Abstract
Introduction: Aggressive MCL has a poor prognosis with a 21-40% complete remission (CR) after CHOP and a duration of response of only 10-16 months. More intense therapy could improve these statistics. Rituximab is effective in MCL and has minimal toxicity.
Methods: A prospective phase II trial of R-HCVAD (considered to be one cycle) alternating every 21 days with R- M/A (considered to be another cycle) as described earlier (Ann Oncol. 13, suppl 2, 2002 #24). Prophylaxis with mesna, calcium leucovorin, prednisone eyedrops, G-CSF, antibacterial, antifungal, and antiviral therapy. CBC with differential and platelet counts X 2-3/week. Re-staging every 2 cycles including upper and lower endoscopies. Patients in complete remission (CR) after 6 courses of a planned 6-8 cycles were not offered consolidation with stem cell transplant. Post-treatment evaluation was performed every 3 months for 1 yr, every 4 months for 2 yrs, every 6 months for 2 years, then annually.
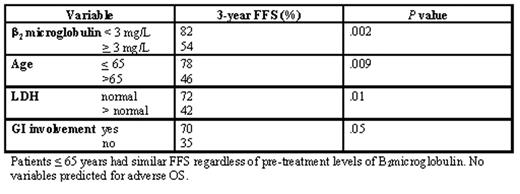
Results: Of 100 patients registered, one was ineligible and two decided to not receive the treatment after registration, leaving 97 evaluable for analysis of response, survival and toxicity. An analysis of response after the first 6 cycles shows an 87% CR/CRu rate. With a median follow up of 40 months, the 3-year FFS and overall survival (OS) were 67% and 81%, respectively. Adverse factors for FFS were:
Grade 4 hematologic toxicity was significant. Five patients died during treatment of sepsis (3), pulmonary hemorrhage (1), and unknown cause (1). Four patients developed myelodysplasia/acute myelogenous leukemia after treatment and while in CR and three have died, for a total of 8 deaths in the study (8%).
Conclusion: R-HCVAD alternating with R-M/A without stem cell transplant is an effective regimen for treatment of aggressive untreated MCL, specially for patients ≤ 65 years old. Toxicity is as expected for an intense regimen. This encouraging data warrants continued follow-up and comparison with existing/new therapies in future trials.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal