Abstract
The molecular biology of lymphatics is only rudimentary owing to the long-standing absence of specific markers, and scanty is the information regarding bladder lymphatic vessels. By using mice with a reporter gene for nuclear factor κB (NF-κB) activity (κB-lacZ) in combination with immunohistochemical staining with a specific lymphatic marker (LYVE-1), we show, for the first time, that NF-κB is constitutively active in lymphatic endothelium in the urinary bladder, uterus, intestine, heart, and airways. Tie2-lacZ mice confirmed that the structures observed in κB-lacZ mice were not blood vessels. In addition, acute instillation of lipopolysaccharide (LPS) or tumor necrosis factor α (TNF-α) into the κB-lacZ mouse bladder revealed the capacity of this transgenic in reporting inducible NF-κB activity. Our findings demonstrate an overriding constitutive NF-κB activity in the lymphatic system. They also provide a working model for detecting lymphatic vessels and evoke testable hypotheses regarding the role of lymphatic vessels in health and disease.
Introduction
The lymphatic vasculature forms a second circulatory system that drains extracellular fluid and provides an exclusive environment in which immune cells can respond to antigens.1 There is only a rudimentary knowledge of the molecular biology of lymphatics.1 In the particular case of the urinary system, most investigators have concentrated their efforts on blood vessels and ignored the lymphatic bed.2
Recently, LYVE-1 (a lymphatic endothelium-specific hyaluronan receptor) that is absent on vascular endothelial cells3,4 has been identified as a marker for lymphatic endothelium. In addition, lymph nodes isolated from mice harboring a lacZ reporter construct driven by nuclear factor κB (NF-κB)–dependent promoter elements (κB-lacZ) have been shown to display β-galactosidase (β-gal) activity correlated with nuclear transcriptionally active NF-κB complexes.5 More recently, other transgenic mice bearing a similar NF-κB reporter were developed,6 which also presented lymph node constitutive NF-κB activity.6
By combining κB-lacZ transgenic mice with LYVE-1 immunohistochemistry (IHC), we assessed whether lymphatics display constitutive NF-κB activity. The results were contrasted with those obtained from Tie2-lacZ mice, which label primarily vascular endothelial cells. The combined data clearly identify lymphatic and not blood vessels as sites of constitutive NF-κB activity.
Study design
κB-lacZ, Tie2-lacZ, and C57BL/6 mice were used in these experiments. κB-lacZ mice harbor a fragment derived from the p105 promoter, which includes 3 functional κB sites inserted upstream of the gene encoding the Escherichia coli β-gal with a nuclear localization sequence.5 In these mice, β-gal activity was directly correlated with nuclear NF-κB–binding activity.5 Tie2-lacZ mice carrying a β-gal reporter gene under the control of the murine Tie2 promoter and expressing β-gal specifically in vascular endothelial cells7 were purchased from Jackson Laboratories (Bar Harbor, ME).
Naive and challenged mice were used. For challenge, 3 groups of female mice were anesthetized and transurethrally catheterized.8 The urinary bladders were instilled with 150 μL saline, E coli lipopolysaccharide (LPS) strain 055:B5 (100 μg/mL), or tumor necrosis factor α (TNF-α;1 μg/mL) and kept in contact with the bladder for 1 hour.8 Twenty-four hours after instillation, tissues were removed and processed. Whole mounts were examined under a dissecting microscope (SMZ 1500, Nikon, Tokyo, Japan), whereas cross-sections were visualized under a compound microscope (Eclipse E600, Nikon). All tissues were photographed at room temperature by a digital camera (DXM1200; Nikon). Exposure times were held constant when acquiring images from different tissues. Lymphatic vessels were quantified by morphometric analysis using Neurolucida workstation (MicroBrightField, Williston, VT; see the Supplemental Materials link at the top of the online article on the Blood website).
β-Galactosidase activity was revealed by X-gal staining at 30°C for 6 hours, as described.5 Afterward, tissues were washed 4 times in phosphate-buffered saline (PBS) and postfixed in 2% paraformaldehyde in piperazine diethanesulfonic acid (PIPES). LYVE-1 IHC was performed as reported.14 Controls included omission of the primary antibody.
Results and discussion
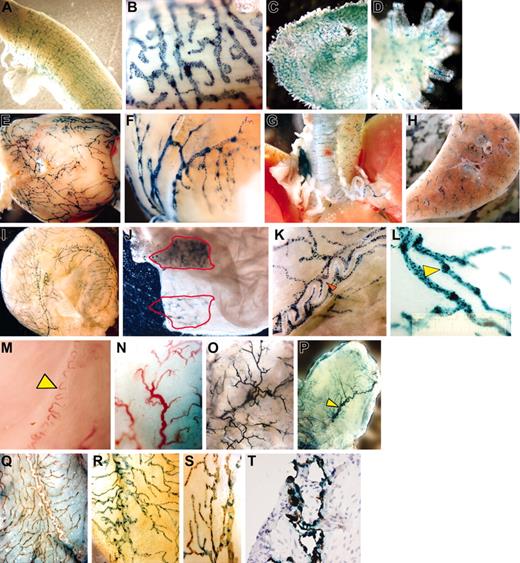
Whole mount preparations of uterus (Figure 1A-B), gastrointestinal tract (Figure 1C-D), heart (Figure 1E-F), airways (Figure 1G-H), and bladder (Figure 1I-L) isolated from naive κB-LacZ mice revealed an interesting pattern of β-gal labeling. Specific staining was observed primarily in a vascular network of blind-ended, thin-walled capillaries that merged to larger collecting ducts. The labeling was interrupted at intervals by constrictions, which gave them a knotted or beaded appearance (Figure 1K-L) suggesting lymphatic vessels.9 The densities of positive vessels (vessel area/tissue area × 100) in decreasing order of magnitude (average ± SEM) were: uterus, 85 ± 1.1; duodenum, 66 ± 0.7; heart, 40.9 ± 0.4; and bladder, 33 ± 0.2 (n = 6 mice).
Results of whole mount preparations. The whole mount preparations of uterus (A-B), duodenum (C-D), heart (E-F), airways (G-H), and urinary bladder (I-L) isolated from naive κB-lacZ mice were labeled with β-gal for 6 hours. The label indicates NF-κB activity primarily in a vascular network of blind-ended, thin-walled capillaries that merged to larger collecting ducts. High-magnification pictures of κB-lacZ bladders indicate that the labeling was interrupted at intervals by constrictions, which gave them a knotted or beaded appearance (K-L). Panel K shows complete absence of β-gal staining in a superficial blood vessel surrounded by lymphatics (red arrow). C57BL/6 bladders (M-N) present typical subserous sigmoid blood vessel (yellow arrow) of similar shape of the main vessel observed in Tie2-lacZ (P), but very different from a typical β-gal staining observed in κB-lacZ bladders (I). On the mucosal site, a dense capillary network was observed in bladders isolated from C57BL/6 (N), which was very similar to the β-gal labeling observed in Tie2-lacZ (O). Interestingly, the bladder mucosa of κB-lacZ mice was devoid β-gal labeling (J). However, when bladder mucosa was lifted away from the detrusor muscle, and the tissue was transilluminated, it was possible to visualize the specific label of adventitial lymphatic vessels (J). Representative photomicrograph of double label with X-gal and LYVE-1 IHC: bladder isolated from C57BL/6 (Q) and κB-LacZ (R-T) mice. Bladders were photographed as whole mounts (Q-S) or cross-section (T). Images were visualized using a Nikon SMZ 1500 microscope equipped with high-resolution Plan 1.6 WD24 objective lenses (Nikon, Tokyo, Japan). The image medium was phosphate-buffered saline at room temperature. Magnifications for individual panels are as follows:A, C, H, N, and O, 10×; B and L, 100×; D, K, and S, 60×;E,G,I,M,andP,7.5×;F,30×; J and Q, 20×;R,40×; and T, 400×.
Results of whole mount preparations. The whole mount preparations of uterus (A-B), duodenum (C-D), heart (E-F), airways (G-H), and urinary bladder (I-L) isolated from naive κB-lacZ mice were labeled with β-gal for 6 hours. The label indicates NF-κB activity primarily in a vascular network of blind-ended, thin-walled capillaries that merged to larger collecting ducts. High-magnification pictures of κB-lacZ bladders indicate that the labeling was interrupted at intervals by constrictions, which gave them a knotted or beaded appearance (K-L). Panel K shows complete absence of β-gal staining in a superficial blood vessel surrounded by lymphatics (red arrow). C57BL/6 bladders (M-N) present typical subserous sigmoid blood vessel (yellow arrow) of similar shape of the main vessel observed in Tie2-lacZ (P), but very different from a typical β-gal staining observed in κB-lacZ bladders (I). On the mucosal site, a dense capillary network was observed in bladders isolated from C57BL/6 (N), which was very similar to the β-gal labeling observed in Tie2-lacZ (O). Interestingly, the bladder mucosa of κB-lacZ mice was devoid β-gal labeling (J). However, when bladder mucosa was lifted away from the detrusor muscle, and the tissue was transilluminated, it was possible to visualize the specific label of adventitial lymphatic vessels (J). Representative photomicrograph of double label with X-gal and LYVE-1 IHC: bladder isolated from C57BL/6 (Q) and κB-LacZ (R-T) mice. Bladders were photographed as whole mounts (Q-S) or cross-section (T). Images were visualized using a Nikon SMZ 1500 microscope equipped with high-resolution Plan 1.6 WD24 objective lenses (Nikon, Tokyo, Japan). The image medium was phosphate-buffered saline at room temperature. Magnifications for individual panels are as follows:A, C, H, N, and O, 10×; B and L, 100×; D, K, and S, 60×;E,G,I,M,andP,7.5×;F,30×; J and Q, 20×;R,40×; and T, 400×.
We then compared tissues isolated from C57BL/6 (Figure 1M-N), Tie2-lacZ (Figure 1O-P), and κB-lacZ (Figure 1I-J) mice. We found a clear correlation of blood vessels in C57BL/6 and Tie2-lacZ mice and a disparity between β-gal labeling of tissues obtained from Tie2-lacZ and κB-lacZ mice. Tie2-lacZ mice presented blood vessels staining in both bladder adventitia (Figure 1P) and mucosal (Figure 1O) layers, whereas κB-lacZ mice exhibited an intense staining in the adventitia (Figure 1I) but not in the mucosa (Figure 1J). Similar to the bladder mucosa, lymphatics were not visualized in the cavity of heart and uterus (data not shown), perhaps because their reduced size precluded detection. However, we were able to observe tiny lacteals of the gastrointestinal tract (Figure 1D). At least in the human bladder, lymphatic vessels are found deeper in the mucosal region rather than superficially10 and in bladder biopsies, lymphatic vessels are seen in the deeper regions of the mucosa but not in the subepithelial layer.11
We used LYVE-1 IHC to further confirm that lymphatic vessels rather than blood vessels were the sites of NF-κB constitutive activity. C57BL/6 bladders (Figure 1Q) revealed a specific LYVE-1 label in the adventitia with a distribution similar to X-gal labeling of κB-lacZ mice (Figure 1I). Moreover, when κB-lacZ bladders were subsequently stained with X-gal and LYVE-1 IHC, a complete overlap of labels was observed both in whole mounts (Figure 1R-S) and in cross-section (Figure 1T). Similar colocalization of stains was found in all tissues studied so far. These results definitively colocalize constitutive NF-κB activity and lymphatic vessels.
Lymphatics are prevalent in organs that come into direct contact with the external environment.12 In this context, our results indicated constitutive NF-κB activity in lymphatics of the respiratory and gastrointestinal systems as well as in other organs that are not in direct contact with the external environment, such as heart, uterus, and bladder (Figure 1).
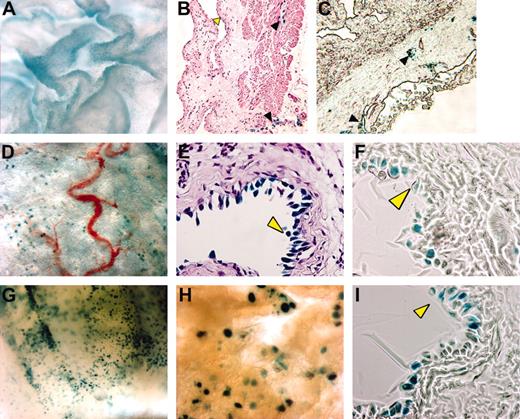
A related question was whether κB-lacZ mice are useful for detecting NF-κB stimulation. Compared to saline (Figure 2A-C), TNF-α induced a strong X-gal staining in the bladder mucosa (Figure 2G-I) without altering the density of lymphatics in the adventitia. Bladder cross-sections confirmed that TNF-α induced a specific lacZ expression in urothelial cells (Figure 2I). Similar results were obtained with acute instillation of LPS (Figure 2D-F). Therefore, we propose that κB-lacZ mice may serve a dual purpose, permitting both visualization of lymphatics and detection of inducible NF-κB activity.
Visualization of TNF-α– and LPS-induced bladder NF-κB activity. Anesthetized female κB-lacZ mice were challenged intravesically with saline (A-C), LPS (100 μg/mL; D-F), or TNF-α (1 μg/mL; G-I). Twenty-four hours after challenge, bladders were removed and stained with X-gal and photographed as whole mounts (A,D,G-H) or cross-sections (B-C,E-F,I). Tissues were stained with hematoxylin and eosin (B,E) or left unstained (C,F,I). Yellow arrowhead indicates urothelial cells and black arrowhead indicates lymphatic vessels. Cross-sections were visualized with a Nikon compound Eclipse E600 microscope equipped with PlanFluor 40×/0.75 DIC m objective lenses (Nikon). Images were viewed in open air at the following magnifications: A, 300×; B and C, 100×;D,30×; E, F, and I, 400×;G,40×; and H, 60×.
Visualization of TNF-α– and LPS-induced bladder NF-κB activity. Anesthetized female κB-lacZ mice were challenged intravesically with saline (A-C), LPS (100 μg/mL; D-F), or TNF-α (1 μg/mL; G-I). Twenty-four hours after challenge, bladders were removed and stained with X-gal and photographed as whole mounts (A,D,G-H) or cross-sections (B-C,E-F,I). Tissues were stained with hematoxylin and eosin (B,E) or left unstained (C,F,I). Yellow arrowhead indicates urothelial cells and black arrowhead indicates lymphatic vessels. Cross-sections were visualized with a Nikon compound Eclipse E600 microscope equipped with PlanFluor 40×/0.75 DIC m objective lenses (Nikon). Images were viewed in open air at the following magnifications: A, 300×; B and C, 100×;D,30×; E, F, and I, 400×;G,40×; and H, 60×.
This study underscores, for the first time, an overriding constitutive NF-κB activity in the lymphatic system and shows the complete topography of the vascular system of the mouse urinary bladder. It remains to be determined why lymphatics and not blood vessels harbor a constitutive NF-κB activity and if there is a specific role for NF-κB–driven gene transcription in lymphatic vessels. In conclusion, κB-lacZ mice along with LYVE-1 antibody will undoubtedly contribute to determine the specific prolymphangiogenic and antilymphangiogenic factors in health and disease.
Prepublished online as Blood First Edition Paper, July 22, 2004; DOI 10.1182/blood-2004-04-1428.
Supported by National Institutes of Health grants DK 55828-01 and DK066101-01 (R.S.), OCAST HR01-127 (R.S.), Institut Pasteur (PTR38; S.M.), and the Institut National de la Santé et de la Recherche Médicale and Ligue Nationale Contre le Cancer (Équipe Labellisée; A.I.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal