Comment on Xia et al, page 3091
In this issue, Xia and colleagues report a novel approach toward enhancing the homing and engraftment of human umbilical cord blood–derived hematopoietic stem and progenitor cells (CB-HSPCs), using targeted “glycosylation engineering” of their cell surfaces.
When the selectins were first discovered, attention was focused mostly on their functions in the trafficking of mature leukocytes and on the nature of their ligands (certain glycoproteins that usually carry a sialic acid and fucose-containing glycan unit called sialyl Lewis x).1 Studies of mice null for selectins2 or for the fucosyltransferases responsible for creating selectin ligands3 eventually provided clues that the selectins also had an impact on hematopoiesis, apparently by facilitating interactions of hematopoietic stem and progenitor cells (HSPCs) with bone marrow endothelium.4 FIG1
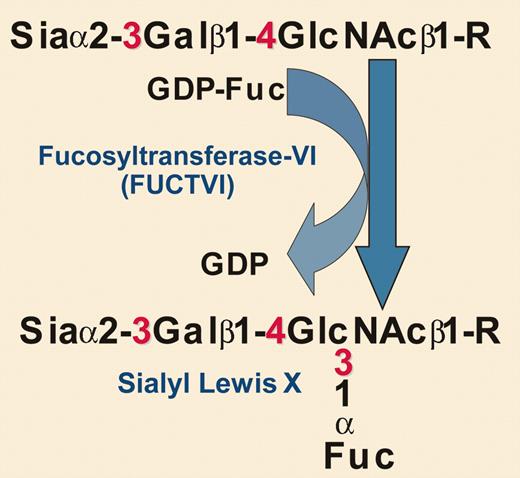
Biochemical details of the glycosylation engineering reaction used by Xia et al. Sia indicates sialic acid; Gal, galactose; GlcNAc, N-acetylglucosamine; Fuc, fucose; and R, glycoprotein.
Biochemical details of the glycosylation engineering reaction used by Xia et al. Sia indicates sialic acid; Gal, galactose; GlcNAc, N-acetylglucosamine; Fuc, fucose; and R, glycoprotein.
Xia and colleagues noted that the poor homing of cord blood (CB)–HSPCs to the bone marrow of immunodeficient mice correlated with low levels of cell surface sialyl Lewis x. In yet another example of how fundamental research can translate into practical applications, the authors took an approach pioneered by Hill and coworkers many years ago—using recombinant soluble glycosyltransferases and high-energy sugar nucleotide donors to modify glycosylation directly on cell surfaces.5 In this case, the authors used an α1-3–specific fucosyltransferase along with the high-energy fucose donor guanosine diphosphate (GDP) fucose to increase the amount of cell surface sialyl Lewis x (see figure). This approach effectively augmented CB-HSPC binding to P-selectin and E-selectin, with evidence for a major contribution by P-selectin glycoprotein ligand-1 (PSGL-1). In doing so, the authors basically mimicked on the cell surface of CB-HSPCs an enzymatic reaction that normally occurs inside the cell (within the lumen of the Golgi apparatus) prior to the delivery of newly synthesized glycoproteins to the cell surface. While the change they make on the cell surface is transient, it lasts long enough to enhance initial homing (and eventually engraftment).
As the authors acknowledge, there are differences between humans and mice with regard to the fine details of the system. Thus, the enhanced engraftment of human CB-HSPCs to the bone marrow of immunodeficient mice that they demonstrate may or may not be recapitulated in a human transplant recipient. Since the reagents and methodology they use are simple and straightforward, one hopes that this recapitulation can be attempted in the near future.
Of note, another recent example of cell-surface glycosylation engineering with potential impact on clinical hematology involves using uridine diphosphate (UDP) galactose and a β1-4–specific galactosyltransferase to add galactose residues to platelet cell surfaces, a procedure that helps alleviate the well-known problem of loss of platelet bioavailability if the platelets are chilled before transfusion.6
Supported by USPHS grant CA38701.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal