Abstract
Levels of serum soluble interleukin 2 receptor (sIL-2R) provide a reliable marker of disease activity in patients with hairy cell leukemia and adult T-cell leukemia/lymphoma. The malignant cells in patients with anaplastic large cell lymphoma (ALCL) express CD30 and are usually positive for expression of CD25. We measured serum sIL-2R and soluble CD30 (sCD30) levels in patients with ALCL treated with EPOCH (etoposide, prednisone, Oncovin, Cytoxan, hydroxydaunorubicin) infusional chemotherapy. Serum sCD30 levels were elevated and decreased in response to therapy as previously reported. Serum sIL-2R levels were elevated in 7 of 9 patients with ALCL and decreased in response to treatment. Baseline serum sIL-2R levels varied but correlated well with serum sCD30 levels (r = 0.97). Patients positive for the anaplastic lymphoma kinase (ALK) gene showed elevated sIL-2R levels, whereas those negative for ALK had normal serum sIL-2R levels and their tumors lacked CD25 expression. Serum sIL-2R levels were elevated in both patients with recurrent disease. (Blood. 2004;104:3355-3357)
Introduction
Anaplastic large cell lymphoma (ALCL) was initially recognized based on its strong and uniform expression of CD30 recognized by the Ki-1 antibody.1,2 A spectrum of neoplasms ranging from benign cutaneous lymphoproliferative disorders to systemic lymphoma express CD30.3,4 Primary systemic ALCL accounted for 2.4% of non-Hodgkin lymphoma diagnoses in a worldwide survey but represents a greater fraction of non-Hodgkin lymphomas in children.5,6 Two forms of ALCL are recognized based on the presence or absence of aberrant expression of the anaplastic lymphoma kinase (ALK) gene located on chromosome 2. Ultimately overexpression of ALK proves to be a defining feature of ALCL, with ALK- cases belonging to one or more different disease entities.7 The most common translocation involves the ALK gene and the nucleophosmin gene located on chromosome 5 leading to the production of an 80-kDa fusion protein,8 although translocation into other genetic loci9,10 or inversion of the ALK locus on chromosome 211 are also seen in ALCL. The t(2;5) translocation occurs in about 72% of patients and the translocation between chromosomes 1 and 2 is the second most common variant.3 There is a strong male predominance and most develop ALK+ ALCL in the second and third decades of life. These patients have a good prognosis with a 77% overall 5-year survival rate. In contrast, ALK- patients have been reported to have an inferior clinical outcome.12,13 These patients tend to be older and there is an equal male-to-female ratio. Soluble CD30 (sCD30), shed from the surface of ALCL cells, can be measured in the serum as a marker of disease activity and higher serum levels were associated with a worse outcome in one study.14-16 Expression of other surface markers shed into the serum may provide additional markers of disease activity.
Serum-soluble interleukin 2 receptor (sIL-2R) levels are increased in patients with malignancies that express CD25, including hairy cell leukemia and adult T-cell leukemia/lymphoma,17 and provide a reproducible measure of disease activity that can be used to predict relapse.18-20 CD25 is expressed by the neoplastic cells in most patients with the systemic form of ALCL.21,22 We measured serum sIL-2R levels in patients with ALCL before, during, and after therapy with dose-adjusted EPOCH (etoposide, prednisone, Oncovin [vincristine], Cytoxan [cyclophosphamide], hydroxydaunorubicin [Adriamycin]) infusional chemotherapy. Patients with ALK+ ALCL have elevated serum sIL-2R levels that correlate with disease activity.
Study design
Patients were treated with dose-adjusted EPOCH chemotherapy as previously described and modified by the addition of filgrastim and adjustment of the dose of chemotherapy based on the level of myelosuppression.23,24 Of 109 patients entered in the study, 10 were diagnosed with ALCL (9.1%). Protocols were approved by the Institutional Review Board of the National Cancer Institute. Informed consent was obtained before patient participation. Serum levels of sIL-2R (R&D Systems, Minneapolis, MN) and sCD30 (Dako, Carpenteria, CA) were measured using commercially available reagents. Immunohistochemistry studies were performed on deparaffinized formalin-fixed, paraffin-embedded (FFPE) tissue sections using a panel of monoclonal antibodies including CD25, CD30 (Novocastra, Newcastle upon Tyne, United Kingdom), and ALK-1 (Dako)25 with the following modification. Deparaffinized slides were placed in a microwaveable pressure cooker containing 1.5 L 10 mM citrate buffer (pH, 6.0) containing 0.1% Tween 20 and microwaved (Model R4A80; Sharp Electronics, Rahwah, NJ) for 40 minutes at 700 W. Antigens were localized using an avidin-biotin-peroxidase method with 3,3′-diaminobenzidine as a chromogen and performed using an automated immunostainer (Ventana Medical Systems, Tucson, AZ). Primary antibody incubation was performed overnight with antibodies to CD25 (1:200), CD30 (1:80), and ALK-1 (1:160). Positive and negative controls were run with all cases and stained appropriately.
Results and discussion
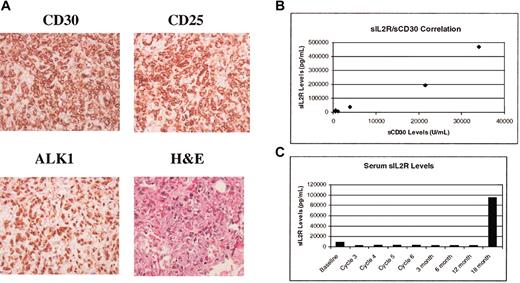
The characteristics of the patients are shown in Table 1. We measured baseline serum sCD30 (normal level, < 18 U/mL) and sIL-2R (normal level, < 3600 pg/mL) levels in patients with ALCL treated with EPOCH infusional chemotherapy. The baseline serum sIL-2R levels were elevated in 7 of 9 patients (median, 18 900 pg/mL; range 6328-470 575 mg/mL) and correlated with elevations in serum sCD30 levels (median, 845 U/mL; range, 217-34 065 U/mL). The correlation between the serum levels of sCD30 and sIL-2R is shown in Figure 1B (r = 0.97). There was a trend toward higher levels of sIL-2R in patients with extensive tumor burden, particularly those with bone marrow involvement. Both patients with ALK- tumors, whose diagnosis was based on morphology and strong uniform CD30 staining, had normal serum sIL-2R levels although sCD30 levels were elevated. In contrast, all of the ALK+ patients tested had elevated sIL-2R levels. The malignant cells from these ALK- patients were negative for CD25 by immunohistochemistry consistent with the serum findings. Eight additional ALK- cases not treated on this study demonstrated variability in expression of CD25 with 5 cases found positive for this marker.
Patient characteristics
Patient no. . | Age, y . | Sex . | Stage . | Response . | CD30+ . | ALK-1+ . | CD25+ . | sCD30 level, U/mL . | sIL-2R level, pg/mL . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | M | IVA | CR | Positive | Negative | Negative | 217 | 517 |
| 2 | 19 | M | IIIA | CR | Positive | ND | ND | 534 | 13 918 |
| 3 | 38 | M | IVB | CR | Positive | Positive* | ND | 34 065 | 470 575 |
| 4 | 21 | M | IIA | CR† | Positive | Positive | Positive | 1 155 | 8 313† |
| 5 | 21 | M | IIB | CR | Positive | Positive* | ND | 508 | 6 328 |
| 6 | 29 | F | IIIA | CR | Positive | Positive | Positive | 3 971 | 39 297 |
| 7 | 34 | M | IV | CR | Positive | Positive | ND | 21 480 | 191 483 |
| 8 | 24 | F | IV | PD | Positive | Positive | Positive | ND | 18 900§ |
| 9 | 68 | F | II | CR | Positive | Positive | ND | ND | ND |
| 10 | 60 | M | IV | CR | Positive | Negative | Negative | 488 | 2 183 |
Patient no. . | Age, y . | Sex . | Stage . | Response . | CD30+ . | ALK-1+ . | CD25+ . | sCD30 level, U/mL . | sIL-2R level, pg/mL . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | M | IVA | CR | Positive | Negative | Negative | 217 | 517 |
| 2 | 19 | M | IIIA | CR | Positive | ND | ND | 534 | 13 918 |
| 3 | 38 | M | IVB | CR | Positive | Positive* | ND | 34 065 | 470 575 |
| 4 | 21 | M | IIA | CR† | Positive | Positive | Positive | 1 155 | 8 313† |
| 5 | 21 | M | IIB | CR | Positive | Positive* | ND | 508 | 6 328 |
| 6 | 29 | F | IIIA | CR | Positive | Positive | Positive | 3 971 | 39 297 |
| 7 | 34 | M | IV | CR | Positive | Positive | ND | 21 480 | 191 483 |
| 8 | 24 | F | IV | PD | Positive | Positive | Positive | ND | 18 900§ |
| 9 | 68 | F | II | CR | Positive | Positive | ND | ND | ND |
| 10 | 60 | M | IV | CR | Positive | Negative | Negative | 488 | 2 183 |
Normal value for sCD30 is less than 18 U/mL and that for sIL-2R less than 3600 pg/mL.
CR indicates complete response; ND, not done; PD, progressive disease.
Cytoplasmic ALK-1 staining, otherwise both cytoplasmic and nuclear staining.
Patient 4 experienced a relapse, at which point his sIL-2R level was measured at 95 661 pg/mL.
Sample was obtained at the start of the second cycle.
Tissue stains and levels of serum sCD30 and sIL-2R. (A) Immunohistochemical staining of tumor tissue for CD30, CD25, ALK1, and hematoxylin and eosin stain of tissue. Images were viewed under an Olympus BX41 microscope equipped with UPlan F1 40 ×/0.75 objective lenses and an Olympus DP12 digital camera (Olympus, Melville, NY). Images were imported with an Olympus Camedia USB Smartmedia card reader into Adobe Photoshop 7.0 (Adobe, San Jose, CA) for processing. (B) Correlation of serum sCD30 and sIL-2R levels in patients with ALCL. Correlation coefficient r = 0.97. (C) Serum sIL-2R levels during therapy with EPOCH infusional chemotherapy. The elevated levels seen before therapy normalized within one cycle of treatment.
Tissue stains and levels of serum sCD30 and sIL-2R. (A) Immunohistochemical staining of tumor tissue for CD30, CD25, ALK1, and hematoxylin and eosin stain of tissue. Images were viewed under an Olympus BX41 microscope equipped with UPlan F1 40 ×/0.75 objective lenses and an Olympus DP12 digital camera (Olympus, Melville, NY). Images were imported with an Olympus Camedia USB Smartmedia card reader into Adobe Photoshop 7.0 (Adobe, San Jose, CA) for processing. (B) Correlation of serum sCD30 and sIL-2R levels in patients with ALCL. Correlation coefficient r = 0.97. (C) Serum sIL-2R levels during therapy with EPOCH infusional chemotherapy. The elevated levels seen before therapy normalized within one cycle of treatment.
Patients with elevated pretreatment serum sIL-2R levels showed a significant reduction in serum sIL-2R levels (median, 1141 pg/mL) at the completion of treatment from a median pretherapy value of 18 900 pg/mL. The time course of the fall in serum sIL-2R levels in one patient is shown in Figure 1C, and other patients showed similar kinetics with normalization of sIL-2R levels by the start of the third cycle of treatment. Similarly, serum sCD30 levels fell following treatment (median, 32 U/mL). Two patients in this group had a relapse; elevated serum sIL-2R levels were detected at the time of relapse.
CD30 belongs to the tumor necrosis factor (TNF) receptor superfamily whose members are involved in signal transduction events that can mediate apoptosis or proliferation. Antibodies directed at the ligand-binding site of CD30, functioning in a fashion similar to CD30 ligand, can transduce signals that mediated apoptosis in the neoplastic cells of patients with ALCL.26 The murine monoclonal antibodies HeFi-1 and M44, directed to the ligand-binding site of CD30, show antitumor activity in mouse models.27 These results suggest that reagents that bind the ligand-binding site of CD30 may be effective in patients with ALCL and clinical trials testing these agents are underway. The availability of alternative markers would be useful because these antibodies invalidate sCD30 as a marker of disease activity.
The clinical outcome in this group of patient is similar to that reported in a previous series.5 One patient failed to respond to treatment with EPOCH chemotherapy and 2 patients had relapses (at 18 months and 8 years) after the initial course of EPOCH chemotherapy. The patient who progressed during therapy with EPOCH responded to treatment with the combination of daclizumab, the humanized monoclonal antibody directed at CD25, and EPOCH chemotherapy and underwent allogeneic transplantation but subsequently progressed. The other patients remain disease free after treatment with this combination; one underwent autologous stem cell transplantation.
In conclusion, high levels of sIL-2R were seen in all patients with ALK+ ALCL at diagnosis and these levels normalized with effective therapy. Serum sIL-2R levels increased at relapse. Lack of expression of CD25 on the malignant cells in ALK- tumors explains the low serum sIL-2R levels in these 2 patients but would be anticipated to be elevated in ALK- cases that express CD25. Larger series of patients should be evaluated for these parameters to extend these results. Serum sIL-2R levels will provide a sensitive measure of disease response in patients treated with antibodies to CD30 that would prevent measurement of serum sCD30 levels.
Prepublished online as Blood First Edition Paper, June 17, 2004; DOI 10.1182/blood-2003-11-3922.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal