Comment on Palumbo et al, page 3052
The role of high-dose therapy in the management of multiple myeloma in patients older than 65 years has, to date, been uncertain. The current article establishes both survival benefit and improved response duration in patients up to the age of 70 years with multiple myeloma.
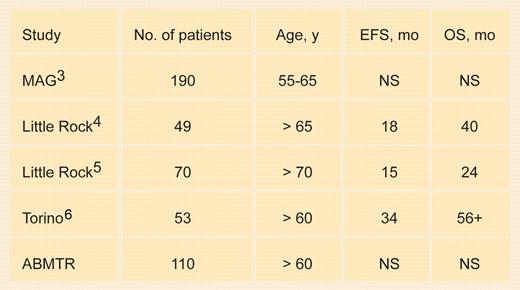
There are 2 studies that have demonstrated the survival advantage of high-dose therapy compared with conventional chemotherapy. In the first of these studies, sponsored by the Intergroupe Francophone du Myélome (IFM),1 patients' median age was 57 years and all were younger than 65 years. In the Medical Research Council (MRC)2 study, the median age was 55 years, and the oldest patient entered was 66 years. These studies do not help make an evidence-based decision on the value of high-dose therapy in patients older than 65 years. In fact, the Myélome-Autogreffe Group (MAG)3 reported patients aged 55 to 65 years where high-dose therapy produced no improvement in event-free or overall survival compared with conventional therapy.FIG1
Transplantation in older patients. EFS indicates event-free survival; OS, overall survival; ABMTR, American Bone Marrow Transplant Registry; and NS, not significant compared with younger patients.
Transplantation in older patients. EFS indicates event-free survival; OS, overall survival; ABMTR, American Bone Marrow Transplant Registry; and NS, not significant compared with younger patients.
A number of retrospective reports on the outcomes following stem cell transplantations in older patients are given in Table 1. The treatment-related mortality in patients older than 65 years is 8% and event-free and overall survival appear shorter in older patients. Most centers, due to high treatment-related mortality, have reduced the conditioning dose of melphalan in older patients to 140 mg/m2. Palumbo (see “Torino” in the table) reported a case-control series of patients receiving 100 mg/m2 melphalan with a contemporaneous group of patients receiving standard therapy and demonstrated a superior event-free and overall survival for the high-dose therapy group. A report from the ABMTR analyzing patients younger than and older than age 60 years showed no difference in outcome. These studies, which establish the safety and feasibility of stem cell transplantation in the elderly, however, do not demonstrate the superiority of high-dose therapy.
An attempt to improve the results with conventional dose therapy was reported in IFM trial 9501. There were 489 patients aged 65 to 75 years randomized to melphalan and dexamethasone; dexamethasone; dexamethasone and interferon; and standard melphalan and prednisone. Dexamethasone-based regimens did not improve overall survival for patients older than 65 years.
In the current issue of Blood, Palumbo and colleagues report patients aged 50 to 70 years who were randomized to standard melphalan/prednisone versus tandem cycles of high-dose melphalan at 100 mg/m2; 46% were older than 65 years. Strictly defined near-complete responses were seen in 25% of patients aged 65 to 70 years. The event-free and overall survival rates for the high-dose group were superior. Patients aged 65 to 70 years had a median survival of 58 months compared with 37 months for patients on standard therapy. This randomized study supports using high-dose therapy for patients older than 65 years.
For patients older than 65 years in the United States, Medicare does not reimburse for tandem cycles of therapy, so it would be difficult to follow this exact protocol. Perhaps a single cycle of therapy at 140 mg/m2 will produce benefits not achievable with standard therapy.
What should the next studies be? Transplant studies demonstrate benefit compared with conventional therapy, however, the nature of conventional therapy is changing. The introduction of thalidomide and bortezomib are altering our concepts of initial therapy for patients. There were 56 newly diagnosed myeloma patients given melphalan and prednisone with the addition of thalidomide at 100 mg per day. A complete response was seen in 22% of patients, with an overall response rate of 90%.7 Addition of thalidomide to dexamethasone in newly diagnosed myeloma patients increased the response rate from 53% to 80%. The next set of studies is destined to compare high-dose therapy with standard therapy containing novel agents. For the time being, however, the concept of offering high-dose therapy to myeloma patients up to the age of 70 years is appropriate and justified.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal