Abstract
The platelet-specific chemokine platelet factor 4 (PF4) is released in large amounts at sites of vascular injury. PF4 binds to heparin with high affinity, but its in vivo biologic role has not been defined. We studied the role of PF4 in thrombosis using heterozygote and homozygote PF4 knock-out mice (mPF4+/– and mPF4–/–, respectively) and transgenic mice overexpressing human PF4 (hPF4+). None of these lines had an overt bleeding diathesis, but in a FeCl3 carotid artery thrombosis model, all showed impaired thrombus formation. This defect in thrombus formation in the mPF4–/– animals was corrected by infusing hPF4 over a narrow concentration range. The thrombotic defect in the mPF4+/– and mPF4–/– animals was particularly sensitive to infusions of the negatively charged anticoagulant heparin. However, the same amount of heparin paradoxically normalized thrombus formation in the hPF4+ animals, although these animals were anticoagulated systemically. Upon infusion of the positively charged protein, protamine sulfate, the reverse was observed with mPF4+/– and mPF4–/– animals having improved thrombosis, with the hPF4+ animals having worsened thrombus formation. These studies support an important role for PF4 in thrombosis, and show that neutralization of PF4 is an important component of heparin's anticoagulant effect. The mechanisms underlying these observations of PF4 biology and their clinical implications remain to be determined.
Introduction
Platelet factor 4 (PF4) is a member of the CXC chemokine subfamily that is synthesized exclusively in megakaryocytes and stored in platelet α-granules.1 PF4 accounts for approximately 2% to 3% of the total protein found in circulating platelets on a molar basis. When platelets are activated, PF4 is released in concentrations that exceed 2 μM (∼12 μg/mL serum) in the vicinity of vessel wall injury.2 The conservation of high levels of PF4 expression in the α-granules of every mammalian species examined suggests that this protein has important biologic activities. However, unlike other CXC chemokines, which have a clear role in inflammation primarily by augmenting neutrophil chemotaxis and activation, the biologic role of PF4 is unclear.
Moreover, whereas other members of the CXC subfamily bind to specific members of the CXCR family of 7 transmembrane G protein–coupled receptors, 3 there had been no well-recognized chemokine receptor for PF4. Binding of PF4 to the chemokine receptor Duffy functions in chemokine clearance, and it has recently been proposed that some vascular beds in humans express an alternatively spliced form of CXCR3, termed CXCR3B, that binds PF4.4 However, the biologic importance of this interaction in vivo has not been explored.
PF4 is known to bind with high affinity to heparin and heparin-like side chains of proteoglycans on membrane surfaces.5-7 PF4 exists as a tetramer at the molar concentrations found at sites of injury. Formation of the tetramer generates an equatorial band of cationic charges, 8 which accounts for its strong avidity for these large negatively charged molecules. Upon its release from activated platelets, PF4 binds to proteoglycans on the surfaces of endothelial cells, 9 platelet membranes, 10 and hepatocytes.11
PF4 was first identified in the 1970s. Since that time, as a result of in vitro studies, PF4 has been implicated in diverse biologic processes, including proposed roles in megakaryopoiesis, 12,13 angiogenesis, 14,15 tumor metastasis, 16 immune responses, 17-20 and thrombosis.21,22 However, these effects were only seen at high PF4 concentrations (5-25 μg/mL or 0.2-0.8 μM). Although these concentrations can be achieved at the site of intense platelet activation, 23 it is unclear which, if any, of these in vitro observations have biologic relevance.
For example, it is still unclear whether PF4 has a role in thrombosis, as both prothrombotic and antithrombotic effects have been described in vitro. Because PF4 neutralizes negatively charged molecules such as heparin and proteoglycans, it had been proposed that PF4 might prevent activation of antithrombin III (ATIII), allowing thrombus extension.24 Since then, numerous in vitro and pharmacologic infusion studies in animal models have pointed to interactions between PF4 and other members of the coagulation system, resulting in both prothrombotic and antithrombotic outcomes. For example, PF4 has been reported to inhibit the activation of factor XII (FXII), a plasma proenzyme that, when activated by negatively charged agents such as glass, ellagic acid, or kaolin, initiates clotting via the intrinsic pathway of thrombin formation.25 The inhibition of FXII by PF4 can be neutralized by heparin.26 More recently, PF4 has been reported to inhibit thrombin activatable fibrinolysis inhibitor (TAFI) in vitro in a single preliminary report, 27 which would be expected to promote thrombus development on the surface of endothelial cells. PF4 can bind directly to negatively charged regions within protein C and thrombomodulin, and thereby greatly enhance the activation of protein C.28,29 Again, both effects were inhibited by heparin. In vivo infusion of PF4 in primate models supports the ability of PF4 to stimulate protein C activity.30
The diverse conclusions drawn from these in vitro studies clearly delineate the need to define the role of PF4 in hemostasis in vivo. Using both a knock-out mouse for PF4 (mPF4–/–) and a transgenic mouse that overexpresses human PF4 (hPF4+)31 we have begun to define the role of PF4 in thrombosis. In this study, we show that PF4 is required to optimize clot formation. Moreover, our studies show that PF4 promotes hemostasis only over a narrow molar range and that negatively charged molecules such as unfractionated high-molecular-weight heparin and positively charged molecules such as protamine sulfate can alter the effect of local PF4 release. Specifically, we show that the presence of high concentrations of PF4 dissociates between the effect of heparin on local thrombus formation and its effect on systemic coagulation. The implications of this finding for the pathogenesis and management of human thrombotic disorders characterized by intense platelet activation is discussed.
Materials and methods
Creation of mice underexpressing and overexpressing PF4
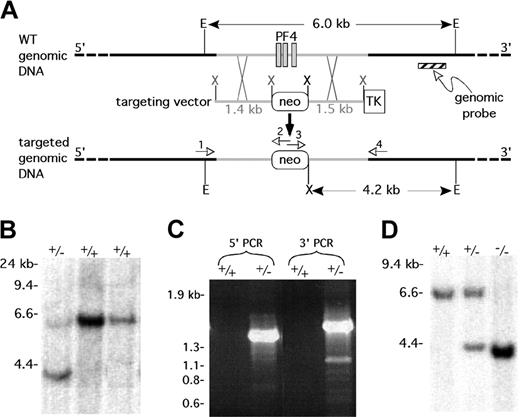
The murine homolog of the PF4 gene locus was cloned from a 129SVJ mouse λ genomic library (ESVJ-1183; Genome Systems, St Louis, MO) and more than 32 kilobase (kb) of the locus was sequenced and submitted to the GenBank (AF349465). On the basis of this information, 1.4-kb upstream and 1.5-kb downstream flanking sequence of the mPF4 gene was polymerase chain reaction (PCR) amplified from the mPF4 λ phage, and inserted into a neomycin-resistant (neo)/thymidine kinase (TK) targeting vector (B. M. Spiegelman, Dana-Farber Cancer Institute, Boston, MA) as a XhoI/XhoI and a BamHI/KpnI restriction fragment, respectively (Figure 1A). The construct removes the entire mPF4-coding region (1.16 kb), which is replaced with the 1.8-kb neo gene. The vector was linearized with NotI prior to electroporation into embryonic stem (ES) cells (Genome Systems). Southern blot analysis on EcoRI/XhoI double-digested genomic DNA isolated from transfected ES cells using an α-32P-dCTP (deoxycytidine triphosphate) random primer labeled probe that extended from 3.97 to 4.20 kb downstream of the last exon of the mPF4 gene was done as previously described32 (Figure 1A). ES cells that were positive for the targeted 4.2-kb band were confirmed by PCR amplification of genomic DNA using the following primer pairs: PF4FD1 5′-CCGTTAGAAGTATAGCTCAGT-3′ and NeoREV 5′-GCGCCAAGTGCCGAGCGGGGC-3′ for the 5′-end, giving a 1.6-kb band, and NeoFD 5′-GCCTGCTC TTTAGTGAAGGCT-3′ and PF4REV1 5′-TTCTAGTGGGACTGTGATGG-3′ for the 3′-end, which generates a 1.8-kb band (Figure 1A). An appropriately targeted ES clone was injected into blastocysts of C57BL/6J mice. Chimeric animals were crossed with wild-type C57BL/6J females, and heterozygotes for the mPF4-targeted gene were detected by genomic Southern blot and PCR analyses as described for the ES cells. mPF4+/– mice were backcrossed onto the C57BL/6J background; and animals described were studied after the sixth cross, and wild-type (WT) littermates were used as controls in all experiments.
Generation of a targeted disruption in the mouse PF4 gene. (A) Strategy for creating a mPF4 knock-out mouse. E = EcoRI, X = XhoI, neo = neomycin resistance gene, and TK = thymidine kinase gene. The numbered arrows refer to the primers used to check for a targeted event by genomic PCR. (B) Southern blot of genomic DNA from ES cells with 1 correctly targeted line (+/–) and 2 wild-type lines (+/+) after a combined EcoRI/XhoI digest and using the genomic probe shown in panel A. (C) Genomic PCR demonstrating correct targeting of the mPF4 gene in the targeted ES line (+/–) and not in the wild-type ES cells (+/+). (D) Southern blot of genomic DNA from mPF4+/+ and mPF4+/– and mPF4–/– mice for the targeted disruption of the mPF4 gene following a EcoRI/XhoI double digestion and using the genomic probe shown in panel A.
Generation of a targeted disruption in the mouse PF4 gene. (A) Strategy for creating a mPF4 knock-out mouse. E = EcoRI, X = XhoI, neo = neomycin resistance gene, and TK = thymidine kinase gene. The numbered arrows refer to the primers used to check for a targeted event by genomic PCR. (B) Southern blot of genomic DNA from ES cells with 1 correctly targeted line (+/–) and 2 wild-type lines (+/+) after a combined EcoRI/XhoI digest and using the genomic probe shown in panel A. (C) Genomic PCR demonstrating correct targeting of the mPF4 gene in the targeted ES line (+/–) and not in the wild-type ES cells (+/+). (D) Southern blot of genomic DNA from mPF4+/+ and mPF4+/– and mPF4–/– mice for the targeted disruption of the mPF4 gene following a EcoRI/XhoI double digestion and using the genomic probe shown in panel A.
The transgenic mice overexpressing hPF4 were described by us previously.31 These animals have approximately 3.5 times the amount of hPF4 per milligram total platelet protein found in human platelets. Analysis of multiple tissues using immunohistochemistry and reverse transcriptase (RT)–PCR showed that hPF4 was expressed exclusively in megakaryocytes and platelets. Positive animals were selected by genomic PCR analysis as previously described.31 hPF4+ mice were backcrossed onto the C57BL/6J background; animals described were studied after the sixth cross, and WT littermates were used as controls in all experiments.
PF4 RNA and protein determinations
Murine platelet total RNAand RT-PCR protocol has been described previously.31 The primer pairs used to detect a 310–base pair (bp) mPF4-amplified product were 5′-GTCCAGTGGCACCCTCTTGA-3′ and 5′-AATTGACATTTAG-GCAGCTGA-3′, and the primer pairs used to detect a 290-bp mPBP-amplified product (as a control) were 5′-GCCTGCCCACTTCATAACCTC-3′ and 5′-GGGTCCAGGCACGTTTTTTG-3′.
For immunoblot analysis, platelets were prepared from blood collected from the inferior vena cava as previously described.33 Aliquots of platelets were suspended in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–buffered saline (pH 7.2) and frozen in the presence of aprotinin (0.5%), leupeptin (100 μM), and phenylmethylsulfonyl fluoride (PMSF; 1 μM). Equal numbers of platelets (2.5 × 106 platelets/well) were lysed in 4 × sample buffer (Invitrogen, Carlsbad, CA), loaded onto a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel and subsequently blotted onto a polyvinylidene diflouride (PVDF) nitrocellulose membrane (Millipore, Bedford, MA). The membrane was blocked with 5% milk and incubated with affinity-purified rabbit anti–human PF4 (1:5000; PeproTech, Rocky Hill, NJ) primary antibody or a rabbit anti–mouse-specific PF4 (1:1000; special order; Bethyl Laboratories, Montgomery, TX) primary antibody followed by a horseradish peroxidase (HRP)–conjugated swine anti–rabbit antibody (DAKO, Carpintera, CA) and developed using the electrochemiluminescence (ECL) kit (PerkinElmer Life Sciences, Boston, MA). Relative intensity of bands was determined by comparing the integrated density value of WT and heterozygous mouse platelets on a densitometer (Alpha Innotech, San Leandro, CA) over the linear density range.
Human PF4 levels were determined by enzyme-linked immunosorbent assay (ELISA) using a rabbit anti–human PF4 antibody (PeproTech). Wells on a 96-well plate were coated overnight at 4°C with a 100-μL solution containing 2 μg/mL primary antibody in 50 mM Na bicarbonate, pH 9.2, and then blocked with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS). After washing 3 times with PBS containing 0.5% Tween-20 (PBS-T), samples were incubated for 1 hour at room temperature. Wells were again washed 3 times with PBS-T followed by incubation for 1 hour at room temperature with peroxidase-conjugated sheep anti–human PF4 antibody (Affinity Biologicals, Ancaster, ON, Canada). OPD (o-phenylenediamine) peroxidase substrate (Sigma, St Louis, MO) was added for 15 minutes, and the plate was read at 450 nm in a THERMOmax microplate reader (Molecular Devices, Sunnyvale, CA)
All animal studies were performed with Institutional Animal Care and Use Committee approval.
Hematologic studies and in vitro coagulation studies
Blood was obtained by retroorbital puncture, and complete blood counts and hematocrit were determined using an automatic cell counter (HEMAVET; Drew Scientific, Dallas, TX). Bleeding times were performed by severing the tail 5 mm from the tip and immersing 10 mm of the residual tail in physiologic saline as previously described.34 The whole-blood clotting time was done as previously described.35 Briefly, non-anticoagulated portal vein blood (600 μL) was equally divided among 3 glass tubes and placed in a 37°C water bath. One tube was tilted every 30 seconds, while the other tubes were left undisturbed. When a solid clot was observed in the first tube, the second tube was tilted every 30 seconds. This was continued with the third tube, and the time it took to form a clot in the third tube was recorded.
Assessment of platelet function was performed in platelet-rich plasma and in washed platelets prepared as previously described using blood collected from the inferior vena cava.36 Aggregation was performed using diluted platelet-rich plasma or with washed platelets supplemented with human fibrinogen and CaCl2 in a lumi-aggregometer (Chronolog, Haverford, PA) as previously described, 37 using either thrombin (0.01-1.0 U/mL), adenosine diphosphate (ADP; 1-10 μM), collagen (1-5 mg/mL), or phorbol myristate acetate (PMA; 200 nM) as agonist. Secretion from platelet-dense granules was measured using radiolabeled serotonin (3H-5HT; Amersham, Piscataway, NJ) incorporated by the platelets as previously described.38
The activated partial thromboplastin time (aPTT) was measured using anticoagulated blood drawn from the inferior vena cava or from the right jugular vein, and centrifuged for 10 minutes at 3000g at 4°C to obtain plasma. To 0.5 mL plasma, 0.2 mL automated aPTT reagent (BioMerieux, Durham, NC) was added, and clotting was initiated by adding of 0.1 mL 2 M CaCl2. Time for clotting to occur was recorded in a BioMerieux recorder.
Carotid artery thrombosis induced by FeCl3
Animals 6 to 10 weeks of age (18-25 g) were used for these studies. The procedure has been previously described.33 Briefly, after anesthetization with pentobarbital 80 mg/kg intraperitoneal, the right carotid artery was exposed by blunt dissection. A patch of number 1 Whatman filter paper, 1 × 2 mm, soaked in 10% FeCl3 was applied to the artery for 2 minutes. After removal of the patch, the artery was washed with PBS, and blood flow was recorded using a small animal blood flow meter (model T106; Transonic Systems, Ithaca, NY) for 30 minutes. Time to initial formation of a complete occlusive thrombus and the stability of occlusions were recorded. Thrombi were considered stable if occlusions lasted longer than 10 minutes. The experimenter was blinded as to the genotype of the animals.
In infusion studies, the right jugular vein as well as the right carotid artery was exposed by blunt dissection. Recombinant hPF4 expressed in Escherichia coli and purified as previously described39 (0-3.5 mg/kg), unfractionated high-molecular-weight heparin (0-150 U/kg; Elkins-Sinn, Cherry Hill, NJ), low-molecular-weight heparin (LMWH; enoxaparin sodium; 0-0.15mg/kg; Aventis Pharmaceuticals Products, Bridgewater, NJ), or protamine sulfate (0-4 mg/kg; American Pharmaceutical Partners, Los Angeles, CA) were infused in various combinations. The infusion products were delivered into the jugular vein in a total volume of 100 to 150 μL 2 minutes prior to applying the FeCl3 patch to the carotid artery except for doses of protamine sulfate more than 2.5 mg/kg, which was infused over 5 minutes. This was necessary because faster rates of infusion of high concentrations of protamine resulted in decreased blood flow as measured by Doppler ultrasound scan and instability of the animals' vital signs.
We devised the following scoring system to assess thrombosis: animals were given a score of 0 if no occlusion occurred, 1 for a complete occlusion that lasted less than 10 minutes, and 2 for a complete occlusion lasting longer than 10 minutes. aPTTs were assessed at baseline in some of these tested animals prior to the infusion of heparin and either 5 minutes after the intravenous infusion of heparin at 75 U/kg or 20 minutes after the application of the FeCl3 patch.
Statistical analysis
The Student t test was used to assess the significance of the time to first complete occlusion. Experimental groups were compared with the mPF4+/+ group. This group consisted of the sum of the PF4+/+ littermates of the mPF4–/– animals and hPF4+ animals because the time to first occlusion did not differ. Animals that did not form occlusions were excluded from this analysis. To assess for significant differences in the ability of the experimental animals to form stable complete occlusions compared with wild-type animals we used a chi-square test, comparing the number that formed stable occlusions with those that did not. In the infusional carotid artery thrombosis studies, statistical analysis was performed using a Student t test, comparing each treatment dose with the baseline untreated animals and again comparing the number that formed stable occlusions with those that did not.
Results
Generation of mPF4 knock-out mice and transgenic hPF4+ mice
The strategy for creating a mPF4 knock-out mouse involved removal of all 3 mPF4 exons (Figure 1A). Genomic DNA extract from 310 potentially targeted ES cell clones were tested by Southern blot (Figure 1B) and PCR analyses (Figure 1C). A single targeted clone was identified and injected into C57BL/6J blastocysts to generate chimeric mice. Two 90% to 100% chimeric mice produced germ line transmission to the offspring. DNA isolated from the founder line used for biologic studies described in “Further characterization of PF4 in the mPF4– and hPF4+ animals” showed correct targeting by genomic Southern blot (Figure 1D) and PCR (not shown) analyses. Heterozygous mPF4+/– animals were backcrossed 6 times onto a C57BL/6J background. The hPF4+ transgenic animals overexpressing hPF431 were also back-crossed onto a C57BL/6J background for the same number of generations.
Further characterization of PF4 in the mPF4– and hPF4+ animals
The expression of mPF4 message in the mPF4+/– and mPF4–/– platelets was compared with wild-type littermate platelets using RT-PCR from platelet total RNA (Figure 2A). mPF4 message was detectable in the mPF4+/– mice but absent in the mPF4–/– mice. The mPF4+/– platelet RNA appeared to be decreased on repeat studies, but the decrease was not further quantified (but see next paragraph).
PF4 message and protein levels. (A) RT-PCR of mPF4 and mPBP (as a positive platelet control) using total platelet RNA message from mPF4+/+, mPF4+/–, and mPF4–/– animals. (B) Immunoblot using a mPF4-specific primary antibody of equal amounts platelet total proteins from mPF4+/+, mPF4+/–, and mPF4–/– animals (top). A Coomassie blue stain done on a parallel gel confirms equal protein loading (bottom). The arrow points to the mPF4 band. (C) Same as in panel B but for hPF4 using a hPF4-specific primary antibody. WT = wild-type mouse. Human refers to human platelet protein control.
PF4 message and protein levels. (A) RT-PCR of mPF4 and mPBP (as a positive platelet control) using total platelet RNA message from mPF4+/+, mPF4+/–, and mPF4–/– animals. (B) Immunoblot using a mPF4-specific primary antibody of equal amounts platelet total proteins from mPF4+/+, mPF4+/–, and mPF4–/– animals (top). A Coomassie blue stain done on a parallel gel confirms equal protein loading (bottom). The arrow points to the mPF4 band. (C) Same as in panel B but for hPF4 using a hPF4-specific primary antibody. WT = wild-type mouse. Human refers to human platelet protein control.
No PF4 was detected in platelets isolated from mPF4–/– animals as assessed by immunoblotting (Figure 2B). Platelets from mPF4+/– mice contained approximately 50% of the mPF4 found in mPF4+/+ animals in each of 3 separate studies (Figure 2B and data not shown). Previous studies by our group showed that platelets from hPF4+ animals had approximately 3.5 times as much hPF4 as human platelet controls per microgram total platelet protein.31 This excess was maintained after the animals had been backcrossed onto the C57BL/6J background, and the PF4 content was compared with an equal amount of total platelet protein extracted from a human platelet control as measured by immunoblotting (Figure 2C). Also, serum from hPF4+ mice contained 3 to 5 times more hPF4 than human serum (depending on individual mice) as measured by ELISA for hPF4 (data not shown).
Initial analysis of the mPF4–/– and hPF4+ mice
The mPF4–/– and hPF4+ mice were grossly normal in appearance, weight, survival, and fertility. The observed distribution of offspring followed expectations according to Mendelian inheritance. Gross anatomy was normal as well. However, there was an inverse relationship between PF4 levels and mean steady-state platelet counts. The platelet counts varied from 1.40 ± 0.12 × 109/mL in mPF4–/– mice (n = 18, P < .000 02 compared with mPF4+/+) to 1.34 ± 0.18 × 109/mL in mPF4+/– mice (n = 17, P < .033 compared with mPF4+/+), to 1.22 ± 0.13 × 109/mL in the mPF4+/+ animals (n = 24), and to 0.95 ± 0.10 × 109/mL in the hPF4+ mice (n = 17 P < .000 001 compared with mPF4+/+). Other hematologic parameters did not show statistically significant differences, including white and red blood cell counts and hematocrit levels (data not shown).
mPF4–/– and hPF4+ animals did not have a noticeable bleeding diathesis, and there was no difference in tail bleeding times between mPF4+/+ animals and any of the experimental groups (average time to cessation of bleeding, 3.0-3.3 minutes; data not shown). Similarly, whole-blood clotting time measured on blood collected from the vena cava without an anticoagulant were within the normal range in all groups (data not shown) as were the aPTTs (data not shown). Platelets from each group of mice aggregated normally, and secretion was normal in response to high doses of thrombin (0.3-1.0 U/mL), ADP (5-10 μM), collagen (1-5 mg/mL), and PMA (200 nM).
FeCl3 carotid artery injury model
For the most part, the studies of coagulation tested the integrity of separate components of the coagulation reaction in vitro, but these responses may not provide a true reflection of the complex interactions that actually occur at a site of injury, nor the interaction of platelets with an injured vascular bed. To study the propensity of our mice to develop thrombi when provoked, we used the FeCl3 model of vascular injury of the carotid artery40 and measured the time to complete occlusion as well as the stability of the occlusion over a 30-minute period after vascular injury. Thrombi were considered stable if they remained occluded for more than 10 minutes.
Of interest, the time to formation of the initial occlusive thrombus was significantly prolonged in the mPF4–/– (n = 10; 10.6 ± 2.9 minutes; P = .03), the mPF4+/– (n = 10; 10.3 ± 1.9 minutes; P = .03), and the hPF4+ (n = 9; 11.7 ± 3.1 minutes; P = .002) mice compared with mPF4+/+ mice (n = 24; 8.7 ± 1.9 minutes). However, this time to initial occlusion tells only part of the story, because a greater proportion of mPF4–/– and hPF4+ mice either formed an unstable thrombus (Figure 3A, gray box) or formed no occlusive thrombus at all (Figure 3A, white box). Mice that did not form occlusive thrombi were excluded from these calculations. Specifically, whereas more than 85% of the mPF4+/+ mice formed stable occlusive thrombi, less than 50% of the mPF4+/– (P < .005), less than 20% of the mPF4–/–, and less than 25% of the hPF4+ (both P < .0001) mice formed such clots.
FeCl3 carotid artery injury model. (A) The percentage of animals with FeCl3-induced carotid injury that developed a stable occlusive thrombus (▪), transient occlusive thrombus (▦), and no occlusive thrombus are shown. The animals are arranged from no PF4 expression on the left to excess PF4 expression on the right. The number tested of each phenotype is indicated at the bottom (P < .005 mPF4+/– versus mPF4+/+; P < .0001 for mPF4–/– versus mPF4+/+ and hPF4+ versus mPF4+/+). (B) Mean thrombosis score of wild-type and littermate mPF4–/– mice treated with increasing doses of recombinant hPF4 infusion (n = 5 animals at each point). A thrombosis score of 2 = stable occlusive thrombus, 1 = transient occlusive thrombus, and 0 = no occlusive thrombus developed. The zero concentration data points are extrapolated from panel A. Numbers indicate levels of heparin infusion in which occlusive thrombi occurred at a significantly different rate from non–heparin-treated mice of that phenotype (P < .01, Student t test; 1 = mPF4+/+, and 2 = mPF4–/–).
FeCl3 carotid artery injury model. (A) The percentage of animals with FeCl3-induced carotid injury that developed a stable occlusive thrombus (▪), transient occlusive thrombus (▦), and no occlusive thrombus are shown. The animals are arranged from no PF4 expression on the left to excess PF4 expression on the right. The number tested of each phenotype is indicated at the bottom (P < .005 mPF4+/– versus mPF4+/+; P < .0001 for mPF4–/– versus mPF4+/+ and hPF4+ versus mPF4+/+). (B) Mean thrombosis score of wild-type and littermate mPF4–/– mice treated with increasing doses of recombinant hPF4 infusion (n = 5 animals at each point). A thrombosis score of 2 = stable occlusive thrombus, 1 = transient occlusive thrombus, and 0 = no occlusive thrombus developed. The zero concentration data points are extrapolated from panel A. Numbers indicate levels of heparin infusion in which occlusive thrombi occurred at a significantly different rate from non–heparin-treated mice of that phenotype (P < .01, Student t test; 1 = mPF4+/+, and 2 = mPF4–/–).
To demonstrate that the observed instability in the test animals was due to the alterations in platelet PF4 concentration, we infused recombinant hPF4 (rhPF4) into the mPF4+/+ and mPF4–/– animals just prior to the FeCl3 injury. Even at the lowest amount infused (1 mg/kg), the rhPF4 interfered with thrombus formation in the mPF4+/+ mice and at higher doses (2.5 mg/Kg) completely abrogated the formation of occlusive thrombi (Figure 3B). However, this same amount of rhPF4 completely corrected the impaired thrombus formation in the mPF4–/– mice, with all of the tested mPF4–/– mice forming stable occlusive thrombi after infusion of 2.5 mg/kg rhPF4. Of interest, infusion of even slightly lower or higher amounts of hPF4 proved to be far less effective in promoting thrombus formation. For instance, at 2.25 mg/Kg, of the 5 mPF4–/– mice tested, 2 of the 5 mPF4–/– mice tested formed stable occlusions, 2 formed unstable occlusions and 1 did not form an occlusive thrombus. The fact that rhPF4 corrects the thrombotic defect in the mPF4–/– animals supports the concept that this defect is due specifically to deficiency of this protein and that hPF4 can substitute for mPF4.
Interaction of PF4 and heparin, LMWH, or protamine sulfate on thrombus formation
The most thoroughly characterized in vitro biologic effect of PF4 is its ability to bind large negatively charged molecules such as heparin.41-45 We therefore examined the impact of altering platelet PF4 content on the anticoagulant potency of heparin. Varying amounts of heparin were infused intravenously immediately before initiating the FeCl3 injury to achieve concentrations both below and above the concentration used clinically.46 In the mPF4+/+ animals, the amount of heparin needed to interfere with the formation of occlusive arterial thrombi was 50 to 75 U/kg, similar to the amount needed clinically (Figure 4A). Both the mPF4+/– and mPF4–/– animals appeared to require lesser amounts of heparin to block formation of occlusive thrombi. These findings in mPF4+/– and mPF4–/– mice are consistent with an interaction between 2 separate defects in thrombosis, one inherited (PF4 deficiency) and the other acquired (heparin treatment). However, the results in the hPF4+ animals are not consistent with a simple additive effect of 2 coagulation defects. In hPF4 overexpressing animals, which at baseline have a thrombotic defect, infusion of heparin in “therapeutic” amounts (50-75 U/kg) promoted the formation of occlusive thrombi. Occlusive thrombi continued to form in hPF4+ mice until twice the usual therapeutic amount of heparin had been infused (150 U/kg). In contrast, the effect of infused LMWH, which does not bind PF4 well, conformed to a simple additive model of coagulation defects in the mPF4–/–, mPF4+/+, and hPF4+ animals (Figure 4B). Heparin and PF4 are able to neutralize each other, such that in our overexpressor animal model, at therapeutic doses of 50 to 75 U/kg, heparin appears to neutralize the excess PF4 released from activated platelets and allows thrombus formation to precede. The anticipated anticoagulant effect of heparin was again seen when infused at concentrations of 125 U/kg or more.
Effects of heparin, LMWH, and protamine sulfate infusions on thrombus formation. Thrombus formation after FeCl3 carotid artery injury preceded by (A) heparin infusion, (B) LMWH (enoxaparin), or (C) protamine sulfate infusion. The thrombosis score is as in Figure 3B. Numbers indicate levels of heparin infusion in which occlusive thrombi occurred at a significantly different rate than in non-heparin-treated mice of that phenotype (P < .05, Student t test; heparin: 1 = mPF4+/+ , 2 = mPF4+/– [▵], 3 = mPF4–/– ⋄, and 4 = hPF4+ [▪]).
, 2 = mPF4+/– [▵], 3 = mPF4–/– ⋄, and 4 = hPF4+ [▪]).
Effects of heparin, LMWH, and protamine sulfate infusions on thrombus formation. Thrombus formation after FeCl3 carotid artery injury preceded by (A) heparin infusion, (B) LMWH (enoxaparin), or (C) protamine sulfate infusion. The thrombosis score is as in Figure 3B. Numbers indicate levels of heparin infusion in which occlusive thrombi occurred at a significantly different rate than in non-heparin-treated mice of that phenotype (P < .05, Student t test; heparin: 1 = mPF4+/+ , 2 = mPF4+/– [▵], 3 = mPF4–/– ⋄, and 4 = hPF4+ [▪]).
, 2 = mPF4+/– [▵], 3 = mPF4–/– ⋄, and 4 = hPF4+ [▪]).
We then asked whether the increased thrombosis in hPF4+ animals after heparin infusion involved the loss of the systemic effects of the infused heparin caused by the excess of locally released PF4. To address this question, we measured aPTTs in mPF4+/+ and hPF4+ animals prior to and 20 minutes after heparin infusion. Both experimental groups had a normal aPTT (mPF4+/+ = 35 ± 4 seconds and hPF4+ = 32 ± 9 seconds, n = 4 each) prior to heparin infusion, and after infusion of 75 U/kg heparin, the aPTT was prolonged to a comparable extent in both groups (both > 300 seconds, n = 4, each). The same results were obtained when heparin was infused and the aPTT was measured 20 minutes after the initiation of carotid artery damage by FeCl3 (both > 300 seconds, n = 4, each). Thus, we observed a clear discrepancy between a systemic prolongation of the aPTT caused by the circulating heparin and heparin's paradoxic effect on thrombus formation at the site of injury in the hPF4+ mice.
As an independent approach to examine whether the effect of PF4 can be explained by neutralization of anionic heparin, we next examined the effect of infusing protamine sulfate, a positively charged molecule that can neutralize heparin47,48 and that in experimental models can compete with PF4 for binding to endothelial cell surfaces.49 The anticoagulant effect of intravenously administered protamine sulfate prevented occlusive thrombi from forming in the mPF4+/+ animals at a dose of 1.0 to 1.5 mg/kg (Figure 4C). Paradoxically, protamine sulfate actually promoted thrombus formation in mPF4+/– and mPF4–/– animals at a dose of 1.0 to 1.5 mg/kg and 1.5 to 3.0 mg/kg, respectively. As we observed with infused rhPF4, promotion of thrombus formation in the mPF4–/– animals occurred only over a narrow range, with loss of its thrombotic effect when higher or lower doses were infused. In contrast, the hPF4+ animals were very sensitive to protamine sulfate infusion, with complete mitigation of thrombus formation even at the lowest dose tested (0.25 mg/kg).
Effect of PF4 on low-dose agonist platelet aggregation studies in vitro
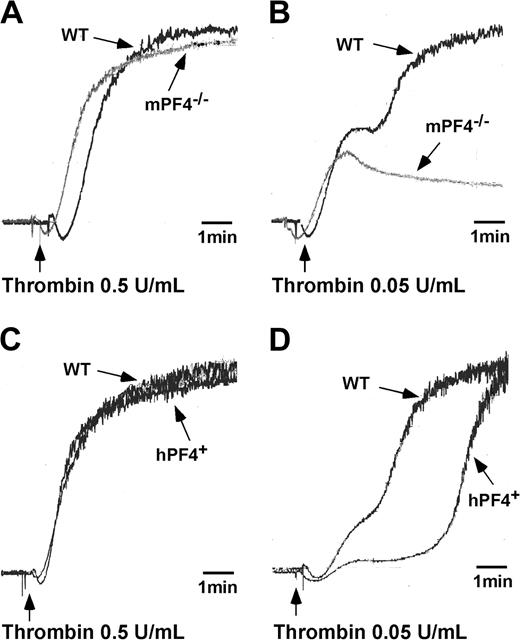
The in vivo thrombosis data would suggest that other aspects of platelet biology should also be impaired in these mice. Specifically, we would have expected impaired platelet aggregation, but platelets isolated from both PF4 knock-out and overexpressor mice respond normally to high concentrations of agonists. We therefore repeated these studies at lower concentrations of agonists, focusing on response to low concentrations of thrombin (0.03-0.1 U/mL). Aggregation of mPF4–/– and hPF4+ washed platelets was normal after addition of 0.5 U/mL thrombin (Figure 5A and C, respectively; n = 3) but was impaired in comparison to littermate WT platelet controls when stimulated by adding 0.05 U/mL thrombin (Figure 5B and D, respectively; n = 3). No second wave of platelet aggregation was observed when platelets isolated from mPF4–/– animals were stimulated with 0.04 to 0.05 U/mL thrombin (data not shown with an n = 3). In contrast, platelets isolated from hPF4+ animals aggregated at low concentrations of thrombin, but the second wave was significantly delayed compared with WT platelets.
Low-dose agonist aggregation of platelets isolated from mPF4–/–, hPF4+, and WT mice. Platelets isolated from mPF4–/– and littermate WT animals were stimulated by the addition of either a high (0.5 U/mL) (A) or low (0.05 U/mL) concentration of thrombin (B). Platelets isolated from hPF4+ and littermate WT animals were stimulated by adding either a high (0.5 U/mL) (C) or low (0.05 U/mL) concentration of thrombin (D). Aggregations were performed at 37°C with continuous stirring, and light transmission was continuously recorded. Data presented are representative of 3 sets of studies with similar outcomes.
Low-dose agonist aggregation of platelets isolated from mPF4–/–, hPF4+, and WT mice. Platelets isolated from mPF4–/– and littermate WT animals were stimulated by the addition of either a high (0.5 U/mL) (A) or low (0.05 U/mL) concentration of thrombin (B). Platelets isolated from hPF4+ and littermate WT animals were stimulated by adding either a high (0.5 U/mL) (C) or low (0.05 U/mL) concentration of thrombin (D). Aggregations were performed at 37°C with continuous stirring, and light transmission was continuously recorded. Data presented are representative of 3 sets of studies with similar outcomes.
Discussion
Megakaryocytes express a number of chemokines, including PF4.50 These proteins are sequestered within platelet α-granules and are secreted at high concentrations at sites where platelets are activated. Several of these chemokines have been implicated in both inflammation and atherosclerosis, but the in vivo biologic function of PF4 has remained unclear. Diverse and seemingly conflicting potential biologic roles have been attributed to PF4 based on in vitro observations. Using genetically modified mice that either lack or overexpress PF4, we now clearly show that PF4 contributes to the evolution of arterial thrombosis.
Mice with either an excess or a deficiency of PF4 had no obvious bleeding diathesis and survived normally into adulthood with no bleeding complications. In vitro studies of plasma coagulation were normal, as were in vivo tail bleeding times. However, the tail bleeding time assay has proven to be an insensitive measure of in vivo defects in platelet biology and can miss significant in vivo thrombotic defects evident in the carotid artery FeCl3 injury model.34 We felt that this model, which depends on the development of a platelet-rich thrombus, might provide a more sensitive and relevant measure of the effects of PF4 during thrombosis. Our data show that thrombus formation and stability are maximal at physiologic levels of PF4; fewer occlusive thrombi and impaired thrombus stability were observed when platelet PF4 levels fell by even 50% or when it exceeded the normal level by less than 4-fold. This conclusion was affirmed by studies in which infusion of recombinant hPF4 into mPF4–/– mice maximized thrombus formation and stability only over a very narrow range of concentrations.
It should be pointed that, as described in “Results,” underexpression and overexpression of PF4 in mice led to increased and decreased platelet counts, respectively. Although such differences could be due to changes in platelet production or their half-life, this observation is in agreement with in vitro studies by our group and others, indicating that PF4 can inhibit megakaryopoiesis.51,52 The platelet count varied by 15% to 30% above (mPF4–/–) or below (hPF4+) that of the mPF4+/+ animals. This variation did not interfere with the FeCl2-induced thrombus formation as normal occlusion could be observed in both hPF4+ and mPF4–/– mice after either injection of 50 U/kg heparin or 2.5 mg/kg rhPF4, respectively.
We propose a simple model to explain the role of PF4 in thrombosis, accounting for our observations after infusions of unfractionated high-molecular-weight heparin, LMWH, and protamine sulfate. This model is based on the known high affinity of PF4 for negatively charged molecules such as heparin and heparan-sulfate glycoproteins that are expressed on cell surfaces. We propose that release of PF4 in large molar amounts neutralizes the negatively charged surfaces of platelets and endothelial cells, allowing closer approximation of platelets to each other and to the endothelial lining, thereby enhancing thrombus formation. Certainly, the existence of electric field differences between the endothelial lining and flowing blood has been previously recognized.53 When insufficient PF4 is released, cell surfaces may retain sufficient negative charges to prevent optimal approximation of platelets and endothelial cells. Conversely, release of excessive amounts of PF4 may cause cell surfaces to become too positively charged for optimal cell-cell approximation.
Our studies with infusions of heparin and protamine sulfate in the setting of FeCl3-induced carotid arterial injury are consistent with this model. The development of thrombi in mPF4+/– and mPF4–/– mice was especially sensitive to heparin infusion (ie, thrombus development was prevented at lower doses of heparin than was required in mPF4+/+ animals). We postulate that less heparin is needed to neutralize the PF4 released in these PF4-deficient animals. More telling, infusion of comparable amounts of heparin enhanced thrombus formation in the hPF4+ overexpressors by neutralizing excess PF4. The converse was observed with protamine sulfate infusions, a positively charged molecule that, similar to PF4, neutralizes large negatively charged molecules such as heparin.54 Protamine sulfate appears to be able to substitute for PF4 to enhance thrombus formation in the mPF4–/– mice. Consistent with the proposed model, hPF4+ mice are particularly sensitive to the antithrombotic effect of protamine sulfate. The absence of a comparable paradoxic effect of LMWH infusion in hPF4 mice is consistent with the proposed model because LMWH has a decreased binding affinity of PF4.42
Also consistent with this model are the results of the platelet aggregations induced by low concentrations of thrombin. When mPF4–/– platelets are stimulated with low concentrations of agonist, we postulate that the remaining excess negative charges prevent optimal aggregation. In the case of hPF4+ platelets, the release of higher then normal levels of positively charged PF4 also prevents optimal approximation of platelets.
Although the results of our studies are consistent with the proposed model, these results may also be consistent with other models. PF4 has been reported not only to inhibit antithrombin III activation by heparin (thus being prothrombotic)55 but also to enhance the activation of protein C (thus being antithrombotic).28,29 If these and perhaps other undefined mechanisms are of biologic relevance, then these competing functions of PF4 may help explain why PF4 promotes clot formation only over a narrow range of concentrations and why both higher or lower PF4 concentrations have similar biologic outcomes. Additional studies in mice with alteration in ATIII and/or the capacity to activate protein C will provide additional insights into their influence on PF4 during thrombosis.
Although the underlying mechanism by which PF4 affects thrombosis remains unclear, several important implications emerge from our findings. First, our data would suggest that patients with either an excess or deficiency of platelet PF4 may have impaired thrombus formation. To date, we are unaware of any studies in which the range of PF4 concentrations present in the healthy population has been examined in detail. Our studies would suggest that this range is likely to be quite narrow. Our studies would also suggest that the mild bleeding diathesis seen in patients with storage pool deficiencies may be due, in part, to a deficiency in PF4.
A second implication of our findings is that the capacity of heparin to neutralize PF4 may be as important as its capacity to inhibit antithrombin III (as assessed by the aPTT). We observed that, although the aPTT is a good indicator of antithrombotic activity at normal platelet PF4 levels, it was nonpredictive of thrombotic propensity in the FeCl3 injury model at low or high platelet PF4 levels. The paradoxic enhancement of thrombosis by heparin in the hPF4+ mice may be of importance in understanding the high risk of thrombus development in heparin-induced thrombocytopenia (HIT). Thrombi typically develop in patients who are receiving heparin in amounts designed to prolong the aPTT. HIT and thrombosis (HITT) develop most commonly in patients with atherosclerosis, after cardiopulmonary bypass or after surgical procedures, settings associated with platelet activation and release of PF4.56,57 Thus, this situation may simulate that seen in the FeCl3 injury model in hPF4+ mice receiving heparin infusions at 50 to 75 U/kg (Figure 4A). Additionally, whether HITT is more likely to develop in patients whose platelets contain or secrete more PF4 is an unanswered question. A similar dissociation between the systemic effect of heparin on systemic measures of coagulation such as the aPTT and a local PF4-dependent enhancement of arterial occlusion may also occur in other disorders associated with intense local platelet activation, including thrombotic thrombocytopenic purpura and acute disruption of atherosclerotic plaques. If excess local PF4 release is contributing to the high incidence of thrombosis seen in HIT, then our studies also suggest that thrombus formation could be decreased by using compounds such as protamine sulfate, even when infused at less than standard therapeutic doses. Whether such a strategy would be useful in the treatment of HIT to avoid thrombosis is presently being pursued in a murine model of this disease.58
Prepublished online as Blood First Edition Paper, February 5, 2004; DOI 10.1182/blood-2003-11-3994.
Supported in part by grants from the American Heart Association (AHA; grant 0255255N, M.A.K.) and the National Heart, Lung, and Blood Institute (NHLBI; grants HL68631, HL54749, and HL54500, M.P.).
Dr Stefan Niewiarowski died on August 25, 2001.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Tammy Kang of the Children's Hospital of Philadelphia for her advice on the statistical analysis in this manuscript and Dr Katya Ravid for generously providing rat PF4 promoter sequence that was useful in cloning the mouse PF4 gene. The help of Dr Valder R. Arruda in setting up aPTT measurements is also greatly appreciated. This paper is dedicated to the memory of Dr Stefan Niewiarowski who was instrumental in the origin of these studies.



![Figure 4. Effects of heparin, LMWH, and protamine sulfate infusions on thrombus formation. Thrombus formation after FeCl3 carotid artery injury preceded by (A) heparin infusion, (B) LMWH (enoxaparin), or (C) protamine sulfate infusion. The thrombosis score is as in Figure 3B. Numbers indicate levels of heparin infusion in which occlusive thrombi occurred at a significantly different rate than in non-heparin-treated mice of that phenotype (P < .05, Student t test; heparin: 1 = mPF4+/+ , 2 = mPF4+/– [▵], 3 = mPF4–/– ⋄, and 4 = hPF4+ [▪]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2003-11-3994/6/m_zh80220469490004.jpeg?Expires=1767740283&Signature=SuGWFhmsoopkOu5K~UfaJL4siLmFiqwOLxfjBp1fEo~WZxSHgpsUUZuCkaaMxrEJn6ttRs52aAGWBPxOvwNC1W20gqD3j1juxA4DBVjy9qidtV0PBOk5KxiOsw2QQI1Ug19oSHYnHR5ZEDjgEQ1GER~DPZPDVqzx8QHaiwxui6q6LmBMW7RtIX6EPvuBYymFacZIDFcPNVfLaKUoRbYi5Gz1LbGAQiC8QDYPjJ0vAeGLV6SO~JdVaVExypfS7cDVPxDAJGxdVfWuVoB4q1zg2BqmwGzhxoQDo6Oua2dpSAtnCXJGDsOWhdZ~I-4T0VCkCA~HlHGLVpAL2GeYoUOKCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal