Abstract
Calcification is a common complication in cardiovascular disease and may affect both arteries and heart valves. Matrix γ-carboxyglutamic acid (Gla) protein (MGP) is a potent inhibitor of vascular calcification, the activity of which is regulated by vitamin K. In animal models, vitamin K antagonists (oral anticoagulants [OACs]) were shown to induce arterial calcification. To investigate whether long-term OAC treatment may induce calcification in humans also, we have measured the grade of aortic valve calcification in patients with and without preoperative OAC treatment. OAC-treated subjects were matched with nontreated ones for age, sex, and disease. Calcifications in patients receiving preoperative OAC treatment were significantly (2-fold) larger than in nontreated patients. These observations suggest that OACs, which are widely used for antithrombotic therapy, may induce cardiovascular calcifications as an adverse side effect.
Introduction
Vitamin K antagonists, also known as oral anticoagulants (OACs), are widely used for the treatment and prophylaxis of thromboembolic diseases.1 Short-term OAC treatment is often applied after deep venous thrombosis, whereas long-term treatment may be required for atrial fibrillation or after prosthetic heart valve implantation. In the Netherlands alone, some 270 000 people (1.7% of the total population) receive long-term OAC treatment. Vitamin K is an essential micronutrient that serves as a cofactor for the transformation of selective glutamic acid (Glu) residues into γ-carboxyglutamic acid (Gla) during the biosynthesis of the so-called Gla proteins, including the vitamin K–dependent coagulation factors.2 In all known Gla proteins, the Gla residues are essential for the function of these proteins. OACs are used to block the γ-carboxylation of the vitamin K–dependent coagulation factors (II, VII, IX, and X) and 3 anticoagulant proteins (C, S, and Z); OAC treatment leads to dysfunctional, undercarboxylated species also known as proteins induced by vitamin Kabsence (PIVKAs). OAC treatment also affects the synthesis and function of a number of other Gla proteins including the noncoagulation protein matrix Gla protein (MGP) in cartilage and the vasculature. The first reports suggesting extrahepatic effects of vitamin K antagonists were published in the 1970s, when it was found that women receiving OAC treatment between the 6th and 12th week of pregnancy gave birth to children with severe bone abnormalities (chondrodysplasia punctata). Presently, it is commonly agreed that the most plausible mechanism underlying this phenomenon is incomplete γ-carboxylation of MGP, resulting in excessive cartilage calcification and subsequent nasal and distal digital hypoplasia, and epiphyseal stippling.3,4
Recent publications have demonstrated that cardiovascular calcifications are the result of an actively regulated process.5-8 One of these regulatory proteins is MGP, a potent inhibitor of soft tissue calcification.9 Transgenic MGP-deficient mice were born to term, but died within 6 to 8 weeks after birth due to massive calcification and rupture of the arteries. Price et al showed that the oral anticoagulant warfarin was capable of inducing a mild form of aortic calcification in rats within 2 weeks of treatment,10 thus confirming the importance of properly carboxylated Gla proteins for adequate calcification inhibition. Since calcification occurred only in young animals, the model may not be representative for OAC treatment in adults and elderly subjects. Recently, however, Sweatt et al directly demonstrated that under-carboxylation of MGP was also associated with aortic calcification in aging rats.11
Whereas OAC treatment is effective for preventing thromboembolic disease, nothing is known of a potential adverse effect of OACs in the vasculature. Here, we have addressed the question of whether coumarin anticoagulation is a risk factor for cardiovascular calcification. Since the etiology of arterial and aortic valve calcification has common pathomechanisms,12 we assembled in this study aortic valves obtained from patients after aortic valve replacement. Since the use of coumarin has not yet been demonstrated with the extent of aortic heart valve calcification, we have compared aortic valve calcification in anticoagulated and nonanticoagulated subjects.
Study design
A total of 45 aortic valves were obtained after routine cardiac replacement surgery. The specimens came from 26 women and 19 men (mean age, 71 years; Table 1) with clinically manifest aortic valve stenosis and/or insufficiency (grades II-III). There were 10 patients who had received a preoperative marcoumar treatment with target international normalized ratio (INR) values between 2 and 3. The duration of treatment varied between 16 and 35 months (mean, 25 months). Histopathologic inspection of the samples showed typical aspects with partial or total valve destructions induced by basophilic-amorphous calcified deposits. Calcification was visualized by von Kossa staining. The calcified area was measured using a microscope coupled to a computized morphometry system (quantimed 570; Leica, Rijswijk, the Netherlands). For morphometric analysis, 5 sections (20 μm apart) were used, and the calcification area was expressed as a percentage of the total section area. All morphometric measurements were conducted by 2 independent persons. The Medical Ethics Committee of the University of Tuebingen approved the study protocol and all subjects gave their written informed consent.
Characteristics of the subjects
. | OAC treatment . | . | . | |
|---|---|---|---|---|
. | No preoperative OAC . | Preoperative OAC . | P . | |
| Subjects, no. | 35 | 10 | na | |
| Age, y, mean ± SD | 70.1 ± 8.5 | 73.4 ± 5.3 | ns | |
| Sex, no. male/no. female | 15/20 | 4/6 | ns | |
| Diabetes mellitus, no. | 3 | 1 | ns | |
| Aortic valve stenosis | ||||
| Grade II, no. | 7 | 3 | ns | |
| Grade III, no. | 28 | 7 | ns | |
| Insufficiency, no. | 19 | 6 | ns | |
| INR, no. | < 1.2 | 2.4 ± 0.4 | < .01 | |
| Calcification score, % | 16 | 37 | < .02 | |
. | OAC treatment . | . | . | |
|---|---|---|---|---|
. | No preoperative OAC . | Preoperative OAC . | P . | |
| Subjects, no. | 35 | 10 | na | |
| Age, y, mean ± SD | 70.1 ± 8.5 | 73.4 ± 5.3 | ns | |
| Sex, no. male/no. female | 15/20 | 4/6 | ns | |
| Diabetes mellitus, no. | 3 | 1 | ns | |
| Aortic valve stenosis | ||||
| Grade II, no. | 7 | 3 | ns | |
| Grade III, no. | 28 | 7 | ns | |
| Insufficiency, no. | 19 | 6 | ns | |
| INR, no. | < 1.2 | 2.4 ± 0.4 | < .01 | |
| Calcification score, % | 16 | 37 | < .02 | |
na indicates not applicable; ns, not significant.
Results and discussion
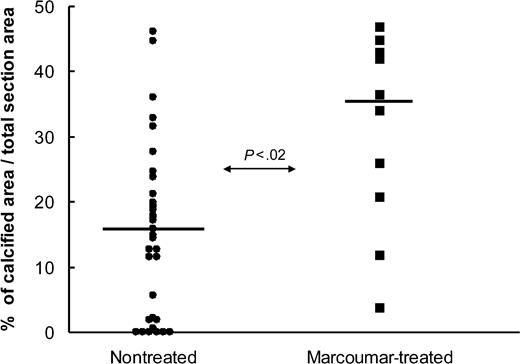
A more than 2-fold difference was observed between the marcoumar-treated and nontreated groups, with a mean calcified area of 16% in the nontreated group and 37% in the OAC-treated group (Figure 1). The difference was statistically significant at P < .02 using the Wilcoxon signed-ranks test. A possible explanation for these observations is that marcoumar treatment results in a decreased protection against tissue calcification due to the impairment of MGP, thus leading to more pronounced valvular calcification. Even low-dose OAC treatment combined with a relatively short period of treatment (like in this study) resulted in significantly more calcification. Many patients, however, receive OACs throughout their lives with INR values well above the range indicated in our study cohort. Since coumarin derivatives are widely used, physicians should be aware of this potential adverse effect on the vasculature.
Difference in calcification score measured in aortic heart valves. Plot represents patients with (▪) and without (•) preoperative marcoumar treatment. Values are expressed as calcification area compared with total section area in percentage. Each value is the mean of 5 measurements per heart valve. Horizontal bar is the median of each group.
Difference in calcification score measured in aortic heart valves. Plot represents patients with (▪) and without (•) preoperative marcoumar treatment. Values are expressed as calcification area compared with total section area in percentage. Each value is the mean of 5 measurements per heart valve. Horizontal bar is the median of each group.
Prepublished online as Blood First Edition Paper, July 20, 2004; DOI 10.1182/blood-2004-04-1277.
Supported by the Netherlands Heart Foundation, grant number 2001.033.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal