Suicide gene transfer into donor T lymphocytes is a promising strategy to avoid severe graft-versus-host disease (GvHD) after allogeneic stem cell transplantation (SCT).1,2 We conducted a phase 1/2 study3 combining infusion of CD34-enriched peripheral blood stem cells (PBSCs) with suicide gene-modified (SGM) donor T lymphocytes. Before our study was put on hold (after the leukemia cases in France4 ), 3 patients had been treated (Table 1).
Patients' characteristics and important clinical data
. | Case 1 . | Case 2 . | Case 3 . |
|---|---|---|---|
| Age, y | 53 | 22 | 58 |
| Sex, patient/donor | Female/female | Male/male | Male/female |
| Diagnosis | CML | CML | MDS |
| Conditioning, mg/kg BW | |||
| Busulfan | 14 | 14 | 12 |
| Cyclophosphamide | 120 | 120 | 120 |
| VP16 | — | — | 30 |
| PBSCT type | Matched related donor | Matched related donor | Matched related donor |
| Mismatches | 1: HLA-A | No | No |
| CD34+ dose/kg | 4.22 × 106 | 4.6 × 106 | 4.3 × 106 |
| Contaminating CD3+ cells | 2 × 103/kg | 1 × 104/kg | 3.6 × 104/kg |
| SGM T cells, CD3+/kg | 4.8 × 106 | 5.46 × 106 | 4.9 × 106 |
| Second infusion (d) | 4.8 × 106 (65) | 5.46 × 106 (58) | No* |
| GvHD prophylaxis | Cyclosporin A | Cyclosporin A | Cyclosporin A |
| Beginning d | −1 | −1 | −1 |
| GvHD (overall grade/d) | Yes (1/23) | No | Yes (11/14) |
| GCV treatment (d) | No | No | Yes (17-26) |
| Outcome | Resolved spontaneously | NA | Resolution |
| Engraftment (chimerism) | Yes (full) | Yes (full) | Yes (full) |
| Neutrophils, d | 11 | 12 | 13 |
| Platelets, d | 10 | 13 | 16 |
| SGM T cells in vivo | Early loss (d 23) after 2nd infusion; only present for 2 d | Present for more than 3 mo | Loss after GCV treatment (d 25) |
| Special remarks | HSV infection during transplantation | Increasing bcr/abl levels at ≈ 1 y | — |
| Clinical outcome | Secondary graft failure (d 156); 2nd allo-PBSCT; severe complications; patient died | DLI for MRD (1 × 107 kg) at 1 y 3 m, alive/well | Secondary graft failure (d 119); 2nd allo-PBSCT; severe complications; patient died |
. | Case 1 . | Case 2 . | Case 3 . |
|---|---|---|---|
| Age, y | 53 | 22 | 58 |
| Sex, patient/donor | Female/female | Male/male | Male/female |
| Diagnosis | CML | CML | MDS |
| Conditioning, mg/kg BW | |||
| Busulfan | 14 | 14 | 12 |
| Cyclophosphamide | 120 | 120 | 120 |
| VP16 | — | — | 30 |
| PBSCT type | Matched related donor | Matched related donor | Matched related donor |
| Mismatches | 1: HLA-A | No | No |
| CD34+ dose/kg | 4.22 × 106 | 4.6 × 106 | 4.3 × 106 |
| Contaminating CD3+ cells | 2 × 103/kg | 1 × 104/kg | 3.6 × 104/kg |
| SGM T cells, CD3+/kg | 4.8 × 106 | 5.46 × 106 | 4.9 × 106 |
| Second infusion (d) | 4.8 × 106 (65) | 5.46 × 106 (58) | No* |
| GvHD prophylaxis | Cyclosporin A | Cyclosporin A | Cyclosporin A |
| Beginning d | −1 | −1 | −1 |
| GvHD (overall grade/d) | Yes (1/23) | No | Yes (11/14) |
| GCV treatment (d) | No | No | Yes (17-26) |
| Outcome | Resolved spontaneously | NA | Resolution |
| Engraftment (chimerism) | Yes (full) | Yes (full) | Yes (full) |
| Neutrophils, d | 11 | 12 | 13 |
| Platelets, d | 10 | 13 | 16 |
| SGM T cells in vivo | Early loss (d 23) after 2nd infusion; only present for 2 d | Present for more than 3 mo | Loss after GCV treatment (d 25) |
| Special remarks | HSV infection during transplantation | Increasing bcr/abl levels at ≈ 1 y | — |
| Clinical outcome | Secondary graft failure (d 156); 2nd allo-PBSCT; severe complications; patient died | DLI for MRD (1 × 107 kg) at 1 y 3 m, alive/well | Secondary graft failure (d 119); 2nd allo-PBSCT; severe complications; patient died |
CML indicates chronic myelogenous leukemia; MDS, myelodysplastic syndrome; PBSCT, peripheral blood stem cell transplantation; GCV, ganciclovir; NA, not applicable; DLI, donor leukocyte infusion; MRD, minimal residual disease.
The protocol allowed a second infusion of 1 to 5 × 106/kg SGM donor T cells on day 60 only if no GvHD higher than grade 1 had developed.
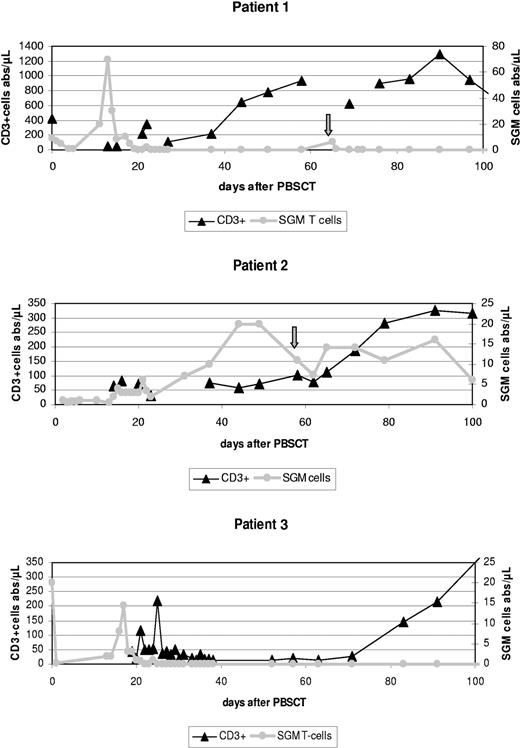
T lymphocytes were transduced5 with the retroviral vector Mo3TIN,6 selected with G418,2 cryopreserved, and safety-tested in a good manufacturing practice (GMP) facility (European Institute for Research and Development of Transplantation Strategies [EUFETS]). CD34 cells were enriched using the Miltenyi Clini-MACS (Miltenyi, Bergisch Gladbach, Germany) (Table 1). Following myeloablative conditioning, each patient received more than 4 × 106 CD34+/kg and approximately 5 × 106/kg body weight (BW) SGM donor T lymphocytes. The latter were immediately detectable in peripheral blood (PB) by quantitative polymerase chain reaction5 (Table 1; Figure 1). Transplantation was well tolerated without acute toxicity; all patients engrafted quickly (Table 1).
Absolute CD3+ T-cell counts (including CD3+CD56+) and numbers of suicide gene-modified (SGM) donor T lymphocytes during the first 100 days after PBSCT in all 3 patients treated according to our protocol. Patient 2 showed stable numbers of SGM cells for about 3 months accompanied by increasing absolute CD3+ counts and full donor chimerism. In contrast, in both patients 1 and 3 early in vivo depletion of SGM donor T lymphocytes was observed, mediated by ganciclovir applied to treat an acute GvHD grade II (patient 3) or most probably as the result of an anti-HSV-thymidine kinase (tk) immune reaction (patient 1). Both patients appeared to have higher absolute CD3+ counts on day 100 compared with patient 2, but developed mixed chimerism (not shown) and eventually rejected their grafts at days 156 and 119. Arrows indicate a second donor SGM T lymphocyte infusion in patient 1 (day 65) and patient 2 (day 58). Note that different Y-axes should be applied to CD3+ and SGM cells.
Absolute CD3+ T-cell counts (including CD3+CD56+) and numbers of suicide gene-modified (SGM) donor T lymphocytes during the first 100 days after PBSCT in all 3 patients treated according to our protocol. Patient 2 showed stable numbers of SGM cells for about 3 months accompanied by increasing absolute CD3+ counts and full donor chimerism. In contrast, in both patients 1 and 3 early in vivo depletion of SGM donor T lymphocytes was observed, mediated by ganciclovir applied to treat an acute GvHD grade II (patient 3) or most probably as the result of an anti-HSV-thymidine kinase (tk) immune reaction (patient 1). Both patients appeared to have higher absolute CD3+ counts on day 100 compared with patient 2, but developed mixed chimerism (not shown) and eventually rejected their grafts at days 156 and 119. Arrows indicate a second donor SGM T lymphocyte infusion in patient 1 (day 65) and patient 2 (day 58). Note that different Y-axes should be applied to CD3+ and SGM cells.
Patient 2, with stable numbers of SGM T cells for more than 3 months, is alive and well more than 2.5 years after transplantation (Table 1).
Patient 1 early on showed a strong increase of SGM cell counts (Figure 1), without GvHD. Thereafter, PB was cleared of SGM T lymphocytes within a few days. This patient received a second dose of SGM T cells (day 65), which vanished within 2 days (Figure 1), strongly indicative of their immune rejection. Indeed, MLR7 data (not shown) confirmed anti-SGM reactivity of this patient's peripheral blood lymphocytes, possibly related to a herpes simplex virus (HSV) reactivation during transplantation.
Patient 3 showed a similar early sharp rise in SGM T cells (Figure 1) associated with acute skin GvHD grade II. Treatment with ganciclovir led to complete resolution of GvHD and rapid disappearance of SGM donor T cells.
Most important, patients 1 and 3 developed secondary graft failure (Table 1). Late graft failure may occur in more than 10% of T-cell-depleted hematopoietic SCTs,8 the amount of CD3+ cells in the transplant being most critical. In our study, numbers of “contaminating” T cells in the CD34+ grafts (Table 1) were far below the suggested threshold of 2 × 105/kg,8 but 5 × 106 SGM T cells/kg was added. Obviously, complete loss of these SGM T lymphocytes about 3 weeks after transplantation in both patients (Figure 1) led to a situation comparable to full T-cell depletion. This, probably in concert with other factors (HLA mismatch + busulphan conditioning for CML, patient 1; slightly reduced busulphan dose, patient 38 ), may have facilitated autologous T-cell recovery, as indicated by T-cell chimerism data from patient 3 (not shown).
In conclusion, we confirmed earlier reports1,2 that the use of SGM T lymphocytes may allow control of acute GvHD. At the same time, we made the troubling observation that early total in vivo depletion of SGM donor T cells may be associated with an increased risk of transplant rejection. This suggests that minimum numbers of donor CD3+ cells are required after transplantation, not only to facilitate engraftment8 but also to prevent late rejection. To preclude the rejection risk associated with HSV-tk-mediated depletion of donor T cells, add-back of limited numbers of nonmodified T lymphocytes (eg, 2 × 105 CD3+/kg) to the CD34-enriched PBSCs8 may be a valuable approach.
One of the authors (K.K.) is employed by a company (EUFETS) whose (potential) products may be related to the present work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal