Abstract
We report in this paper that a novel small molecule, JTZ-132, induced growth and differentiation of megakaryocytic progenitor cells and improved thrombocytopenia in myelosuppressed mice. JTZ-132 stimulated proliferation of UT-7/TPO cells, a cell line highly sensitive to thrombopoietin (TPO), and exhibited full efficacy comparable to TPO with an approximate EC50 (median effective concentration) value of 0.43 μM, whereas little proliferation was observed in a TPO-insensitive cell line, UT-7/EPO, and human carcinoma cell line, HCT116. Signal transduction studies revealed that JTZ-132 induced tyrosine phosphorylation of c-Mpl, Janus kinase-2 (JAK2), and signal transducers and activators of transcription 5 (STAT5) in UT-7/TPO cells as well as TPO. JTZ-132 increased the number of megakaryocyte-specific marker, CD61+ and CD41+, cells in cultures of mouse and human bone marrow cells, respectively, and the colonyforming unit megakaryocytes in mouse bone marrow cells. In vivo experiments in x-ray irradiation– or busulfan injection–induced myelosuppressed mice demonstrated that subcutaneously injected JTZ-132 at 30 mg/kg showed significantly higher platelet number at nadir and accelerated platelet recovery without affecting white blood cell number. These data suggest that JTZ-132 is a novel stimulator of megakaryocytopoiesis and thrombocytopoiesis in vitro and in vivo with TPO mimetic activities and that it is useful for the treatment of thrombocytopenia.
Introduction
Although applications of granulocyte colony-stimulating factor have been successful for neutropenia and erythropoietin (EPO) for anemia,1-4 platelet transfusions have frequently been applied to patients of thrombocytopenia. However, transmission of both viral and bacterial infections by way of the transfusions remains a concern. Furthermore, thrombocytopenia can mandate a decrease in the dose intensity of cytotoxic therapy by causing either delays or dose reductions in therapy administration. Thrombopoietin (TPO), the ligand for the c-Mpl receptor, has been considered to be the principal physiologic regulator of megakaryopoiesis and platelet production.5-8 TPO induces megakaryocytopoiesis and thrombocytopoiesis, acting primarily on the proliferation of megakaryocyte colony-forming unit (CFU-Meg) and the maturation of megakaryocytes as the major target cells in megakaryocytic lineage.9-12 Clinical trials of recombinant human (rh) TPO or pegylated recombinant human megakaryocyte growth and development factor in patients receiving myelosuppressive therapy have been conducted, and their efficacies have been demonstrated.13,14 However, there may be a risk of producing neutralizing antibody for such protein-related drugs.15,16 Thus, a nonpeptidyl small molecular TPO mimetic that could be used safely would present important medical advantages for the treatment of thrombocytopenia. Although there are some reports that identify TPO-mimetic peptides,17,18 and small molecular nonpeptide compounds,19,20 pharmacologic properties of these compounds have not been demonstrated to produce platelets in thrombocytopenic animal models in vivo.
UT-7/TPO was established as a TPO-dependent subline of UT-7/GM, a human leukemia cell line, and shows high sensitivity to TPO in cell proliferation.21 However, UT-7/EPO was established as an EPO-sensitive cell line, and it shows little response to TPO.22 Therefore, in comparison to UT-7/EPO, there is great advantage in using UT-7/TPO to identify TPO mimetic because of its high response to TPO. Using these cell lines, we finally identified a novel synthetic compound called JTZ-132, a specific stimulator of UT-7/TPO, which may exhibit TPO-mimetic activities.
In this study, we assessed the biologic activities of JTZ-132 as a TPO mimetic in studies of UT-7/TPO cell proliferation as well as c-Mpl and JAK/STAT (Janus kinase/signal transducers and activators of transcription) protein phosphorylation in UT-7/TPO cells and bone marrow cell differentiation. Furthermore, we investigated thrombocytopoietic activity of JTZ-132 in x-ray–irradiated and chemotherapy-induced thrombocytopenic mice in vivo.
Materials and methods
Reagents
RhTPO, rhEPO, and rh interleukin-11 (rhIL-11; NEUMEGA) were obtained from PeproTech (London, United Kingdom), Sankyo (Tokyo, Japan), and Genetics Institute (Cambridge, MA), respectively. JTZ-132 ((S)-7-hydroxyimino-3-(3-methyl-[1, 2, 4]oxadiazol-5-yl)-5-propyl-4, 5, 6, 7-tetrahydrobenzo[c]thiophene-1-sulfonamide, Figure 1) was chemically synthesized by Japan Tobacco Inc Central Pharmaceutical Research Institute (Osaka, Japan). JTZ-132 was prepared in nano-sized particles by Skye Pharma (Muttenz, Switzerland). WST-1 was obtained from Roche (Mannheim, Germany). Busulfan was from Wako Pure Chemical Industries. (Osaka, Japan). Monoclonal mouse antiphospho-STAT5A/B antibody and bovine serum albumin (BSA) were from Sigma (St Louis, MO). Protein G-Sepharose beads were from Pharmacia Biotech (Uppsala, Sweden). Hyperfilm enhanced chemiluminescence (ECL) and ECL plus detection reagent were from Amersham Biosciences (Buckinghamshire, England). Anti-JAK2 antisera, horseradish peroxidase–coupled goat antirabbit immunoglobulin G (IgG), rabbit antihuman c-Mpl antibody, and anti–P-Tyr antibody py99 were from Upstate Biotechnology (Lake Placid, NY), Kirkegaard and Perry Laboratories (Gaithersburg, MD), IBL (Gunma, Japan), and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Antimouse CD16/32 antibody, R-phycoerythrin (PE)–conjugated hamster antimouse CD61 antibody, and PE-conjugated hamster antihuman CD41 antibody were obtained from BD Pharmingen (San Diego, CA).
Cell lines
UT-7/TPO cells were maintained in liquid culture with Iscoves modified Dulbecco medium (IMDM; Invitrogen, San Diego, CA) containing 10% fetal calf serum (FCS; Moregate Biotech, Melbourne, Australia) and 10 ng/mL rhTPO. UT-7/EPO cells were maintained in the same culture condition as UT-7/TPO cells except for the addition of 1 IU/mL rhEPO instead of rhTPO. Human colon carcinoma cell line, HCT116, was obtained from the American Type Culture Collection (Manassas, VA). The cells were maintained in McCoy 5A medium modified (Invitrogen) supplemented with 10% FCS.
Animals
Seven- or 8-week-old male Balb/c mice were obtained from Charles River Japan (Yokohama, Japan) and given free access to food and water.
Cell proliferation assay
UT-7/TPO and UT-7/EPO cells were starved of corresponding cytokines at 37° C for 24 hours. The cells were resuspended at a density of 1 × 104 cells/0.2 mL IMDM containing 10% FCS and incubated in 96-well plates in the presence of various concentrations of JTZ-132, rhTPO, rhEPO, or vehicle (0.1% dimethyl sulfoxide) at 37° C for 3 days. The assay measurements were done in duplicate, and 3 independent experiments were performed in UT-7/TPO cell assay. HCT116 cells were plated at a density of 3 × 103 cells/0.2 mL McCoy 5A medium modified containing 10% FCS or 0.5% BSA and incubated in 96-well plate in the presence of various concentrations of JTZ-132 or vehicle (0.1% dimethyl sulfoxide) at 37° C for 3 days. The assay was done by hexaplicate measurements. WST-1 was used for the measurement of cell proliferation in accordance with the protocol of the assay kit. The mean proliferation value over control was expressed as a percentage of the maximum response induced by rhTPO, rhEPO, and 10% FCS in UT-7/TPO, UT-7/EPO, and HCT116 cell proliferation assay, respectively.
Western blotting of tyrosine-phosphorylated proteins in UT-7/TPO
UT-7/TPO cells were starved of rhTPO for 24 hours. The cells were washed and resuspended at 2.5 × 106 cells/mL with IMDM and were exposed to JTZ-132, rhTPO, or vehicle (0.1% dimethyl sulfoxide) at 37° C for 10 minutes. After washing with ice-cold phosphate-buffered saline (PBS), the cells were lysed on ice with lysis buffer (pH 7.0) composed of 20 mM N-2-hydroxyethyl piperazine-N′-2-ethanesulphonic acid; 1 mM sodium pyrophosphate, sodium fluoride, sodium orthovanadate and phenylmethyl sulfonylfluoride; and 1% Triton X-100 at 4° C for 30 minutes. Insoluble materials were removed by centrifugation at 15 000g at 4° C for 10 minutes. For the detection of phosphorylated c-Mpl, the supernatants were immunoprecipitated with protein G-Sepharose–conjugated anti–P-Tyr antibody py99 at 4° C for 3 hours. The immunoprecipitates were collected by a brief centrifugation, washed 3 times with lysis buffer, and boiled in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer for 3 minutes. After a brief centrifugation, the supernatants (20 μL/lane) were separated by SDS-PAGE and then electroblotted onto nitrocellulose membrane. The blots were blocked with 3% skim milk in PBS at room temperature for 1 hour and then incubated with primary antibody (rabbit antihuman c-Mpl antibody at 1:50) at room temperature for 1 hour. After washing with PBS containing 0.1% Tween 20, the blots were probed with secondary antibody (horseradish peroxidase–coupled goat antirabbit IgG at 1:5000) at room temperature for 1 hour. After a second wash, the blots were exposed to Hyperfilm ECL and ECL plus detection reagent to enable visualization of phosphotyrosine-containing proteins. For the detection of phosphorylated JAK2, the supernatants from UT-7/TPO cell lysates were immunoprecipitated with protein G Sepharose-conjugated rabbit anti-JAK2 antisera at 4° C for 3 hours. The supernatants of the immunoprecipitated proteins collected as mentioned earlier (20 μL/lane) were separated by SDS-PAGE and then electroblotted onto nitrocellulose membranes. The blots were incubated with py99 as primary antibody and then probed with a 1:5000 dilution of goat antimouse horseradish peroxidase–coupled second antibody and exposed to Hyperfilm ECL to enable visualization of immunoreactive bands. For the detection of phosphorylated-STAT5, the supernatants of the cell lysates mixed with SDS-PAGE sample buffer were separated by SDS-PAGE (20 μL/lane), immunoblotted with a 1:2000 dilution of monoclonal mouse antiphospho-STAT5A/B antibody, and probed with a 1:5000 dilution of goat antimouse horseradish peroxidase–coupled second antibody.
Megakaryocyte differentiation from bone marrow cells
To collect mouse bone marrow cells, mice were killed by cervical dislocation, femurs were harvested, and single-cell suspensions were prepared. Bone marrow cells were collected by centrifugation at 100g at 4° C for 10 minutes, resuspended in IMDM supplemented with 20% BIT9500 (StemCell Technologies, Vancouver, Canada), 5.5 × 10–5 M 2-mercaptoethanol as a serum-free medium. Human bone marrow cells (BioWhittaker, Walkersville, MD) were suspended separately in the same medium as mouse bone morrow cells. The mouse and human bone marrow cells were plated at a concentration of 1 × 106 cells/mL in 24-well plates followed by the addition of JTZ-132, rhTPO, or vehicle control (0.1% dimethyl sulfoxide) and cultured at 37° C for 6 days and 10 days, respectively. The mouse bone marrow cells were preincubated with antimouse CD16/32 (FcγR-blocking antibody) at 4° C for 20 minutes, then incubated with PE-conjugated hamster antimouse CD61 antibody. The human bone marrow cells were incubated with PE-conjugated hamster antihuman CD41 antibody at 4° C for 20 minutes. After washing with PBS containing 0.1% BSA, the cells were resuspended in the same buffer, and the fluorescence intensity was measured by FACSort (Becton Dickinson, Oxford, CA) to analyze cell differentiation. Two experiments were done in duplicate measurements, and the data are expressed as the percentage of CD61+ or CD41+ cells calculated by taking the number of vehicle control cells as 100%. The EC50 (median effective concentration) values of JTZ-132 were calculated from the maximum responses induced by JTZ-132.
CFU-Meg assay
Mouse bone marrow cells were suspended at a concentration of 1 × 106 cells/mL in IMDM supplemented with 20% BIT9500 and 5.5 × 10–5 M 2-mercaptoethanol, and the 0.75-mL suspensions were cultured with 100 ng/mL rhTPO or 1, 3, and 10 μM JTZ-132 in 1.1mg/mL collagen gel of double-chamber slides (StemCell Technologies) at 37° C for 7 days. Collagen gels were dehydrated, fixed, and stained with 10 mg acetylthiocholiniodide in 20 mL of 75 mM sodium phosphate buffer (pH 6.0) containing 5 mM sodium citrate, 3 mM copper sulfate, and 0.5 mM potassium ferricyanide at room temperature for 3.5 hours. The assay was done by using 3 mice with duplicate measurements in each mouse, and the number of colonies (orange-brown) were counted by using a stereoscopic microscope. CFU-Meg was defined as containing at least 3 megakaryocytes per colony.
X-ray irradiation induced hematocytopenia in mice
Mice were exposed to 5 Gy irradiation by using a soft x-ray ionization chamber (M-150WE; Softex, Tokyo, Japan) at day 0. The mice (8 mice per group) received subcutaneous injections of vehicle for rhTPO and rhIL-11 (0.1% BSA/saline), rhTPO (1 μg/0.2 mL/mouse), or rhIL-11 (10 μg/0.2 mL/mouse) once a day, or vehicle for JTZ-132 (30% polyethylene glycol-400/7% hydroxypropyl β-cyclodextrin; 6.67 mL/kg) or JTZ-132 (1, 3, 10 mg/6.67 mL/kg) thrice a day for 4 consecutive days from day 1. Heparinized blood was obtained from the orbital sinus of the mice. The number of platelets, white blood cells (WBCs), and red blood cells (RBCs) were counted at day 9, 11, 14, and 17 for rhTPO and rhIL-11, and at day 8, 10, 13, 15, and 20 for JTZ-132 by using Sysmex F-800 (Toa Medical Electronics, Kobe, Japan).
Busulfan-induced hematocytopenia in mice
Busulfan (6 mg/mL in polyethylene glycol-400) was injected to mice intraperitoneally at 15 mg/kg at day 0 and 3. The mice (8 mice per group) were treated with subcutaneous injections of vehicle for rhIL-11 (0.1% BSA/saline) or rhIL-11 (500 μg/10 mL/kg) or vehicle for JTZ-132 (5% glucose containing 0.02% Simulsol) or JTZ-132 (nano particles; 7.5-30 mg/10 mL/kg), once daily for 8 consecutive days from day 4. Blood cell counts were performed following the same procedure as described earlier at day 10, 12, 15, 18, 22, and 29.
Statistical analysis
Data are expressed as mean ± SEM and were analyzed by using a 1-way analysis of variance (ANOVA) with Student t test or Dunnett test. P values less than .05 were considered to be statistically significant.
Results
Screening of chemical library with UT-7/TPO cells and the proliferation of UT-7/TPO cells but not UT-7/EPO and HCT116 cells by JTZ-132
We have screened more than 100 000 library compounds using UT-7/TPO and UT-7/EPO, cell lines highly sensitive to TPO and EPO, respectively.21,22 The compounds were evaluated in UT-7/TPO cell proliferation assay, then the positive compounds were further tested in UT-7/EPO proliferation assay. The active compounds in UT-7/TPO but not in UT-7/EPO were selected as the precursor molecules and were followed by chemical modifications to have more potency and selectivity in UT-7/TPO than in UT-7/EPO. We named one of the active compounds as JTZ-132. The cell proliferative activity of JTZ-132 in comparison with rhTPO is shown in Figure 2. The maximum proliferation with rhTPO at 100 ng/mL and rhEPO at 1 IU/mL expressed as optical density at 450 nm in WST-1 assay were 1.6 and 2.2, respectively. JTZ-132 exhibited concentration-dependent UT-7/TPO cell proliferation in the concentration range between 0.03 and 3 μM. The maximum effect was achieved at 3 μM, which was comparable to that of rhTPO at 100 ng/mL. The EC50 value of JTZ-132 calculated as a percentage of the maximum response induced by JTZ-132 was 0.43 μM. However, the proliferative response by JTZ-132 in TPO-insensitive UT-7/EPO cells was very weak even at high concentrations (5% of rhEPO at 10 μM). Proliferation of human colon carcinoma cell line, HCT116 in 10% FCS-containing and serum-free medium measured by WST-1 assay were 1.52 and 0.29, respectively, and were not significantly affected by JTZ-132 at concentrations of 0.01 to 10 μM in both conditions. The percentage of proliferation (percentage of FCS control) by JTZ-132 at 10 μM in FCS-containing and serum-free condition were 3.2% and 4.9%, respectively.
Proliferative response of UT-7/TPO cells to JTZ-132. UT-7/TPO cells were deprived of rhTPO overnight and then plated at a density of 1 × 104cells/well in IMDM containing 10% FCS and incubated in the presence of JTZ-132 or rhTPO. After 3 days, the absorbance was measured by the WST-1 assay in duplicate. Each value is calculated as a percentage of that induced by maximal rhTPO (100 ng/mL) or rhEPO (1 IU/mL) response. Data shown are the mean ± SEM of 3 independent experiments in UT-7/TPO cells and single experiment in UT-7/EPO cells.
Proliferative response of UT-7/TPO cells to JTZ-132. UT-7/TPO cells were deprived of rhTPO overnight and then plated at a density of 1 × 104cells/well in IMDM containing 10% FCS and incubated in the presence of JTZ-132 or rhTPO. After 3 days, the absorbance was measured by the WST-1 assay in duplicate. Each value is calculated as a percentage of that induced by maximal rhTPO (100 ng/mL) or rhEPO (1 IU/mL) response. Data shown are the mean ± SEM of 3 independent experiments in UT-7/TPO cells and single experiment in UT-7/EPO cells.
JTZ-132 induces the tyrosine phosphorylation of c-Mpl, JAK2, and STAT5 in UT-7/TPO cells
TPO activates cells through the receptor encoded by the protooncogene, c-Mpl,6 and uses JAK2 and STAT5 by tyrosine phosphorylation of the signals.23-25 The expression of c-Mpl and the same signal transduction was also demonstrated in UT-7/TPO cells.21 Immunoprecipitation with antiphosphotyrosine antibody revealed that c-Mpl was tyrosine phosphorylated by the addition of JTZ-132 at 30 μM and rhTPO at 100 ng/mL in UT-7/TPO cells (Figure 3A). Tyrosine phosphorylation of JAK2 was also detected by JTZ-132 at 30 μM and rhTPO at 1 and 10 ng/mL (Figure 3B). Tyrosine phosphorylation of STAT5 was clearly revealed by JTZ-132 at a concentration of 0.1 μM, increasing concentration dependently, and by rhTPO at 100 ng/mL (Figure 3C). In all cases, phosphorylation of the proteins induced by JTZ-132 was less than that induced by rhTPO at the concentrations used.
Tyrosine phosphorylation of c-Mpl, JAK2, and STAT5 in UT-7/TPO cells stimulated by JTZ-132. UT-7/TPO cells (2.5 × 106 cells/tube) stimulated with JTZ-132 or rhTPO for 10 minutes were lysed by the addition of lysis buffer. (A) Tyrosine phosphorylated proteins were immunoprecipitated with py99, separated by SDS-PAGE, and transferred onto nitrocellulose membrane. The immunoblots were probed with specific antibody of c-Mpl and detected by a horseradish peroxidase–coupled second antibody. (B) JAK2 was immunoprecipitated with specific anti-JAK2 antisera. Immune complexes were separated by SDS-PAGE and transferred onto nitrocellulose membrane. The immunoblots were probed with py99 and horseradish peroxidase–coupled second antibody. (C) Cell lysates were separated by SDS-PAGE and were immunoblotted with antiphospho-STAT5A/B antibody and probed with horseradish peroxidase–coupled second antibody. In all assays, 20 μL of each sample was applied to SDS-PAGE separation. The proteins were visualized by using chemiluminescence detection system.
Tyrosine phosphorylation of c-Mpl, JAK2, and STAT5 in UT-7/TPO cells stimulated by JTZ-132. UT-7/TPO cells (2.5 × 106 cells/tube) stimulated with JTZ-132 or rhTPO for 10 minutes were lysed by the addition of lysis buffer. (A) Tyrosine phosphorylated proteins were immunoprecipitated with py99, separated by SDS-PAGE, and transferred onto nitrocellulose membrane. The immunoblots were probed with specific antibody of c-Mpl and detected by a horseradish peroxidase–coupled second antibody. (B) JAK2 was immunoprecipitated with specific anti-JAK2 antisera. Immune complexes were separated by SDS-PAGE and transferred onto nitrocellulose membrane. The immunoblots were probed with py99 and horseradish peroxidase–coupled second antibody. (C) Cell lysates were separated by SDS-PAGE and were immunoblotted with antiphospho-STAT5A/B antibody and probed with horseradish peroxidase–coupled second antibody. In all assays, 20 μL of each sample was applied to SDS-PAGE separation. The proteins were visualized by using chemiluminescence detection system.
JTZ-132 stimulates human and mouse megakaryocytopoiesis
TPO stimulates platelet production by differentiation and proliferation of bone marrow megakaryocytes and their progenitor cells.6-10 Therefore, we tested whether JTZ-132 affects megakaryocytopoiesis in mouse and human bone marrow cells. We used rhTPO instead of recombinant mouse TPO, because of the cross-reactivity of rhTPO in cell stimulation between mouse and human and the availability of purchasing. Serum-free culture of bone marrow cells with rhTPO (100 ng/mL) for 6 days in mouse and 10 days in human developed increased numbers of CD61+ and CD41+ cells from the mouse and human cells, respectively. Similar results were obtained with JTZ-132 (0.1-3 μM) in a concentration-dependent manner. The full efficacy of JTZ-132 observed at 1 to 3 μM was comparable to that of rhTPO at 100 ng/mL and even higher than rhTPO in human bone marrow cells. The EC50 values of JTZ-132 in mouse and human bone marrow cell assay were 0.23 and 0.27 μM, respectively (Figure 4).
Effect of JTZ-132 on mouse (A) and human (B) bone marrow cell differentiation to megakaryocytes. Mouse and human bone marrow cells were cultured in IMDM supplemented with BIT9500 and 2-mercaptoethanol in the presence of JTZ-132 or rhTPO for 6 days and 10 days, respectively. Megakaryocytes were identified after labeling with PE-antimouse CD61 (mouse) and PE-antihuman CD41 (human) monoclonal antibody. Samples were analyzed with FACSort. The values shown are the means of the results from 2 experiments.
Effect of JTZ-132 on mouse (A) and human (B) bone marrow cell differentiation to megakaryocytes. Mouse and human bone marrow cells were cultured in IMDM supplemented with BIT9500 and 2-mercaptoethanol in the presence of JTZ-132 or rhTPO for 6 days and 10 days, respectively. Megakaryocytes were identified after labeling with PE-antimouse CD61 (mouse) and PE-antihuman CD41 (human) monoclonal antibody. Samples were analyzed with FACSort. The values shown are the means of the results from 2 experiments.
JTZ-132 stimulates mouse CFU-Meg
To evaluate whether JTZ-132 affects early stage of thrombopoiesis, primary mouse bone marrow cells were cultured in collagen gel with various concentrations of JTZ-132, and the CFU-Meg activity was compared with that of rhTPO. Culture (7 days) of the bone marrow cells with rhTPO resulted in 23 CFU-Meg colonies. JTZ-132 generated the colonies in a concentration-dependent manner at 1 to 10 μM, and the activity of JTZ-132 at 10 μM was comparable to that of rhTPO (Figure 5).
Effect of JTZ-132 on mouse CFU-Meg. Mouse bone marrow cells (7.5 × 105 cells/chamber) were cultured with 100 ng/mL rhTPO or 1, 3, and 10 μmol/L JTZ-132 in collagen gel of double-chamber slides at 37°C for 7 days. Collagen gels were dehydrated, fixed, and stained with acetylthiocholiniodide at room temperature for 3.5 hours. The numbers of colonies (orange-brown) were counted with use of a stereoscopic microscope. CFU-Meg was defined as containing at least 3 megakaryocytes per colony. Data represent mean ± SEM of the results from 3 mice.
Effect of JTZ-132 on mouse CFU-Meg. Mouse bone marrow cells (7.5 × 105 cells/chamber) were cultured with 100 ng/mL rhTPO or 1, 3, and 10 μmol/L JTZ-132 in collagen gel of double-chamber slides at 37°C for 7 days. Collagen gels were dehydrated, fixed, and stained with acetylthiocholiniodide at room temperature for 3.5 hours. The numbers of colonies (orange-brown) were counted with use of a stereoscopic microscope. CFU-Meg was defined as containing at least 3 megakaryocytes per colony. Data represent mean ± SEM of the results from 3 mice.
Effect of JTZ-132 on x-ray irradiation-induced thrombocytopenia in mice
The mice exposed to x-ray irradiation showed platelet nadir (< 100 000/μL) at days 8 to 10, and the platelet number recovered approximately 80% by day 20. Subcutaneous injection of rhTPO (1 μg/mouse/d) or rhIL-11 (10 μg/mouse/d) for 4 consecutive days from day 1 showed significantly higher platelet number at nadir at day 9 and accelerated the platelet recovery over the experiment period compared with vehicle-treated control (Figure 6A). Neither cytokine showed drastic changes in WBC or RBC number (Figure 6B-C). Following subcutaneous injections of JTZ-132 (3, 9, 30 mg/kg/d) for 4 consecutive days, significant effect was observed at 30 mg/kg from day 10, and the platelet recovery accelerated through days 13 to 15 (Figure 6D). RBCs were slightly increased only at day 13 in 3 mg/kg/d JTZ-132–treated animals (Figure 6F), whereas WBCs were not significantly affected by JTZ-132 (Figure 6E). Animal body weights in vehicle- and JTZ-132 30 mg/kg/d-treated groups were 19.9 ± 0.6 g and 19.5 ± 0.5 g at day 4, and 24.2 ± 0.3 g and 23.9 ± 0.3 g at day 20, respectively. There were no significant differences between the 2 groups.
Thrombocytopoietic activity of rhTPO, rhIL-11, and JTZ-132 in myelosuppressed mice induced by x-ray irradiation. Changes of platelet (A,D), WBC (B,E), and RBC (C,F) numbers in mice that received 5 Gy irradiation using a soft x-ray ionization chamber at day 0. (A-C) Vehicle for rhTPO and rhIL-11 (0.1% BSA/saline; ○), rhTPO (1 μg/mouse/day, •), or rhIL-11 (10 μg/mouse/day, ▴), and (D-F) vehicle for JTZ-132 (30% polyethylene glycol-400/7% hydroxypropyl β-cyclodextrin, ○) or JTZ-132 (3 mg/kg/day, •; 9 mg/kg/day, ▴; 30 mg/kg/day, ▪) were dosed subcutaneously from days 1 to 4. Heparinized blood was obtained from the sinus vein of the mice at indicated days, and immediately measured the blood cell count by Sysmex F-800. Data represent mean ± SEM of 8 mice. Statistical analysis was performed by Student t test (# indicates P < .05; ##, P < .01) or Dunnett test (*indicates P < .05; **, P < .01) versus corresponding vehicle treatment.
Thrombocytopoietic activity of rhTPO, rhIL-11, and JTZ-132 in myelosuppressed mice induced by x-ray irradiation. Changes of platelet (A,D), WBC (B,E), and RBC (C,F) numbers in mice that received 5 Gy irradiation using a soft x-ray ionization chamber at day 0. (A-C) Vehicle for rhTPO and rhIL-11 (0.1% BSA/saline; ○), rhTPO (1 μg/mouse/day, •), or rhIL-11 (10 μg/mouse/day, ▴), and (D-F) vehicle for JTZ-132 (30% polyethylene glycol-400/7% hydroxypropyl β-cyclodextrin, ○) or JTZ-132 (3 mg/kg/day, •; 9 mg/kg/day, ▴; 30 mg/kg/day, ▪) were dosed subcutaneously from days 1 to 4. Heparinized blood was obtained from the sinus vein of the mice at indicated days, and immediately measured the blood cell count by Sysmex F-800. Data represent mean ± SEM of 8 mice. Statistical analysis was performed by Student t test (# indicates P < .05; ##, P < .01) or Dunnett test (*indicates P < .05; **, P < .01) versus corresponding vehicle treatment.
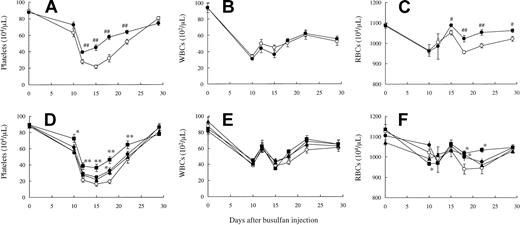
Effect of JTZ-132 on busulfan-induced thrombocytopenia in mice
In the second model of thrombocytopenia, mice were myelosuppressed by cancer chemotherapy agent, busulfan. The busulfan-treated mice showed platelet nadir (< 200 000/μL) at days 15 to 18, and then the platelet number gradually recovered to approximately 50% by day 22. JTZ-132 treatment at 30 mg/kg showed significantly higher platelet number at the nadir and accelerated the platelet recovery compared with vehicle treatment (Figure 7D). This thrombocytopoietic activity of JTZ-132 was almost equipotent to that of rhIL-11 examined in the same experiment (Figure 7A). WBCs were also decreased by busulfan treatment; however, in contrast to the profound effect of JTZ-132 on platelets, the WBCs were not significantly changed by JTZ-132 and rhIL-11 (Figure 7B,E). The busulfan-induced decrease in RBCs was modest, and significant increase was observed compared with vehicle control from day 15 to 29 by rhIL-11, and day 18 at 15 mg/kg/d and at day 18 and 22 at 30 mg/kg/d by JTZ-132 (Figure 7C,F). No significant differences in body weights were observed between vehicle- and JTZ-132–treated animals (the body weights in vehicle- and JTZ-132 30 mg/kg/d-treated groups were 27.0 ± 0.5 g and 27.5 ± 0.5 at day 11, and 28.6 ± 0.4 g and 29.2 ± 0.4 g at day 29, respectively).
Thrombocytopoietic activity of rhIL-11 and JTZ-132 in myelosuppressed mice induced by busulfan. Changes of platelet (A,D), WBC (B,E), and RBC (C,F) numbers in mice that received busulfan. Busulfan was administered intraperitoneally with 2 sequential injections (15 mg/2.5 mL/kg) at day 0 and day 3. (A-C) Vehicle for rhIL-11 (0.1% BSA/saline, ○) or rhIL-11 (500 μg/kg/day, •), and (D-F) vehicle for JTZ-132 (0.02% Simulsol/5% glucose, ○) or JTZ-132 (7.5 mg/kg/d, •; 15 mg/kg/d, ▴;30 mg/kg/d, ▪) were administered subcutaneously from days 4 to 11. Heparinized blood was obtained from the sinus vein of the mice at indicated days, and the blood cell count was immediately measured by Sysmex F-800. Data represent mean ± SEM of 8 mice. Statistical analysis was performed by Student t test (# indicates P < .05; ##, P < .01) and Dunnett test (* indicates P < .05; **, P < .01) versus corresponding vehicle treatment.
Thrombocytopoietic activity of rhIL-11 and JTZ-132 in myelosuppressed mice induced by busulfan. Changes of platelet (A,D), WBC (B,E), and RBC (C,F) numbers in mice that received busulfan. Busulfan was administered intraperitoneally with 2 sequential injections (15 mg/2.5 mL/kg) at day 0 and day 3. (A-C) Vehicle for rhIL-11 (0.1% BSA/saline, ○) or rhIL-11 (500 μg/kg/day, •), and (D-F) vehicle for JTZ-132 (0.02% Simulsol/5% glucose, ○) or JTZ-132 (7.5 mg/kg/d, •; 15 mg/kg/d, ▴;30 mg/kg/d, ▪) were administered subcutaneously from days 4 to 11. Heparinized blood was obtained from the sinus vein of the mice at indicated days, and the blood cell count was immediately measured by Sysmex F-800. Data represent mean ± SEM of 8 mice. Statistical analysis was performed by Student t test (# indicates P < .05; ##, P < .01) and Dunnett test (* indicates P < .05; **, P < .01) versus corresponding vehicle treatment.
Discussion
It has been widely recognized that TPO is involved in megakaryocytopoiesis and thrombocytopoiesis activities in the differentiation and proliferation of platelet-producing cells, megakaryocytes.9-12 Although developing rhTPO for the treatment of thrombocytopenia, many investigators have made efforts to the search for small molecule compounds that can mimic the activity of TPO.17-20 However, to our knowledge, there has been no report that shows in vivo thrombocytopoietic activity by nonpeptide TPO-mimetic compounds in animal models.
We chose to use UT-7/TPO cells, which are dependent on and highly sensitive to TPO. UT-7/TPO cells have morphologically mature megakaryocytic characteristics and are very useful for the study of TPO, which stimulates growth and signal transduction in the cells.21 However, UT-7/EPO cells are EPO-dependent cell lines and do not respond to TPO.22 While using these cell lines to discover the TPO mimetic in our chemical library, we found a series of novel compounds. Following chemical modifications of the selected compounds, we identified JTZ-132 as a synthetic low-molecular-weight TPO mimetic. It was found that JTZ-132 exhibited an EC50 of 0.4 μM in UT-7/TPO cell proliferation assay, showing full efficacy comparable to rhTPO. It is important to note that JTZ-132 showed little effect on TPO-insensitive UT-7/EPO, a subline of UT-7, whereas EPO strongly stimulates cell proliferation of UT-7/EPO21,22 and on HCT116. These observations strongly suggest that JTZ-132 is not a simple cell growth stimulator of human leukemia cells and carcinoma cells. We next proved that JTZ-132 induced phosphorylation of c-Mpl, JAK2, and STAT5 in UT-7/TPO cells in a similar manner to that of rhTPO. A signal transduction study conducted in UT-7/TPO cells indicates that JTZ-132 may stimulate the cell-signaling cascade by initiating phosphorylation of c-Mpl and can mimic an activity of TPO. The phosphorylation of c-Mpl, JAK2, and STAT5 by JTZ-132 was slightly weak even at 30 μM compared with rhTPO, despite the observation of full efficacy of JTZ-132 in UT-7/TPO proliferation at 3 μmol/mL. It is possible that the c-Mpl-stimulating mechanism of JTZ-132 is different from that of TPO. As to receptor binding, we have not yet ascertained whether JTZ-132 directly binds to the same part of the extracellular domain of c-Mpl as rhTPO. Recent information26 about the receptor-binding domain of rhTPO could be of help toward identifying the binding site of JTZ-132. In addition, future studies, including the kinetics of signaling and the extent of receptor dimerization by JTZ-132 in comparison to TPO, would be useful to explain the difference.
TPO supports not only the proliferation of megakaryocytic progenitors but also the differentiation of hematopoietic stem cells and immature megakaryocytes lineage to mature megakaryocytes. It was demonstrated that JTZ-132 stimulated the generation of megakaryocytes as indicated by increased numbers of CD41- and CD61-expressing cells in human and mouse primary bone marrow cells, respectively, and the efficacy of JTZ-132 was almost equal to that of TPO. Furthermore, CFU-Meg was also increased by JTZ-132 in mouse primary bone marrow cells as well as rhTPO. The results suggest that JTZ-132 expands megakaryocytes by stimulating differentiation and proliferation. These activities observed in vitro thus raised the possibility that JTZ-132 may exert thrombocytopoietic effect in vivo.
In our previous experiments, we demonstrated in normal mice that 4 daily subcutaneous injections of JTZ-132 at a dosage of 30 mg/kg/d increased the platelet number by 20% compared with vehicle treatment (data not shown). We then used 2 different animal models of thrombocytopenia induced by x-ray irradiation and by the anticancer drug busulfan in mice. In x-ray irradiation-induced thrombocytopenic model, we observed significant effect in increasing platelet number in peripheral blood at nadir and recovery periods by JTZ-132 injection. The effect of JTZ-132 was considered to be comparable to those of rhTPO and rhIL-11. RhIL-11 is known to be a multifunctional hematopoietic cytokine, affecting lymphopoietic and myeloid/erythroid cells.25 In fact, the thrombocytopoietic activities of rhIL-11 were demonstrated,27,28 and it was developed as the first drug for thrombocytopenic patients in clinics.29 We then studied the effect of JTZ-132 further by comparison with that of rhIL-11 in a cancer chemotherapeutic drug-induced thrombocytopenia model, which shows longer platelet nadir than x-ray irradiation, without severe neutropenia. Because, by injecting subcutaneously more than 8 consecutive days, we found that the vehicle (30% polyethylene glycol-400/7% hydroxypropyl β-cyclodextrin) itself slightly increased platelet number, we used a nanoparticle formulation of JTZ-132 in the busulfan-treated thrombocytopenic model to effect treatment longer than 8 days. The thrombocytopoietic effect of JTZ-132 was also observed in this model; JTZ-132 showed significant effect on the platelet nadir and accelerated platelet recovery. The thrombocytopoietic efficacy of JTZ-132 was equivalent to that of rhIL-11, examined in the same experiment.
In these in vivo experiments, significant increases in RBCs were observed in JTZ-132–treated animals (3 mg/kg at day 13 in x-ray irradiation model, and 15 and 30 mg/kg at day 18 in busulfan model). It is well known that TPO not only stimulates thrombocytopoiesis but also supports erythropoiesis, by expanding erythroid progenitors and inhibiting apoptosis of the progenitors.30-32 In fact, it was demonstrated in several reports that TPO accelerates RBC recovery in myelosuppressed mice.33,34 Therefore, the increase in RBCs observed by JTZ-132 treatment in our myelosuppressed mice may reflect the TPO mimetic activity of JTZ-132. Although a similar effect by rhTPO was not clear in our present study, we previously experienced an increase in RBCs by recombinant mouse TPO treatment in a mouse x-ray irradiation model (data not shown). The species difference in TPO as materials could be the reason for the different effect on RBCs in mice. Importantly, JTZ-132 did not show significant effect on WBCs in the experiments. This may exclude the possibility that JTZ-132 increased the platelet number by nonspecific irritation after subcutaneous injection. It seems that JTZ-132 may act directly on thrombocytopoiesis in bone marrow cells.
Although the thrombocytopoietic dose (30 mg/kg/d) of JTZ-132 in these mice models seems to be rather high, JTZ-132 did not show significant effects at least on animal body weights at all doses tested during the experiments. Although there is no other toxicologic data than the animal body weights, these results suggest that JTZ-132 could be beneficial for the treatment of thrombocytopenia in cancer patients.
In conclusion, we showed in this report that JTZ-132 can mimic the activity of TPO in megakaryocyte proliferation and differentiation in vitro and can significantly increase platelet number at nadir and accelerated platelet recovery in thrombocytopenic mice in vivo. To our knowledge, this is the first report of a nonpeptide small molecular TPO mimic, exhibiting thrombocytopoietic activity in animal models.
Prepublished online as Blood First Edition Paper, March 4, 2004; DOI 10.1182/blood-2003-10-3623.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank J. Maruhashi and N. Miyagawa for their technical assistance, and H. Watanabe for his research coordination over this study.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal