Abstract
The safety and feasibility of adoptive immunotherapy using ex vivo-expanded differentiated human effector T cells that express tumor-specific chimeric receptors are being evaluated in clinical trials. Typically, these T cells are CCR7neg and bear a T-cell receptor of unknown specificity. To improve the therapeutic potential of genetically engineered T cells in general, and CD19-specific T cells in particular, strategies are needed to improve their ability traffic to sites of residual/macroscopic disease where infused T cells can be specifically activated for proliferation, cytokine secretion, and tumor-lysis. To accomplish these goals we have generated a selection process that uses genetically modified T cells, expressing influenza A matrix protein 1 (MP1) or CMV pp65, to act as antigen presenting cells (T-APC) in order to expand autologous viral-specific T cells in vitro and in vivo. The viral-specific effector T cells can then be genetically modified with a CD19-specific chimeric immunoreceptor (CD19R), which recognizes CD19 on malignant B cells, independent of MHC. By using these viral-specific T cells as a platform for the introduction of CD19R, we now demonstrate that bi-specific T cells express the chemokine receptor CCR7, which is implicated in the trafficking of T cells to lymph nodes. We demonstrate that this chemokine receptor functions to directionally chemotax the genetically modified bi-specific T-cells along concentration gradients of CCL19 or CCL21. We further demonstrate that both the endogenous and introduced chimeric immunoreceptor continue to function in CCR7+ bi-specific T cells. Indeed, the bi-specific T cells are capable of augmented cytokine production and proliferation upon docking with both CD19 and MP1 antigens, compared with these same T cells interacting with either CD19 or MP1 alone. This enhanced activation is an explanation for the enhanced in vivo anti-tumor activity demonstrated by bi-specific T-cells when stimulated with MP1+ T-APC in treating CD19+ lymphoma in NOD/scid mice. An advantage of this methodology is that the CCR7+ bi-specific T cells and T-APC can be genetically modified and expanded in compliance with current good manufacturing practice (cGMP) for 2nd generation Phase I/II clinical trials to test their ability to traffic to sites of lymphoma providing potent regional/local T-cell activation.
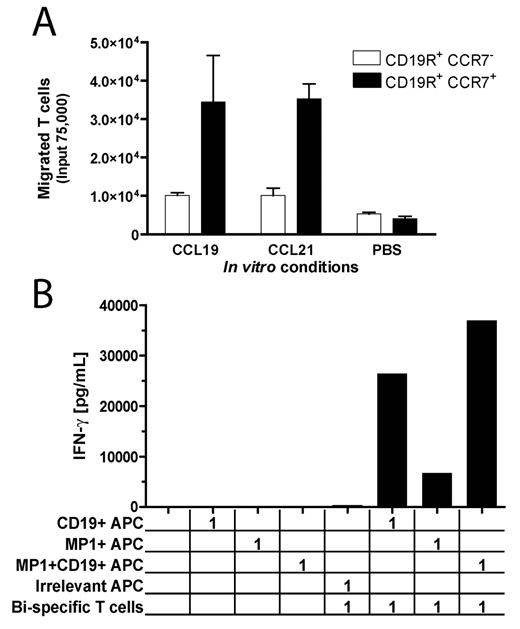
Legend: (A) CCR7+ viral- and CD19-bi-specific T cells migrate along recombinant CCL19 and CCL21 concentration gradients, whereas CCR7neg CD19-specific T cells do not. (B) Stimulation of both introduced chimeric immunoreceptor and endogenous T-cell receptor on CD19- and MP1- bi-specific T-cells, using artificial APC, results in augmented cytokine production.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal