Abstract
A proliferation-inducing ligand (APRIL) is a new tumor necrosis factor family member implicated in tumor cell proliferation. We investigated the role of APRIL in megakaryocytopoiesis, a developmental hematopoietic process responsible for progenitor cell differentiation to megakaryoblasts and megakaryocytes, leading to platelet formation. APRIL is not expressed in CD34+ progenitor cells from healthy donors, but it is massively up-regulated during the proliferative phase of megakaryocytic cell differentiation. Exogenous APRIL expression in primary cells increases megakaryocytic cell growth, suggesting that APRIL acts as a proliferative factor in megakaryocytopoiesis. More importantly, neutralization of endogenous APRIL was able to dramatically reduce megakaryocyte expansion and platelet production. Thus, our data provide evidence that APRIL acts as a growth factor for terminal megakaryocytopoiesis and may promote physiologic platelet production.
Introduction
The production of mature megakaryocytes (MKs) from multipotent hematopoietic progenitor cells requires the coordinated activity of different cytokine signaling pathways to ensure controlled cell proliferation, survival, and differentiation.1-3
Thrombopoietin (TPO) is a major hematopoietic growth factor that supports early hematopoietic cells and all the developmental stages of megakaryocytopoiesis.4,5 Several other cytokines can promote MK growth and platelet production in physiologic or pathologic circumstances, including stem cell factor (SCF), interleukin 1 (IL-1), IL-3, IL-6, and IL-11.1,2 Increased platelet production is observed in essential thrombocythemia and during inflammation, as the inflammatory environment promotes the formation of MKs.2 The tumor necrosis factor (TNF) family is a group of cytokines involved in the regulation of cell death, proliferation, activation, and differentiation. Some members of the TNF family bind and activate death receptors, a group of surface proteins able to transduce apoptotic signals on ligand binding.6-8 Death receptor ligands are likely to inhibit the megakaryocytopoietic process, as shown by the ability of CD95 (Fas/APO-1) ligand to block MK maturation through the specific targeting of key developmental transcription factors.9 Recently, a proliferation-inducing ligand (APRIL) has been identified as a new member of the TNF family proteolytically processed in the Golgi compartment by a furin convertase and released as a soluble form.10,11 Furin is involved in the maturation of growth factors and integrins synthesized by MKs and appears as a key enzyme for megakaryocytopoiesis and thrombocytopoiesis.12,13 APRIL mRNA levels are reportedly low in normal tissues. In contrast, high mRNA levels have been detected in tumor cell lines and in several primary tumor tissues.14-16 Such expression has been proposed to promote cancer cell survival and proliferation because exogenous expression of APRIL increased tumor cell growth in vitro and in vivo.10 Although APRIL can bind to B-cell maturation antigen (BCMA) and transmembrane activator and calcium modulator and cyclophillin ligand (CAML) interactor (TACI) receptors, the protumor effect of this cytokine seems mediated by at least one additional, yet unidentified, receptor.15 Similarly, recent evidence supports the presence of a functional receptor, different from BCMA and TACI, in fibroblast and epithelial cell lines.10,15 The existence of another functional receptor is further corroborated by the inability of BLys/BAFF, another member of the TNF family capable of binding BCMA and TACI, to promote the proliferation of APRIL-sensitive tumor cells.14,15,17
We speculate that the ability of APRIL to act as autocrine growth factor was not confined to tumor cells. Therefore, we investigated APRIL expression and function in hematopoiesis. Here, we show that MKs express considerable levels of APRIL and that targeting endogenous APRIL production severely affects MK proliferation and platelet generation.
Study design
Cell cultures and gene transfer studies
CD34+ cells were purified from cord blood samples by Ficoll gradient centrifugation and 3 rounds of microbead sorting (Miltenyi Biotec, Bergish-Gladbach, Germany). Megakaryocytic unilineage cultures were obtained by cultivating CD34+ cells in serum-free medium supplemented with 100 ng/mL TPO.18 Platelet production was evaluated as previously described.19
APRIL and BLyS cDNAs were cloned into the retroviral vector, PINCO-GFP (green fluorescent protein).20 CD34+ cells were subjected to 6 infection cycles and placed in growth medium supplemented with cycling growth factors (IL-3, 100 U/mL; SCF, 100 ng/mL; Flt3 100 ng/mL) for 48 hours before sorting. GFP+ cells were separated by flow cytometry and cultivated in serum-free medium containing 100 ng/mL TPO to obtain virtually pure transduced MK populations.
RT-PCR and immunoblot analysis
cDNA amplification was obtained by 35 polymerase chain reaction (PCR) cycles. PCR products were verified by sequencing, analyzed by Southern blotting, and normalized by amplification of the S26 gene. APRIL forward primer: 5′TAAAAAGTGGCTCCCAGCTT3′; reverse primer 5′GGTGGGAAT-GAAAAGGGAAA3′; probe: 5′CATCTTCCTGGGTTTGGCTCCCCGTTC-CTC3′. BCMA forward primer: 5′TTTCTTTGGCAGTTTTCGTG3′; reverse primer: 5′GATGCAGTCTTCACAGGTGC3′; probe: 5′GGTCTCCTGGGCAT-GGCTAACATTGACCTG3′. TACI forward primer: 5′CCAAGGATTGGAG-CACAGA3′; reverse primer: 5′GGCAGGCACACACACAAT3′; probe: 5′GCCCCGCTCAAGGCCCCGTCAAAGTCAAAGTCCGGC3′.
Real-time reverse transcription–PCR (RT-PCR) was performed by TaqMan technology, using the ABI PRISM 7700 DNA sequence detection system (Applied Biosystems, Foster City, CA). Thermal cycling conditions included 40 cycles at 95°C for 15 seconds and at 60°C for 1 minute. Original input RNA amounts were calculated with relative standard curves for APRIL and glyceraldehyde phosphate dehydrogenase (GAPDH) RNA. Gene expression values were reported as the normalized percentage derived by dividing the copy numbers of APRIL by GAPDH, using cells transduced with APRIL cDNA as reference control. Commercial ready-to-use primers/probe mixes were used (Assay-on-Demand Gene Expression products, Hs00601664_g1; Applied Biosystems).
Protein extracts were prepared by resuspending cell pellets in 1% nonidet P-40 (NP40) lysis buffer. Equal amounts of proteins were analyzed by standard immunoblot procedure. The rabbit polyclonal anti-APRIL antibody was produced as previously described11 and used at 5 μg/mL. Anti-BLyS polyclonal rabbit antibody (BD PharMingen, Los Angeles, CA) was used at a dilution of 1:1000.
Cell surface binding of APRIL was assessed by incubating 2 × 105 cells with 100 ng/mL Flag-APRIL (APRIL MEGA ligand; Alexis, Lausen, Switzerland) for 1 hour at 37°C. After incubation, cells were washed 3 times in phosphate-buffered saline (PBS) and APRIL receptor expression was evaluated by immunofluorescence staining using anti–Flag-M2 (Sigma, St Louis, MO) and a fluorescein isothiocyanate (FITC)–conjugated antimouse IgG antibody (Molecular Probes, Eugene, OR).
Results and discussion
The megakaryocytic unilineage liquid culture system recapitulates physiologic megakaryocytopoiesis and allows the study of different steps of the differentiation process.18
In TPO-supplemented culture of cord blood CD34+ cells, morphologic evaluation indicates the presence of putative mononuclear megakaryoblasts at day 2 to 3, then at day 10 to 12 cells mature in binuclear and polynucleated MKs and begin to produce platelets.19
We analyzed megakaryocytic cells at various differentiation stages for the expression of APRIL and its receptors. Flow cytometry analysis showed that APRIL binding to its putative receptors was absent in CD34+ cells, but gradually increased during MK differentiation (Figure 1A). Similarly, APRIL mRNA was not present in CD34+ cells or in day 3 megakaryoblasts but was easily detectable from day 5 until the end of the culture, reaching considerably high levels around day 9 (Figure 1B-C). Moreover, immunoblot analysis revealed the presence of both full-length and soluble APRIL in MKs (Figure 1D). In contrast, we were not able to detect TACI or BCMA mRNA at any stage of megakaryocytic differentiation (Figure 1B), suggesting that APRIL binding to MKs depends on the presence of a different receptor.
Expression of APRIL and its receptors during MK differentiation. (A) Flow cytometry analysis of cord blood CD34+ cells undergoing unilineage MK differentiation (from day 3 to day 14) labeled with recombinant Flag-APRIL (open histograms) as compared with staining controls (black histograms). Jurkat and 293 cells were used as positive and negative controls, respectively. (B-C) Semiquantitative RT-PCR (B) and real-time (C) analyses of CD34+ and differentiating megakaryocytic cells. Peripheral blood mononuclear cells (PBMCs) and hematopoietic progenitors transduced with empty vector (PINCO) or with APRIL cDNA (APRIL) were used as controls. Error bars indicate ± standard deviation (SD). (D) Immunoblot analysis of APRIL expression in CD34+ and differentiating megakaryocytic cells. 293 cells transfected with empty vector (P) or with APRIL cDNA (APRIL) were used as controls.
Expression of APRIL and its receptors during MK differentiation. (A) Flow cytometry analysis of cord blood CD34+ cells undergoing unilineage MK differentiation (from day 3 to day 14) labeled with recombinant Flag-APRIL (open histograms) as compared with staining controls (black histograms). Jurkat and 293 cells were used as positive and negative controls, respectively. (B-C) Semiquantitative RT-PCR (B) and real-time (C) analyses of CD34+ and differentiating megakaryocytic cells. Peripheral blood mononuclear cells (PBMCs) and hematopoietic progenitors transduced with empty vector (PINCO) or with APRIL cDNA (APRIL) were used as controls. Error bars indicate ± standard deviation (SD). (D) Immunoblot analysis of APRIL expression in CD34+ and differentiating megakaryocytic cells. 293 cells transfected with empty vector (P) or with APRIL cDNA (APRIL) were used as controls.
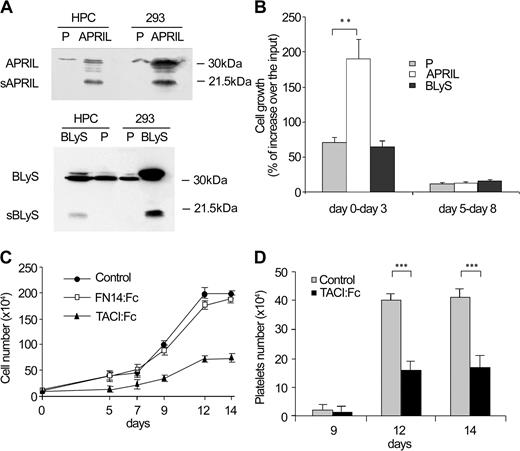
The expression of APRIL in megakaryoblasts prompted us to investigate whether APRIL could influence the growth of megakaryocytic cultures. We therefore transduced cord blood CD34+ progenitor cells with a retrovirus carrying the APRIL cDNA and GFP as reporter gene, so that the positive cells could be sorted by fluorescence-activated cell sorting. Both the empty vector and a vector containing the cDNA of BLyS/BAFF were transduced as negative controls. Expression of exogenous genes in GFP+ cells was confirmed by immunoblot analysis (Figure 2A). Sorted cells were cultivated in MK unilineage medium for 8 days because the previous exposure to the cycling factors during retroviral transduction reduces the time required for the complete maturation of hematopoietic precursors.9 Megakaryocytic culture quantification revealed a significantly increased expansion of APRIL-transduced cells during the proliferative phase as compared with the control populations, whereas during the late differentiation period the differentially transduced cells showed a comparable modest increase in number (Figure 2B). Thus, exogenous APRIL promotes the growth of megakaryocytocytic cells during their expansion phase.
APRIL promotes MK expansion and platelet production. (A) Cycling cord blood CD34+ cells were transduced with empty vector (P), with APRIL (APRIL) or BLyS (BLyS) cDNA and analyzed by immunoblot for APRIL and BLyS expression after cytofluorimetric sorting based on GFP+ cells. 293 cells transfected with empty vector (P) or with APRIL (APRIL) or BLyS (BLyS) cDNA were used as controls. (B) Cell growth of cells transduced and sorted as described in panel A and cultured in unilineage MK system from day 0 to day 3 and from day 5 to day 8. (C) Cell growth of cord blood CD34+ cells cultured in standard unilineage MK system (control) or in the presence of TACI-Fc or Fn14-Fc recombinant soluble proteins (Alexis). (D) Platelet production from unilineage MK culture or in the presence or in the absence of recombinant soluble TACI-Fc. Error bars indicate ± SD. *P < .01, **P < .001.
APRIL promotes MK expansion and platelet production. (A) Cycling cord blood CD34+ cells were transduced with empty vector (P), with APRIL (APRIL) or BLyS (BLyS) cDNA and analyzed by immunoblot for APRIL and BLyS expression after cytofluorimetric sorting based on GFP+ cells. 293 cells transfected with empty vector (P) or with APRIL (APRIL) or BLyS (BLyS) cDNA were used as controls. (B) Cell growth of cells transduced and sorted as described in panel A and cultured in unilineage MK system from day 0 to day 3 and from day 5 to day 8. (C) Cell growth of cord blood CD34+ cells cultured in standard unilineage MK system (control) or in the presence of TACI-Fc or Fn14-Fc recombinant soluble proteins (Alexis). (D) Platelet production from unilineage MK culture or in the presence or in the absence of recombinant soluble TACI-Fc. Error bars indicate ± SD. *P < .01, **P < .001.
To determine whether the production of endogenous APRIL contributes to the megakaryopoietic process, cord blood CD34+ cells were grown in MK unilineage culture in the presence of soluble human recombinant TACI-Fc fusion protein, which binds APRIL and neutralizes its proliferative activity. As shown in Figure 2C, exposure to TACI-Fc fusion protein dramatically reduced the growth of megakaryocytic cells, whereas the Fn14-Fc fusion protein that interacts with TWEAK, another member of the TNF family, was ineffective. Similarly, the addition of TACI-Fc fusion protein in the culture medium from day 5 resulted in a significant decrease of platelet production (Figure 2D), confirming the contribution of autocrine APRIL production to megakaryocytopoiesis. On exposure to TACI-Fc, we did not observe a significant increase in apoptosis nor a decreased production of platelets per single MK (results not shown). Thus, the contribution of APRIL on megakaryocytopoiesis seems to be the result of a direct increase in MK proliferation.
Data presented here indicate that APRIL acts as an autocrine growth factor for megakaryocytic cells. During in vitro differentiation, APRIL is expressed from the promegakaryocytic stage until terminal maturation. The strong reductions of MK growth and platelet production obtained after neutralization of endogenous activity suggest that APRIL may promote the physiologic megakaryocytopoiesis.
Although original reports have focused their attention on the role of APRIL in tumorigenesis, APRIL expression has been found in monocytes and dendritic cells.21,22 Moreover, recent data indicate that APRIL modulates B- and T-cell immunity, 14,22,23 suggesting that APRIL may act on different hematopoietic cells.
BCMA and TACI expression appears to be restricted to B lymphocytes. Accordingly, no expression of TACI or BCMA could be detected in MKs. Therefore, we conclude that APRIL binding and growth signaling in MKs are mediated by another unknown receptor, as supposed by different authors in other systems.15,17 Different cytokines can increase MK growth and platelet production in concert with TPO. These promegakaryocytic factors are generally produced by other cell types, such as leukocytes, fibroblasts, and bone marrow stroma cells. However, MKs can produce cytokines that promote their differentiation program, such as platelet-derived growth factor and von Willebrand factor.24 Moreover, we have recently shown that autocrine-paracrine vascular endothelial growth factor (VEGF) loops can increase MK polyploidization through the stimulation of Flt1 receptor.25 The ability of endogenous APRIL to contribute to MK expansion suggests that it can constitute a major autocrine growth factor for megakaryocytopoiesis. Thus, it is likely that the activity of self-produced cytokines is not restricted to the accomplishment of the MK differentiation program but may play a considerable role in MK growth, eventually contributing to determine the amount of platelet production.
Prepublished online as Blood First Edition Paper, April 22, 2004; DOI 10.1182/blood-2003-11-3861.
Supported by funding from the Italian Association for Cancer Research (AIRC) and Association of International Cancer Research (AICR).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Paola Di Matteo, Stefano Guida, Gualtiero Mariani, and Giuseppe Loreto for their contribution to the paper.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal