity of hematopoietic progenitors to GM-CSF. To examine this hypothesis, we subcloned the WT Shp-2 cDNA and three mutant Shp-2 cDNAs found in leukemic cells from children with JMML (E76K, D61V, and D61Y), into the murine stem cell retroviral plasmid pMIEG3 in tandem with EGFP. Each vector was sequenced to verify the desired point mutation and to rule-out unwanted mutations. Murine bone marrow low density mononuclear cells were subjected to fibronectin-assisted retroviral transduction and sorted for EGFP positive cells using fluorescence activated cell sorting. EGFP positive cells were plated into progenitor assays with increasing concentrations of GM-CSF (0, 0.01 0.1, 1, and 10 ng/mL). As predicted, transduction with each of the Shp-2 mutations induced hypersensitivity to GM-CSF compared to vector alone or to WT Shp-2, as evidenced by significantly higher % maximal colony formation at each GM-CSF dose tested (Figure 1).
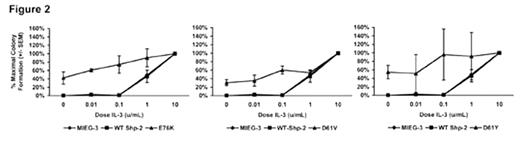
We next conducted progenitor assays using EGFP positive cells with increasing concentrations of IL-3 (0. 0.01, 0.1, 1, and 10 U/mL). Although hematopoietic progenitors from JMML patients have not been described to be hypersensitive to IL-3, surprisingly, we have observed in preliminary studies that introduction of each of the Shp-2 mutations induced hypersensitivity to IL-3 in a similar fashion to that observed with GM-CSF (Figure 2).
These data demonstrate that somatic PTPN11 mutations observed in children with JMML induce hematopoietic cell growth aberrancies in a manner overlapping with the classical description of JMML, yet also induce unique hematopoietic cell growth characteristics based on the observed hypersensitivity to IL-3. These findings suggest that signaling pathways in addition to the Ras-MAPK cascade may be dysregulated in PTPN11 mutation-bearing hematopoietic cells and provide a novel model for the investigation of new therapeutics in JMML. Current studies are ongoing to examine the effect of these mutations on in vivo hematopoiesis and leukemogenesis.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal