Abstract
Recent evidence suggests that protease release by neutrophils in the bone marrow may contribute to hematopoietic progenitor cell (HPC) mobilization. Matrix metalloproteinase-9 (MMP-9), neutrophil elastase (NE), and cathepsin G (CG) accumulate in the bone marrow during granulocyte colony-stimulating factor (G-CSF) treatment, where they are thought to degrade key substrates including vascular cell adhesion molecule-1 (VCAM-1) and CXCL12. To test this hypothesis, HPC mobilization was characterized in transgenic mice deficient in one or more hematopoietic proteases. Surprisingly, HPC mobilization by G-CSF was normal in MMP-9–deficient mice, NE × CG-deficient mice, or mice lacking dipeptidyl peptidase I, an enzyme required for the functional activation of many hematopoietic serine proteases. Moreover, combined inhibition of neutrophil serine proteases and metalloproteinases had no significant effect on HPC mobilization. VCAM-1 expression on bone marrow stromal cells decreased during G-CSF treatment of wild-type mice but not NE × CG-deficient mice, indicating that VCAM-1 cleavage is not required for efficient HPC mobilization. G-CSF induced a significant decrease in CXCL12α protein expression in the bone marrow of Ne × CG-deficient mice, indicating that these proteases are not required to down-regulate CXCL12 expression. Collectively, these data suggest a complex model in which both protease-dependent and -independent pathways may contribute to HPC mobilization.
Introduction
The use of hematopoietic progenitor cells (HPCs) to reconstitute hematopoiesis following myeloablative therapy has significantly improved the clinical outcome for patients with a variety of diseases. Recently, mobilized peripheral blood HPCs instead of bone marrow–derived HPCs have been used because of reduced engraftment times and relative ease of collection. Although the great majority of HPCs reside within the bone marrow, a small number of HPCs also circulate in the peripheral blood. The number of circulating HPCs can be dramatically increased, or mobilized, by a wide variety of stimuli including hematopoietic growth factors, chemotherapy, and chemokines.1,2 Currently, granulocyte colony-stimulating factor (G-CSF) is the most commonly used agent to mobilize HPCs because of its potency and lack of serious toxicity. However, the mechanisms that mediate G-CSF–induced HPC mobilization are incompletely understood.
We previously showed that expression of the G-CSF receptor (G-CSFR) on HPCs is not required for their mobilization into the blood in response to G-CSF.3 This observation suggests that G-CSF induces HPC mobilization indirectly through the generation of trans-acting signals. Recent studies suggest that hematopoietic proteases released by neutrophils into the bone marrow microenvironment may represent such a signal. A highly proteolytic microenvironment is induced in the bone marrow during HPC mobilization by G-CSF.4 In particular, matrix metalloproteinase-9 (MMP-9 or gelatinase B), neutrophil elastase (NE), and cathepsin G (CG) accumulate in the bone marrow of mice during treatment with G-CSF with kinetics that mirror that for HPC mobilization.4 MMP-9 is highly expressed in neutrophils, monocytes, and macrophages.5 In neutrophils, MMP-9 is stored in gelatinase granules and can be rapidly released following activation.6 Evidence for a direct role for MMP-9 in HPC mobilization is provided by the observation that neutralizing antibodies to MMP-9 partially block HPC mobilization by interleukin-8 (IL-8) in nonhuman primates.7 Moreover, a recent study reported that HPC mobilization by G-CSF was impaired in MMP-9–deficient mice.8 Collectively, these data suggest the hypothesis that MMP-9 is a key mediator of HPC mobilization by G-CSF or IL-8.
Neutrophils express 3 serine proteases: NE, CG, and proteinase 3.9 These proteases are stored in primary granules of neutrophils and can be released following neutrophil activation.9 We previously reported that during HPC mobilization by G-CSF, NE and CG are released into the bone marrow microenvironment and are capable of proteolytic cleavage of vascular cell adhesion molecule-1 (VCAM-1).10 Importantly, during mobilization with G-CSF, VCAM-1 expression in the bone marrow stroma is strongly reduced. VCAM-1 is thought to be the major ligand for α4β1 integrin (very late antigen-4 [VLA-4]), a key integrin expressed on HPCs.11 These observations suggest a model in which proteases released by activated neutrophils cleave VCAM-1 on stromal cells leading to the disruption of its key adhesive interaction with VLA-4 on HPCs.
More recently, evidence supporting an alternative mechanism by which NE and CG may regulate HPC mobilization has been generated.12,13 NE and CG are capable of proteolytic cleavage of both CXCL12 (stromal cell–derived factor-1) and its receptor, CXCR4.12 The interaction of CXCL12 with CXCR4 on HPCs is thought to be a key signal regulating HPC trafficking in the bone marrow.14,15 We and others showed that CXCL12 protein expression in the bone marrow is significantly decreased following HPC mobilization with G-CSF.12,13,16 Moreover, during G-CSF–induced HPC mobilization the amino-terminus of CXCR4 on HPCs is cleaved.12 Collectively, these data suggest the hypothesis that G-CSF may induce HPC mobilization through the proteolytic inactivation of both CXCL12 and CXCR4 by NE and CG. In support of this hypothesis, Petit et al13 showed that treatment of mice with an NE inhibitor partially blocked HPC mobilization by G-CSF.
To test these hypotheses, neutrophil mobilization by G-CSF was characterized in transgenic mouse lines deficient in one or more hematopoietic proteases or in mice following treatment with a broad-spectrum metalloproteinase inhibitor. We show that HPC mobilization by G-CSF is normal in mice lacking MMP-9 or functional neutrophil serine proteases.
Materials and methods
Animals
MMP-9–deficient, NE-deficient, CG-deficient, and dipeptidyl peptidase-I (DPP-I)–deficient mice, all inbred onto a 129 genetic background, were produced as previously described.17-21 NE- and CG-deficient mice were intercrossed to produce NE × CG-deficient mice using standard breeding strategies. Southern blot analyses were performed to confirm the presence of homozygous null alleles for NE and CG (data not shown). The lack of functional NE and CG in these mice was confirmed using NE- or CG-specific chromogenic substrate assays, as previously described (data not shown).22 In some experiments, DPP-I–deficient mice backcrossed for 10 generations onto a C57BL/6 background were used. All mice were housed in a specific pathogen–free environment and examined twice weekly by veterinary staff for signs of illness. All studies were approved by the animal studies committee at Washington University, St Louis, MO.
Mobilization regimens
Recombinant human IL-8, a generous gift from Searle (now part of Pfizer, New York, NY), was given by a single intraperitoneal injection at a dose of 30 μg per mouse and peripheral blood was analyzed at the indicated times. Recombinant human G-CSF (Amgen, Thousand Oaks, CA) diluted in phosphate-buffered saline (PBS) with 0.1% low endotoxin bovine serum albumin (BSA; Sigma, St Louis, MO) was administered by daily subcutaneous injection at a dose of 250 μg/kg per day for 5 days. Mice were analyzed 4 hours after the final injection of G-CSF. Cyclophosphamide (CY; Sigma) was reconstituted in sterile water and administered as a single intraperitoneal injection at a dose of 200 μg/kg.
Administration of MMI270
MMI270, a broad-spectrum metalloproteinase inhibitor, was a generous gift from Novartis Pharma KK (Ibaraki, Japan). MMI270 was administered by continuous subcutaneous infusion using an implantable miniosmotic pump (Alza Pharmaceuticals, Palo Alto, CA) at a rate of 30 mg/kg per day for 6 days. Briefly, MMI270 was reconstituted in a 1:1 ratio of dimethylsulfoxide (DMSO)/polyethylene glycol 300 at a concentration of 37.5 mg/mL, loaded into a miniosmotic pump, and surgically inserted into the subcutaneous tissue on the backs of mice. Serum levels of MMI270 were analyzed at the time of animal harvest using a previously described high-perfomance liquid chromatography (HPLC) assay.23
Gelatin zymography
The amount of MMP-9 present in the serum of mice at the indicated times after treatment with IL-8 or G-CSF was measured by gelatin zymography, as described previously.24
Colony-forming cell (CFU-C) assay
Blood, bone marrow, and spleen cells were harvested from mice using standard techniques, and the number of nucleated cells in these tissues was quantified using a Hemavet automated cell counter (CDC Technologies, Oxford, CT). Blood (10 μL-20 μL), nucleated spleen cells (1 × 105), or nucleated bone marrow cells (2.5 × 104) were plated in 2.5 mL methylcellulose media supplemented with a cocktail of recombinant cytokines (MethoCult 3434; Stem Cell Technologies, Vancouver, BC, Canada). Cultures were plated in duplicate and placed in a humidified chamber with 6% CO2 at 37° C. Colonies containing at least 50 cells were scored on day 7 of culture.
In vitro cleavage of recombinant human VCAM-1 by bone marrow extracellular fluid
Bone marrow (BM) extracellular fluid was obtained by flushing both femurs of a mouse with a total of 1 mL ice-cold PBS and then collecting the cell-free supernatant following centrifugation at 500g for 2 minutes. BM extracellular fluid (10 μL) was mixed with an equal volume of PBS containing 0.1 μg recombinant extracellular domain of human VCAM-1 (R&D Systems, Minneapolis, MN) and incubated at 37° C for 30 minutes. Digestions were stopped by adding 20 μL of 125 mM Tris-HCl, pH 6.8, 20% glycerol, 4% sodium dodecyl sulfate, and boiling for 5 minutes. Samples were separated on 10% polyacrylamide gels, transferred onto nitrocellulose, and stained with a goat antihuman VCAM-1 serum (R&D Systems) as previously described.10
VCAM-1 immunohistochemical staining
Bone marrow was flushed from femurs directly onto poly-l-lysine–coated glass slides and processed as previously described.10 Following fixation in ice-cold acetone, slides were air-dried and kept at room temperature until staining. Prior to staining, slides were rehydrated in PBS Tween 20 (PBST) for 2 hours and blocked for 2 hours in PBST containing 5% donkey serum (PBSTDS). Slides were incubated overnight at 4° C with either M/K2-7 hybridoma supernatant mixed with an equal volume of PBSTDS or 2 μg/mL nonimmune rat IgG1 in 50% PBSTDS/50% hybridoma growth medium. Following 4 washes with PBSTDS, slides were incubated for 2 hours at room temperature with 1:250 biotinylated donkey F(ab′)2 fragments antirat IgG non–cross-reacting with mouse proteins (Jackson ImmunoResearch Laboratories, West Grove, PA) in PBSTDS. Following 4 washes in Tris-buffered saline Tween 20 (TBST), slides were incubated for 1 hour in TBST, 0.1% BSA, 1:400 alkaline phosphatase–conjugated streptavidin (Amersham). Following 4 washes in TBST, slides were stained with fast red TR, naphtol AS-MX phosphate, and levamisole, then counterstained with hematoxylin and mounted with Aquamount (Fronine Laboratory Supplies, Riverston, Australia) as previously described.10 Microscopy was performed using a Zeiss Axiokop 2 microscope (Carl Zeiss Microimaging, Thornwood, NY) with a Zeiss Plan-Neoflur × 20 objective (numerical aperture 0.5). Digital images were obtained using a Diagnostic Instruments Spot RT Slider camera (Sterling Heights, MI) and analyzed with Spot Software Windows version 4.0.2 imaging software (http://www.spotsoftware.nl).
CXCL12α ELISA
CXCL12α protein concentration in BM extracellular fluid was measured by enzyme-linked immunosorbent assay (ELISA), as described previously.16 BM extracellular fluid was diluted 1:3 in ELISA diluent (0.1% BSA, TBST). Recombinant human recombinant CXCL12α was used to generate a standard curve (data not shown). All ELISA reagents were purchased from R&D Systems.
Progenitor transmigration assay
Transwell migration of HPCs in response to CXCL12α was performed, essentially as described previously.12 In brief, mononuclear cells from the bone marrow or peripheral blood were isolated using a Lympholyte-Mammal density gradient, per manufacturer's recommendations (Cedarlane Laboratories, Hornby, ON, Canada). Cells were incubated in RPMI supplemented with 10% fetal calf serum at 37° C for 1 hour to allow time for resensitization of potentially desensitized CXCR4. Cells (2 × 105) were loaded into the upper chamber of 5-μm pore transwell inserts (Corning Costar, Cambridge, MA). Recombinant human recombinant CXCL12α was added to the lower chamber at a concentration of 200 mg/mL and the chamber was incubated for 4 hours at 37° C. The CFU-C content in the original cell preparation and in cells that transmigrated to the lower chamber was determined using the CFU-C assay.
Statistical analysis
Data are presented as the mean plus or minus the standard deviation (SD). Statistical significance was assessed by 2-tailed Student t test.
Results
MMP-9 is not required for HPC mobilization by IL-8 or G-CSF in mice
Recent data suggest that MMP-9 is a critical mediator of HPC mobilization by G-CSF and IL-8.7,8 To test this hypothesis, we first determined whether treatment of mice with IL-8 or G-CSF results in the release of MMP-9 by measuring the amount of MMP-9 in the plasma of animals after treatment with these agents (Figure 1A). Indeed, at the time of peak HPC mobilization (15 minutes after IL-8 administration or after 5 days of G-CSF treatment), a significant increase in the amount of MMP-9 in the serum was detected relative to untreated mice. The identity of the MMP-9 band was confirmed by its specific loss in MMP-9–deficient mice treated with G-CSF. Moreover, the MMP-9 band was abolished by incubation of the gel in the presence of EDTA (ethylenediaminetetraacetic acid; data not shown). We next measured MMP-9 release in G-CSFR–deficient mice after treatment with IL-8. We previously showed that HPC mobilization by IL-8 is markedly impaired in G-CSFR–deficient mice.25 Interestingly, no significant increase in serum MMP-9 was detected in these mice following IL-8 administration (Figure 1B). Finally, MMP-9 release during HPC mobilization by CY was examined in wild-type mice. Treatment of mice with CY results in robust HPC mobilization that peaks on day 8 after administration. Surprisingly, no increase in serum MMP-9 was detected at the time of peak HPC mobilization by CY (1Figure 1C). Collectively, these data show that an increase in the plasma level of MMP-9 is detectable during HPC mobilization by some, but not all, agents.
Plasma MMP9 levels during HPC mobilization. (A) Plasma was collected from wild-type mice at baseline (control), 15 minutes after IL-8 administration (30 μg, intraperitoneal; IL-8), or 5 days after treatment with G-CSF (250 μg/kg per day for 5 days; G-CSF). As a control, MMP-9–deficient mice treated with G-CSF also were analyzed. (B) Plasma was collected from wild-type mice after IL-8 administration or from G-CSFR–deficient mice at baseline or after IL-8 administration. (C) Plasma was collected from wild-type mice at baseline, after IL-8 administration, or 8 days after treatment with cyclophosphamide (200 mg/kg; CY). In each case, equal amounts of plasma proteins were analyzed by gelatin zymography. Results are representative of 3 independent experiments. Molecular size markers are indicated at left in kDa.
Plasma MMP9 levels during HPC mobilization. (A) Plasma was collected from wild-type mice at baseline (control), 15 minutes after IL-8 administration (30 μg, intraperitoneal; IL-8), or 5 days after treatment with G-CSF (250 μg/kg per day for 5 days; G-CSF). As a control, MMP-9–deficient mice treated with G-CSF also were analyzed. (B) Plasma was collected from wild-type mice after IL-8 administration or from G-CSFR–deficient mice at baseline or after IL-8 administration. (C) Plasma was collected from wild-type mice at baseline, after IL-8 administration, or 8 days after treatment with cyclophosphamide (200 mg/kg; CY). In each case, equal amounts of plasma proteins were analyzed by gelatin zymography. Results are representative of 3 independent experiments. Molecular size markers are indicated at left in kDa.
To more directly assess the role of MMP-9 in HPC mobilization, HPC mobilization by IL-8 or G-CSF was studied in MMP-9–deficient mice. Of note, the MMP-9–deficient and wild-type mice used in this study were inbred on a 129 genetic background. As reported previously,26 treatment of wild-type mice with IL-8 results in a rapid (peak 15-30 minutes after administration) but modest (3.4-fold increase over baseline) increase in circulating CFU-Cs (Figure 2A). Surprisingly, the kinetics and magnitude (5.0-fold over baseline) of the increase in peripheral blood CFU-Cs after IL-8 administration were similar in MMP-9–deficient mice. In addition, a similar increase in circulation neutrophils was observed in wild-type and MMP-9–deficient mice (data not shown).
HPC mobilization by IL-8 or G-CSF in MMP-9–deficient mice. (A) Wild-type (WT; ♦) or MMP-9–deficient mice (KO; □) were treated with a single intraperitoneal injection of 30 μg human recombinant IL-8 at time zero, and the number of colony-forming cells (CFU-Cs) in the blood was quantified at the indicated time points. A minimum of 3 mice were analyzed at each time point. (B) Wild-type or MMP-9–deficient mice were treated with saline alone (□; n = 4) or with G-CSF (▪; 250 μg/kg per day for 5 days; n = 10), and the number of CFU-Cs in the bone marrow, blood, and spleen was quantified 4 hours after the last injection. Data represent the mean ± SD.
HPC mobilization by IL-8 or G-CSF in MMP-9–deficient mice. (A) Wild-type (WT; ♦) or MMP-9–deficient mice (KO; □) were treated with a single intraperitoneal injection of 30 μg human recombinant IL-8 at time zero, and the number of colony-forming cells (CFU-Cs) in the blood was quantified at the indicated time points. A minimum of 3 mice were analyzed at each time point. (B) Wild-type or MMP-9–deficient mice were treated with saline alone (□; n = 4) or with G-CSF (▪; 250 μg/kg per day for 5 days; n = 10), and the number of CFU-Cs in the bone marrow, blood, and spleen was quantified 4 hours after the last injection. Data represent the mean ± SD.
We next examined G-CSF–induced HPC mobilization in MMP-9–deficient mice (Figure 2B). As reported previously, treatment with G-CSF (250 μg/kg per day for 5 days) resulted in a significant increase in blood (35-fold) and spleen (19-fold) CFU-Cs in wild-type mice.25 A similar increase in the number of blood (31-fold) and spleen (18-fold) CFU-Cs was observed in MMP-9–deficient mice. Likewise, a similar increase in the number of blood and bone marrow neutrophils was observed in wild-type and MMP-9–deficient mice (data not shown). Of note, no significant difference in the number of CFU-Cs in the bone marrow of MMP-9–deficient or wild-type mice was observed. Collectively, these data demonstrate that MMP-9 is not required for IL-8– or G-CSF–induced HPC mobilization in mice.
NE and CG are not required for HPC mobilization by G-CSF in mice
Neutrophil serine proteases, specifically NE and CG, recently have been implicated as key mediators of G-CSF–induced HPC mobilization.4,10 To test this hypothesis, HPC mobilization by G-CSF was examined in NE × CG-deficient mice inbred on a 129 background. Of note, basal hematopoiesis is normal in these mice.22 G-CSF treatment induced a similar increase in blood and spleen CFU-Cs in wild-type and NE × CG-deficient mice. In the blood, 21-fold and 28-fold increases in CFU-Cs were detected in wild-type and NE × CG-deficient mice, respectively. Likewise, a similar increase in spleen CFU-Cs was detected in wild-type (15-fold) and NE × CG-deficient mice (15-fold).
In addition to NE and CG, neutrophils contain one additional serine protease, proteinase 3. To determine whether the protease activity of proteinase 3 contributes to HPC mobilization in the absence of NE and CG, we studied DPP-I–deficient mice. DPP-I is an enzyme required for the processing of many proteases. Loss of DPP-I leads to the functional inactivation of a broad range of hematopoietic serine proteases, including NE, CG, and proteinase 3.22 Of note, DPP-I–deficient mice inbred on a 129 genetic background have normal basal hematopoiesis.19 These mice were treated with G-CSF for 5 days and the number of CFU-Cs in the blood, bone marrow, and spleen was quantified (Figure 3B). Robust HPC mobilization similar to that seen in wild-type mice was observed. There is considerable heterogeneity in the mobilization response to G-CSF among different inbred strains of mice. To determine whether the contribution of neutrophil serine proteases to HPC mobilization is strain dependent, we studied DDP-I–deficient mice inbred onto a C57BL/6 background. As reported previously, HPC mobilization by G-CSF was reduced in wild-type C57BL/6 mice compared with 129 mice (1113 ± 534 CFU-Cs per mL of blood on day 5 of G-CSF treatment). A similar mobilization response was observed in DDP-I–deficient C57BL/6 mice (1763 ± 531 CFU-Cs per mL). Collectively, these data show that the protease activity of neutrophil serine proteases is not required for HPC mobilization by G-CSF in mice.
HPC mobilization by G-CSF in NE × CG-deficient and DPP-I–deficient mice. Wild-type and NE × CG-deficient mice (A) or wild-type and DPP-I–deficient mice (B) were treated with 250 μg/kg G-CSF per day for 5 days, and the number of CFU-Cs in the bone marrow, blood, and spleen was quantified 4 hours after the final dose of G-CSF (n = 4 mice each group). Data represent the mean ± SD.
HPC mobilization by G-CSF in NE × CG-deficient and DPP-I–deficient mice. Wild-type and NE × CG-deficient mice (A) or wild-type and DPP-I–deficient mice (B) were treated with 250 μg/kg G-CSF per day for 5 days, and the number of CFU-Cs in the bone marrow, blood, and spleen was quantified 4 hours after the final dose of G-CSF (n = 4 mice each group). Data represent the mean ± SD.
Combined inhibition of a broad spectrum of metalloproteinases and neutrophil serine protease activity does not result in impaired HPC mobilization by G-CSF
It is possible that MMP-9 and neutrophil serine proteases provide redundant proteolytic activities required for HPC mobilization. Thus, the loss of a single protease activity may not result in impaired HPC mobilization. To address this possibility, we treated DPP-I–deficient mice with MMI270, a broad-spectrum metalloproteinase inhibitor. MMI270 inhibits MMP1, MMP2, MMP3, MMP9, and MMP13 with an inhibitory concentration (IC50) of less than or equal to 50 nm.23 DDP-I–deficient mice were treated with MMI270 (30 mg/kg) or vehicle alone by continuous subcutaneous infusion for 6 days. G-CSF was given on days 2 to 6 and CFU-Cs were quantified after the final dose of G-CSF on day 6. The plasma concentration of MMI270 at the time of animal harvest was 454 ± 203 nM, well above the IC50 for the indicated metalloproteinases. As shown in Figure 4, treatment of DPP-I–deficient mice with MMI270 had no effect on G-CSF–induced HPC mobilization. Thus, the combined inhibition of a broad spectrum of metalloproteinases and neutrophil serine proteases had no significant effect on HPC mobilization by G-CSF.
HPC mobilization by G-CSF in DPP-I–deficient mice treated with a broad-spectrum metalloproteinase inhibitor. DPP-I–deficient mice (n = 3, each) were treated with 30 mg/kg MMI270 or vehicle alone (control) by continuous subcutaneous infusion for 6 days. On days 2 to 6, mice received 250 μg/kg G-CSF per day for 5 days, and the number of CFU-Cs in the bone marrow, blood, and spleen was quantified 4 hours after the final dose of G-CSF. Data represent the mean ± SD.
HPC mobilization by G-CSF in DPP-I–deficient mice treated with a broad-spectrum metalloproteinase inhibitor. DPP-I–deficient mice (n = 3, each) were treated with 30 mg/kg MMI270 or vehicle alone (control) by continuous subcutaneous infusion for 6 days. On days 2 to 6, mice received 250 μg/kg G-CSF per day for 5 days, and the number of CFU-Cs in the bone marrow, blood, and spleen was quantified 4 hours after the final dose of G-CSF. Data represent the mean ± SD.
G-CSF–induced cleavage of VCAM-1 on bone marrow stromal cells is dependent on NE and CG but is not required for HPC mobilization
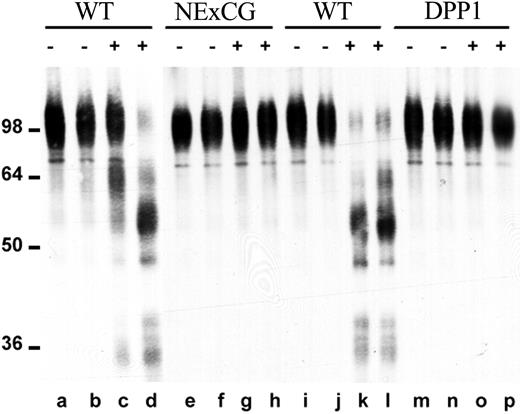
We previously showed that NE and CG are able to cleave VCAM-1 in vitro.10 Since interaction of VCAM-1 on stromal cells with VLA-4 on HPCs is thought to be a key adhesive interaction regulating HPC trafficking in the bone marrow,11 this observation suggests that VCAM-1 cleavage by NE or CG may be a key step in HPC mobilization. To further examine this possibility, we studied VCAM-1 cleavage in NE × CG-deficient and DPP-I–deficient mice. The release of proteases capable of cleaving VCAM-1 was assessed by incubating bone marrow extracellular fluid with recombinant human VCAM-1 (Figure 5). As previously reported by our group,10 in wild-type mice, G-CSF induced the release of proteases capable of cleaving VCAM-1 (Figure 5, lanes c, d, k, l). In contrast, no VCAM-1 cleavage was observed in bone marrow extracellular fluid harvested from G-CSF–treated, NE × CG-deficient (Figure 5, lanes g, h) or DPP-I–deficient (Figure 5, lanes o, p) mice. These data confirm our previous observation that NE and CG are the major VCAM-1 cleaving proteases released into bone marrow extracellular fluids during G-CSF treatment.
VCAM-1 cleaving activity in bone marrow extracellular fluid. Bone marrow extracellular fluid was harvested from wild-type mice (WT; lanes a-d and i-l), NE × CG-deficient mice (NE × CG; lanes e-h), or DPP-I–deficient mice (lanes m-p) after treatment with saline (–; lanes a, b, e, f, i, j, m, n) or G-CSF (+; lanes c, d, g, h, k, l, o, p). Cleavage of recombinant human VCAM-1 after incubation with bone marrow extracellular fluid was assessed as described in “Materials and methods.” Molecular size markers are indicated at left in kDa.
VCAM-1 cleaving activity in bone marrow extracellular fluid. Bone marrow extracellular fluid was harvested from wild-type mice (WT; lanes a-d and i-l), NE × CG-deficient mice (NE × CG; lanes e-h), or DPP-I–deficient mice (lanes m-p) after treatment with saline (–; lanes a, b, e, f, i, j, m, n) or G-CSF (+; lanes c, d, g, h, k, l, o, p). Cleavage of recombinant human VCAM-1 after incubation with bone marrow extracellular fluid was assessed as described in “Materials and methods.” Molecular size markers are indicated at left in kDa.
We next examined VCAM-1 expression on bone marrow stromal cells in vivo by immunohistochemical staining of VCAM-1 in the bone marrow (Figure 6). As reported previously by our group, G-CSF treatment induced a significant decrease in VCAM-1 expression in the bone marrow.10 Interestingly, VCAM-1 expression in the bone marrow was consistently increased in NE × CG-deficient mice at baseline. However, after G-CSF very little change in VCAM-1 expression was detected. These data suggest that VCAM-1 cleavage on bone marrow stromal cells is not required for efficient HPC mobilization by G-CSF.
Immunohistochemical staining of VCAM-1 in the bone marrow. Wild-type and NE × CG-deficient mice were treated with saline or G-CSF for 5 days, bone marrow touch preparations were generated, and immunostaining for VCAM-1 was performed. Data are representative of 3 separate experiments. Original magnification, × 62.
Immunohistochemical staining of VCAM-1 in the bone marrow. Wild-type and NE × CG-deficient mice were treated with saline or G-CSF for 5 days, bone marrow touch preparations were generated, and immunostaining for VCAM-1 was performed. Data are representative of 3 separate experiments. Original magnification, × 62.
G-CSF induces a significant decrease in CXCL12α protein expression in the bone marrow and a decrease in functional CXCR4 expression on HPCs that is not dependent on NE or CG
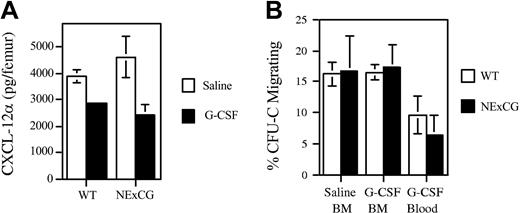
Accumulating evidence suggests that disruption of the interaction of CXCL12α with its cognate receptor, CXCR4, on HPCs is a key step in mobilization. We and others previously showed that the level of CXCL12α protein in the bone marrow decreases following G-CSF treatment.12,13,16 Two recent reports suggested that this decrease in CXCL12 expression is mediated by NE- and CG-dependent proteolysis of CXCL12α.12,13 Moreover, we recently showed that CXCR4 on HPCs is cleaved during G-CSF treatment, resulting in a nonfunctional receptor, and providing further evidence implicating these proteases. To test this hypothesis, CXCL12α protein expression and functional CXCR4 expression on HPCs were examined in NE × CG-deficient mice. As reported previously, a significant decrease in CXCL12α protein was detected in bone marrow extracellular fluid of wild-type mice following G-CSF treatment (Figure 7A). Surprisingly, a similar decrease in CXCL12α protein was detected in NE × CG-deficient mice. Thus, down-regulation of CXCL12α protein expression in the bone marrow by G-CSF is not dependent on NE or CG. Expression of functional CXCR4 on HPCs was measured using a CXCL12α chemotaxis assay. As reported previously, the percentage of CXCL 12–responsive CFU-Cs in the blood following G-CSF treatment was reduced compared with BM resident CFU-Cs (Figure 7B). Similar data were observed in NE × CG-deficient mice. These data show that the decrease in functional CXCR4 expression on HPCs mobilized by G-CSF also is not dependent on NE or CG.
CXCL12α protein and functional CXCR4 expression on HPCs following G-CSF treatment. Wild-type or NE × CG-deficient mice were treated with saline or G-CSF (n = 3-4, each). (A) The amount of CXCL12α protein in the bone marrow extracellular fluid was measured by ELISA. (B) The chemotaxis of CFU-Cs to CXCL12α was measured; the percentage of CFU-Cs migrating in response to CXCL12α is shown. Data represent the mean ± SD.
CXCL12α protein and functional CXCR4 expression on HPCs following G-CSF treatment. Wild-type or NE × CG-deficient mice were treated with saline or G-CSF (n = 3-4, each). (A) The amount of CXCL12α protein in the bone marrow extracellular fluid was measured by ELISA. (B) The chemotaxis of CFU-Cs to CXCL12α was measured; the percentage of CFU-Cs migrating in response to CXCL12α is shown. Data represent the mean ± SD.
Discussion
Recent studies have implicated hematopoietic proteases as potentially key mediators of HPC mobilization.4,7,8,10,12,13,27 In the present study, we have examined this hypothesis through the characterization of HPC mobilization in selected protease-deficient mice. There is strong evidence that specific hematopoietic proteases accumulate in the bone marrow during HPC mobilization. We recently showed that NE and CG activity in bone marrow extracellular fluid increased 8- to 100-fold following treatment with G-CSF or CY.4 Importantly, the peak in NE and CG activity coincided with maximal HPC mobilization. Pruijt et al7 showed that treatment of rhesus monkeys with IL-8 induces a dramatic and rapid increase in plasma levels of MMP-9 that correspond with HPC mobilization. We previously showed that the level of MMP-9 also is significantly increased in bone marrow extracellular fluid following treatment with G-CSF or CY.4 However, the kinetics of the increase in MMP-9 were delayed, particularly with CY, where the peak of MMP-9 in the bone marrow extracellular fluid was on day 10 following CY injection when the number of circulating HPCs returns to baseline values.4 Consistent with these observations, in the present study we show that the plasma level of MMP-9 increases following treatment with G-CSF or IL-8 in mice. However, no detectable increase in plasma MMP-9 was detected following CY treatment, despite robust HPC mobilization. These data raise the possibility that MMP-9 may not be required for CY-induced mobilization, even though we previously showed that mobilization by this agent is dependent on G-CSF receptor signals.25
Recent evidence suggests a direct role for MMP-9 in HPC mobilization. Pruijt et al7 showed that pretreatment of rhesus monkeys with neutralizing antibodies to MMP-9 partially blocked HPC mobilization by IL-8. In a subsequent report, the authors showed that depletion of neutrophils prior to IL-8 administration also blocked HPC mobilization in mice, suggesting that neutrophils may be the primary source of protease release.28 However, consistent with a previous report,28 we show in the current study that IL-8–induced HPC mobilization is normal in MMP-9–deficient mice. Collectively, these data raise the possibility that species-specific differences in the requirement for MMP-9 to mediate IL-8–induced HPC mobilization may exist.
MMP-9 also has been implicated in G-CSF–induced HPC mobilization in mice. Heissig et al8 reported that HPC mobilization by G-CSF was significantly impaired in MMP-9–deficient mice. Moreover, treatment of mice with CGS27 023A (MMI270), a broad-spectrum metalloproteinase inhibitor, nearly completely blocked HPC mobilization by G-CSF. The authors showed that MMP-9 cleaved Kit-ligand from the surface of bone marrow stromal cells, providing a potential mechanism for its effects on HPC trafficking. In contrast, in the present study, we show that HPC mobilization by G-CSF is normal in MMP-9–deficient mice. Since Heissig et al used a lower dose of G-CSF (50 μg/kg per day for 5 days), we repeated our studies using this G-CSF regimen. Again, no difference in HPC mobilization between wild-type and MMP-9–deficient mice was observed (data not shown). Consistent with our data, a recent report also showed that G-CSF–induced HPC mobilization is normal in MMP-9–deficient mice.29 To begin to address the possibility of redundant protease activity, we treated DDP-I–deficient mice with MMI270, the same broad-spectrum metalloproteinase inhibitor used by Heissig et al. In contrast to their results, treatment with MMI270 had no significant effect on HPC mobilization by G-CSF. In the present study, a plasma concentration of MMI270 of 454 nM was achieved (similar data are not provided by Heissig et al). Although direct proof that MMP-9 was inhibited in our mice is not provided, this plasma concentration of MMI270 is well above the IC50 for MMP-9 (8 nM), and it is above the IC50 (200 nM) for the inhibition of angiogenesis in a rat aorta model, a model that is dependent on metalloproteinases. Of note, treatment of wild-type mice with BB-94/Batimastat30,31 (British Biotechnology, Oxford, United Kingdom), another broad-spectrum metalloproteinase inhibitor, also had no significant effect on G-CSF–induced HPC mobilization (data not shown). Although the reason for the discrepancy between these 2 studies is not clear, several potentially important differences have been identified. First, different dosing regimens for MMI270 were used: 30 mg/kg per day for 6 days by continuous subcutaneous infusion in the present study and 60 mg/kg given subcutaneously every 3 days in the study by Heissig et al. Second, different genetic strains of mice were used. In the present study, 129 mice were used, whereas Heissig el al used mice outbred on a CD1 genetic background. This latter possibility is consistent with previous reports showing considerable differences in G-CSF–induced HPC mobilization among different mouse genetic strains.32 There is nearly a 12-fold difference in the magnitude of HPCs mobilized into the blood between poor responders (for example, C57BL/6 mice) and good responders (for example, DBA mice). 129 mice display an intermediate phenotype. Unfortunately, there are no published reports comparing HPC mobilization in outbred CD1 mice with inbred strains of mice.
We previously showed that VCAM-1 expression on bone marrow stromal cells is markedly decreased following HPC mobilization by G-CSF. VCAM-1 is thought to be a key ligand for VLA-4, a major integrin expressed on HPCs.33 The importance of VCAM-1–VLA-4 interactions to HPC trafficking in the bone marrow has been confirmed by studies showing that antibodies directed against VCAM-1 or VLA-4 lead to HPC mobilization.11,34,35 Moreover, a recent report showed that the inducible deletion of α4 integrin in adult mice results in a 7-fold increase of circulating CFU-Cs that was maintained over 20 weeks.36 We previously showed that NE and CG are able to cleave VCAM-1 in vitro.10 Since NE and CG are induced in the bone marrow during G-CSF treatment, this observation suggested that NE- and CG-mediated cleavage of VCAM-1 on bone marrow stromal cells is a key step in HPC mobilization. In the present study, we show that VCAM-1 cleaving activity in bone marrow extracellular fluid and the down-regulation of VCAM-1 expression on bone marrow stromal cells in vivo is markedly reduced in NE × CG-deficient mice, confirming that NE and CG are the major proteases mediating VCAM-1 cleavage on bone marrow stroma in vivo and directly regulate VCAM-1 expression in this tissue. However, G-CSF–induced HPC mobilization is normal in NE × CG-deficient mice, despite continued high expression of VCAM-1 in the bone marrow. These data strongly suggest that, although the disruption of VCAM-1–mediated interactions in vivo is sufficient to elicit mobilization,11,34,35,37 VCAM-1 cleavage is not required for efficient HPC mobilization by G-CSF in mice.
Accumulating evidence suggests that interaction between CXCL12 and CXCR4 may play a key role in regulating HPC trafficking from the bone marrow. CXCL12 is constitutively produced by bone marrow stromal cells and CXCR4 is broadly expressed on hematopoietic cells.38-40 Studies of mice lacking CXCL12 or CXCR4 have established that CXCL12 is necessary for the normal migration of hematopoietic progenitors from the fetal liver to the bone marrow.14,41 Elevation of CXCL12 levels in the blood by administration of CXCL12 or by injection of an adenoviral vector expressing CXCL12 is associated with a significant mobilization of hematopoietic progenitors.42,43 Finally, recent data show that treatment of humans with AMD3100, a specific CXCR4 antagonist, leads to rapid and robust HPC mobilization.44 These data suggest that CXCL12 may be a key retention signal in the bone marrow for HPCs. We and others previously showed that G-CSF treatment results in a significant decrease in CXCL12α protein in the bone marrow of wild-type mice.12,13,15,16 Evidence that the decrease in CXCL12α protein expression in the bone marrow may be secondary to its proteolytic degradation by NE and CG in vitro and in vivo has recently been provided.12,13 Proteases present in bone marrow extracellular fluid from G-CSF–mobilized but not untreated mice are able to cleave, and inactive CXCL12 and specific inhibitors for both NE and CG abolish this activity.12 Moreover, treatment of mice with a peptide inhibitor of NE partially blocked G-CSF–induced HPC mobilization.13 In the present study, we show that a similar decrease in CXCL12α protein expression in the bone marrow is observed in wild-type and NE × CG-deficient mice. Thus, there are efficient NE- and CG-independent mechanisms to down-regulate CXCL12α protein expression during G-CSF–induced HPC mobilization. We recently showed that the amino-terminus of CXCR4 is cleaved on HPCs during G-CSF–induced HPC mobilization, resulting in receptor inactivation and further disrupting CXCL12-CXCR4 interactions. In the present study, we show that functional CXCR4 expression on mobilized blood HPCs is reduced to a similar degree in both wild-type and NE × CG-deficient mice, indicating that the proteolytic cleavage of CXCR4 also does not require NE or CG. Interestingly, in contrast to the situation with humans, no decrease in functional CXCR4 expression was observed in bone marrow–resident HPCs following G-CSF treatment. Nonetheless, these data are consistent with the hypothesis that perturbation of CXCL12-CXCR4 interactions is a key step in HPC mobilization by G-CSF.
What role do hematopoietic proteases play in HPC mobilization? Our data suggest several possibilities. First, it is possible that functional redundancy exists among proteases contributing to HPC mobilization such that the loss of any single protease is well compensated. Our finding that G-CSF–induced HPC mobilization is normal in DPP-I–deficient mice (which are functionally deficient in all neutrophil serine proteases), or in DDP-I–deficient mice treated with a broad-spectrum metalloproteinase inhibitor, argues against this possibility. Second, other proteases not inhibited in our studies may contribute to HPC mobilization. A recent study showed that MMP-9 expressed on the cell surface of neutrophils is resistant to inhibition by tissue inhibitor of metalloproteinases (TIMPs).45 Thus, it is possible that only partial MMP-9 inhibition was achieved in mice treated with MMI270. Recently, Christopherson et al27 reported that CD26 (dipeptidylpeptidase IV) a membranebound extracellular protease expressed on a subset of HPCs, may contribute to G-CSF–induced HPC mobilization. They showed that CXCL12 is cleaved and inactivated by CD26. Moreover, G-CSF–induced HPC mobilization is defective in CD26 deficient mice or in wild-type mice treated with a specific CD26 inhibitor.27,46 Third, non–protease-dependent mechanisms may contribute to HPC mobilization. Consistent with this possibility, we recently showed that expression of CXCL12 mRNA in bone marrow cells is significantly reduced during HPC mobilization by G-CSF (C.L. Semerad, M. Christopher, F. Liu, et al, manuscript submitted). In fact, the magnitude and kinetics of the decrease in CXCL12 mRNA closely mirrored the decrease in CXCL12α protein. Collectively, current evidence supports a complex model in which multiple protease-dependent and -independent mechanisms contribute to HPC mobilization.
Prepublished online as Blood First Edition Paper, March 9, 2004; DOI 10.1182/blood-2003-05-1589.
Supported by grants from the National Institutes of Health National Heart, Lung, and Blood Institute (R01 HL60772-01A1 [D.C.L.]; RO1 HL47328 [R.S.]; RO1 AI49261-02 [C.P.]) and from the National Health and Medical Research Council of Australia (080193 [J.-P.L. and P.J.S.]).
J.-P.L. and F.L. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Jill Woloszynek and Jean Hendy for their expert technical assistance. We also thank Shinichi Koizumi and Hiroaki Fukaya (Novartis Pharma KK, Tsukuba Research Institute) for their assistance in obtaining MMI270 and measuring its concentration in mouse plasma samples.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal