Abstract
Dendritic cells (DCs) are fundamental for immunity. We investigated reconstitution of plasmacytoid DC (PDC) and myeloid DC (My-DC) precursors in the first 2 months after allogeneic hematopoietic stem cell transplantation (Allo-HSCT). Circulating DCs were monitored from the earliest phase of hematopoietic reconstitution in 43 children given standard therapy to prevent graft-versus-host disease (GVHD) and either treated or untreated with granulocyte colony-stimulating factor (G-CSF) after HSCT. In patients without GVHD, both My-DCs and PDCs reached consistently high absolute values during the initial phase. Time of engraftment did not differ between My-DCs and PDCs, regardless of administration of G-CSF. Treatment with G-CSF (1) accelerated early recovery of My-DC absolute numbers; (2) was associated with lower numbers of both My-DCs and PDCs in the later phase; and (3) significantly reduced the proportion of interleukin-12 (IL-12)–secreting cells. In some patients who developed acute GVHD, we found high numbers of circulating DC precursors during the early phase of this complication. However, treatment with steroids invariably induced rapid decrease of PDCs. Altogether, these data provide an evaluation of DC release after Allo-HSCT, indicate that postgrafting administration of G-CSF impairs the appearance of IL-12–producing DCs, and suggest that DC homeostasis may be disrupted at onset of GVHD.
Introduction
Allogeneic hematopoietic stem cell transplantation (Allo-HSCT) after myeloablative conditioning constitutes the treatment of choice for several hematologic malignancies and congenital diseases, but it can be severely affected by major complications.1 Myeloablative conditioning abrogates generation of leukocytes by recipient hematopoietic stem cells, and patients have to face profound aplasia, leading to high risk of developing opportunistic infections. During such a limited but crucial time interval, neogenerated leukocytes released into peripheral blood and participating in innate immune response are of fundamental importance for conferring early capability to resist environmental pathogens. Dendritic cells (DCs) constitute one of the key cellular components of innate immunity.2 DCs become activated and exert disparate functions either when pattern-recognition receptors on their surface recognize distinctive pathogen-associated molecular patterns on the surface of microorganisms3 or when they perceive endogenous danger signals.4 The innate response provided by DCs consists mainly of release of cytokines such as type-I interferons (IFNs), tumor-necrosis factor alfa (TNF-α), interleukin-12 (IL-12), among others.5 Furthermore, myeloid DCs expressing high levels of CD16 (Fc receptor γIII) can act as effectors by exerting potent antibody-dependent cell cytotoxicity.6 As innate resistance to common infections depends, at least in part, on availability of DCs, it would be important to know how efficiently they are reconstituted after Allo-HSCT.
A life-threatening complication occurring after Allo-HSCT is graft-versus-host disease (GVHD). GVHD is thought to be mediated by donor T cells that must be sensitized by DCs whose origin is still questioned.7 Intriguingly, DCs resistant to myeloablative conditioning can persist within tissues of recipients in numbers sufficient to induce acute GVHD in mice.8 However, a recent study in human patients has clearly shown that, regardless of the clinical history and of the conditioning regimen, peripheral blood DCs are completely replaced by donor cells within 2 weeks after transplantation.9 These donor-derived DCs, present in the peripheral blood of patients, may be contributed by mature cells transferred with the graft and/or by recapitulated DC myelopoiesis. Several pieces of evidence indeed suggest that DC subtypes administered with grafts somehow can influence the incidence and severity of GVHD in patients who underwent transplantation.10,11 Nonetheless, DCs directly transferred with the graft cannot contribute to stable reconstitution because of their deficiency in replicative potential12 and their short survival.13 Ultimately, recovery of circulating DCs is provided by neogenerated hematopoiesis from progenitors of donor origin.9 Circumstantial evidence concurs to suggest that there is a relationship between neogenerated DC subtypes and incidence of GVHD.14 However, there are no human data so far available on this issue.
The study of DC subtypes in vivo in humans has been made difficult by issues concerning their functional plasticity15 and because, in contrast to what happens with mice, blood is the only readily available human source of DCs. Human blood is a fertile source of both DC precursors and immature DCs that are heterogeneous for expression of a range of markers,16 but many of these differences may simply reflect variability either in maturation or in activation state. Despite such limitations, recent studies on human blood DC subsets have shown that there exist at least 2 well-distinct subtypes of DC precursors, namely myeloid DC (My-DC) and plasmacytoid DC (PDC) precursors, each bearing distinct toll-like receptors (TLRs) and producing different innate cytokine responses.17 In particular, PDCs express TLR-9, which binds unmethylated cytosine-guanine (CpG) motifs of microbial origin18 and produces massive amounts of type-I IFN.19 Thus, the notion that PDC and My-DC subtypes have distinct functions is consistent with putatively separated developmental pathways, but it is currently unknown whether they regenerate separately after Allo-HSCT.
In this study, we have closely monitored by ex-vivo analysis PDC and My-DC precursors in patients treated with Allo-HSCT. While PDC precursors were evaluated as a unique population, My-DC precursors were evaluated as a heterogeneous population consisting of 3 partially separated subsets, as we have already described elsewhere.20 Of these, CD14+ monocytes are by far the most abundant My-DC precursors, but 2 smaller myeloid mononuclear subsets, expressing CD16 or CD2 and rather negligible levels of CD14 surface antigen, have also been shown to differentiate into mature My-DCs that closely resemble those resident in secondary lymphoid organs and in most peripheral tissues.12,16,21-23 We analyzed separately patients who did or did not experience GVHD and who did or did not receive granulocyte colony-stimulating factor (G-CSF), in order to dissect the influence of these 2 variables on DC recovery.
Patients, materials, and methods
Study subjects and clinical protocols
In the period between May 2000 and November 2003, 43 patients undergoing Allo-HSCT after a myeloablative conditioning regimen at the Pediatric Hematology/Oncology Unit of the Policlinico S. Matteo in Pavia were enrolled in this study. Blood samples of patients and healthy subjects were collected after obtaining informed consent from parents, and this study was approved by the institutional review board. In patients with malignancies who received an allogeneic hematopoietic stem cell (Allo-HSC) transplant from a family donor, GVHD prophylaxis consisted of cyclosporin-A alone (Cs-A, 1-3 mg/kg per day intravenously for the first 28 days and then orally at a dosage of 3-5 mg/kg per day for 4-6 further months), whereas recipients of an unrelated donor allograft received a combination of Cs-A, short-term methotrexate, and horse anti–thymocyte globulin24 (ATG, 7-11.25 mg/kg from day –4 to day –2). Patients with nonmalignant disorders who received transplants from an HLA-compatible family donor were given GVHD prophylaxis with Cs-A and short-term methotrexate according to the schedule previously described elsewhere.25 The majority of patients studied (n = 25) was treated with G-CSF after transplantation starting from day 7 after transplantation until recovery of an absolute neutrophil count (ANC) higher than 2 × 109/L,26 whereas the remaining 18 patients did not receive any growth factor after transplantation. Myeloid and platelet engraftment were defined as the first 3 consecutive days with an ANC higher than 0.5 × 109/L and unsupported platelet count higher than 50 × 109/L, respectively. Acute GVHD was diagnosed and graded according to previously reported criteria.27 All patients surviving more than 7 days after transplantation were considered at risk for developing acute GVHD. At the time of the investigations, all patients were in complete hematopoietic chimerism as demonstrated through study of genetic polymorphism of variable number of tandemly repeated short DNA sequences, according to a protocol described elsewhere.25,28 Patients were considered assessable for evaluation of the reconstitution kinetics of PDC and My-DC precursors if they were exempt from GVHD, steroid treatment, and other major life-threatening complications during the 2 months following hematopoietic transplantation. Table 1 summarizes characteristics of such selected cohort composed of 12 and 14 patients who did or did not receive G-CSF, respectively.
Characteristics of patients who did not have GVHD or did not require treatment with steroids
Patient no. . | Age, y + mo . | Sex . | Disease . | Donor (sex) . | Graft source . | Cells/kg, × 108 . | ATG . | Growth factor . | ANC recovery, d . | PLT recovery, d . | Conditioning regimen . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 + 5/12 | F | ALL | UD (M) | BM | 4.0 | Y | G-CSF | +15 | +21 | TBI/TT/CY | Dead (relapse) |
| 2 | 8 + 4/12 | M | ALL | UD (F) | BM | 6.3 | Y | G-CSF | +18 | +22 | TBI/TT/CY | Alive |
| 3 | 10 + 5/12 | M | SAA | UD (F) | BM | 6.8 | Y | G-CSF | +17 | +21 | CY/FLU | Alive |
| 4 | 19 | M | Thal | Sibl (M) | BM | 3.0 | N | G-CSF | +18 | +29 | BU/TT/FLU | Alive |
| 5 | 16 | F | SAA | UD (F) | BM | 3.0 | Y | G-CSF | +18 | +22 | CY/FLU | Alive |
| 6 | 5 | M | SAA | Sibl (F) | BM | 6.6 | N | G-CSF | +14 | +19 | CY | Alive |
| 7 | 9 + 3/12 | M | Thal | Sibl (M) | BM | 2.2 | N | G-CSF | +11 | +21 | BU/TT/FLU | Alive |
| 8 | 18 + 7/12 | F | MDS | UD (F) | BM | 2.5 | Y | G-CSF | +18 | +27 | BU/CY/L-PAM | Alive |
| 9 | 9 + 2/12 | F | Thal | Sibl (M) | BM | 4.0 | N | G-CSF | +12 | +16 | BU/TT/FLU | Alive |
| 10 | 7 + 6/12 | M | AML | Sibl (M) | BM | 5.0 | N | G-CSF | +13 | +25 | BU/CY/L-PAM | Alive |
| 11 | 13 + 2/12 | F | ALL | Sibl (M) | BM | 4.8 | N | G-CSF | +9 | +14 | TBI/TT/CY | Alive |
| 12 | 17 + 7/12 | M | ALL | UD (F) | BM | 6.8 | Y | G-CSF | +16 | +34 | TBI/TT/CY | Alive |
| 13 | 1 + 2/12 | F | AML | Sibl (F) | BM | 7.0 | N | Nil | +17 | +22 | BU/CY/L-PAM | Alive |
| 14 | 7 + 6/12 | F | Thal | Sibl (M) | CB | 0.38 | N | Nil | +12 | +17 | BU/TT/FLU | Alive |
| 15 | 7 + 4/12 | M | AML | UD (M) | BM | 2.6 | Y | Nil | +17 | +22 | BU/CY/L-PAM | Alive |
| 16 | 17 + 3/12 | M | ALL | UD (M) | BM | 3.8 | Y | Nil | +17 | +23 | TBI/TT/CY | Dead (relapse) |
| 17 | 14 | M | Thal | Sibl (F) | BM | 4.5 | N | Nil | +16 | +32 | BU/TT/FLU | Alive |
| 18 | 14 + 10/12 | M | SAA | Sibl (M) | BM | 2.2 | N | Nil | +21 | +26 | CY | Alive |
| 19 | 16 + 2/12 | M | AML | Sibl (F) | BM | 2.5 | N | Nil | +17 | +35 | BU/CY/L-PAM | Alive |
| 20 | 17 | F | AML | Sibl (M) | BM | 2.5 | N | Nil | +14 | +27 | BU/CY/L-PAM | Alive |
| 21 | 13 + 3/12 | F | Thal | Sibl (M) | BM | 4.5 | N | Nil | +20 | +20 | BU/TT/FLU | Alive |
| 22 | 3 + 1/12 | M | MDS | UD (F) | BM | 15.0 | Y | Nil | +17 | +22 | BU/CY/L-PAM | Alive |
| 23 | 18 | F | Thal | Sibl (F) | BM | 4.5 | N | Nil | +21 | +33 | BU/TT/FLU | Alive |
| 24 | 13 + 11/12 | F | ALL | UD (M) | BM | 6.0 | Y | Nil | +22 | +22 | TBI/TT/CY | Alive |
| 25 | 8 + 6/12 | F | ALL | UD (M) | BM | 6.3 | Y | Nil | +22 | +29 | TBI/TT/CY | Alive |
| 26 | 11 | M | ALL | Sibl (M) | BM | 9.0 | N | Nil | +13 | +15 | TBI/TT/CY | Alive |
Patient no. . | Age, y + mo . | Sex . | Disease . | Donor (sex) . | Graft source . | Cells/kg, × 108 . | ATG . | Growth factor . | ANC recovery, d . | PLT recovery, d . | Conditioning regimen . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 + 5/12 | F | ALL | UD (M) | BM | 4.0 | Y | G-CSF | +15 | +21 | TBI/TT/CY | Dead (relapse) |
| 2 | 8 + 4/12 | M | ALL | UD (F) | BM | 6.3 | Y | G-CSF | +18 | +22 | TBI/TT/CY | Alive |
| 3 | 10 + 5/12 | M | SAA | UD (F) | BM | 6.8 | Y | G-CSF | +17 | +21 | CY/FLU | Alive |
| 4 | 19 | M | Thal | Sibl (M) | BM | 3.0 | N | G-CSF | +18 | +29 | BU/TT/FLU | Alive |
| 5 | 16 | F | SAA | UD (F) | BM | 3.0 | Y | G-CSF | +18 | +22 | CY/FLU | Alive |
| 6 | 5 | M | SAA | Sibl (F) | BM | 6.6 | N | G-CSF | +14 | +19 | CY | Alive |
| 7 | 9 + 3/12 | M | Thal | Sibl (M) | BM | 2.2 | N | G-CSF | +11 | +21 | BU/TT/FLU | Alive |
| 8 | 18 + 7/12 | F | MDS | UD (F) | BM | 2.5 | Y | G-CSF | +18 | +27 | BU/CY/L-PAM | Alive |
| 9 | 9 + 2/12 | F | Thal | Sibl (M) | BM | 4.0 | N | G-CSF | +12 | +16 | BU/TT/FLU | Alive |
| 10 | 7 + 6/12 | M | AML | Sibl (M) | BM | 5.0 | N | G-CSF | +13 | +25 | BU/CY/L-PAM | Alive |
| 11 | 13 + 2/12 | F | ALL | Sibl (M) | BM | 4.8 | N | G-CSF | +9 | +14 | TBI/TT/CY | Alive |
| 12 | 17 + 7/12 | M | ALL | UD (F) | BM | 6.8 | Y | G-CSF | +16 | +34 | TBI/TT/CY | Alive |
| 13 | 1 + 2/12 | F | AML | Sibl (F) | BM | 7.0 | N | Nil | +17 | +22 | BU/CY/L-PAM | Alive |
| 14 | 7 + 6/12 | F | Thal | Sibl (M) | CB | 0.38 | N | Nil | +12 | +17 | BU/TT/FLU | Alive |
| 15 | 7 + 4/12 | M | AML | UD (M) | BM | 2.6 | Y | Nil | +17 | +22 | BU/CY/L-PAM | Alive |
| 16 | 17 + 3/12 | M | ALL | UD (M) | BM | 3.8 | Y | Nil | +17 | +23 | TBI/TT/CY | Dead (relapse) |
| 17 | 14 | M | Thal | Sibl (F) | BM | 4.5 | N | Nil | +16 | +32 | BU/TT/FLU | Alive |
| 18 | 14 + 10/12 | M | SAA | Sibl (M) | BM | 2.2 | N | Nil | +21 | +26 | CY | Alive |
| 19 | 16 + 2/12 | M | AML | Sibl (F) | BM | 2.5 | N | Nil | +17 | +35 | BU/CY/L-PAM | Alive |
| 20 | 17 | F | AML | Sibl (M) | BM | 2.5 | N | Nil | +14 | +27 | BU/CY/L-PAM | Alive |
| 21 | 13 + 3/12 | F | Thal | Sibl (M) | BM | 4.5 | N | Nil | +20 | +20 | BU/TT/FLU | Alive |
| 22 | 3 + 1/12 | M | MDS | UD (F) | BM | 15.0 | Y | Nil | +17 | +22 | BU/CY/L-PAM | Alive |
| 23 | 18 | F | Thal | Sibl (F) | BM | 4.5 | N | Nil | +21 | +33 | BU/TT/FLU | Alive |
| 24 | 13 + 11/12 | F | ALL | UD (M) | BM | 6.0 | Y | Nil | +22 | +22 | TBI/TT/CY | Alive |
| 25 | 8 + 6/12 | F | ALL | UD (M) | BM | 6.3 | Y | Nil | +22 | +29 | TBI/TT/CY | Alive |
| 26 | 11 | M | ALL | Sibl (M) | BM | 9.0 | N | Nil | +13 | +15 | TBI/TT/CY | Alive |
ANC indicates absolute neutrophil count (day of recovery after transplantation); PLT, platelets (day of recovery after transplantation); F, female; ALL, acute lymphoblastic leukemia; UD, unrelated donor; BM, bone marrow; Y, yes; TBI, total body irradiation; TT, thiotepa; CY, cyclophosphamide; M, male; SAA, severe aplastic anemia; FLU, fludarabine; Thal, thalassemia major; Sibl, sibling; N, no; BU, busulfan; MDS, myelodysplastic syndrome; L-PAM, melphalan; AML, acute myelogenous leukemia; and CB, cord blood.
Among the remaining 17 patients considered not fully assessable for evaluation of the reconstitution kinetics of DCs, 2 and 1 died of acute and chronic GVHD, respectively. We studied the DC subsets on 6 of these 17 patients at the time of onset of GVHD and Table 2 reports their characteristics.
Characteristics of patients who had GVHD and were treated with steroids
Patient no. . | Age, y + mo . | Sex . | Disease . | Donor (sex) . | Cells/kg, × 108 . | ATG . | Growth factor . | ANC recovery, d . | PLT recovery, d . | Conditioning regimen . | Acute GVHD, grade . | Chronic GVHD . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 + 8/12 | M | HLH | UD (M) | 10.0 | Y | G-CSF | +14 | +18 | BU/TT/FLU | II | No | Alive |
| 2 | 10 | M | ALL | UD (F) | 4.7 | Y | G-CSF | +18 | +22 | TBI/TT/CY | I | Yes, limited | Dead |
| 3 | 9 + 2/12 | M | ALL | UD (M) | 6.2 | Y | G-CSF | +15 | +21 | TBI/TT/CY | II | No | Alive |
| 4 | 7 | M | ALL | UD (F) | 3.0 | Y | G-CSF | +18 | +50 | TBI/TT/CY | II | Yes, extensive | Dead |
| 5 | 17 | F | ALL | Sibl (F) | 3.0 | N | NIL | +16 | +30 | TBI/TT/CY | III | Yes, limited | Alive |
| 6 | 6 + 1/12 | F | AML | UD (M) | 8.0 | Y | NIL | +10 | +29 | BU/CY/L-PAM | II | Nonevaluable | Alive |
Patient no. . | Age, y + mo . | Sex . | Disease . | Donor (sex) . | Cells/kg, × 108 . | ATG . | Growth factor . | ANC recovery, d . | PLT recovery, d . | Conditioning regimen . | Acute GVHD, grade . | Chronic GVHD . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 + 8/12 | M | HLH | UD (M) | 10.0 | Y | G-CSF | +14 | +18 | BU/TT/FLU | II | No | Alive |
| 2 | 10 | M | ALL | UD (F) | 4.7 | Y | G-CSF | +18 | +22 | TBI/TT/CY | I | Yes, limited | Dead |
| 3 | 9 + 2/12 | M | ALL | UD (M) | 6.2 | Y | G-CSF | +15 | +21 | TBI/TT/CY | II | No | Alive |
| 4 | 7 | M | ALL | UD (F) | 3.0 | Y | G-CSF | +18 | +50 | TBI/TT/CY | II | Yes, extensive | Dead |
| 5 | 17 | F | ALL | Sibl (F) | 3.0 | N | NIL | +16 | +30 | TBI/TT/CY | III | Yes, limited | Alive |
| 6 | 6 + 1/12 | F | AML | UD (M) | 8.0 | Y | NIL | +10 | +29 | BU/CY/L-PAM | II | Nonevaluable | Alive |
The graft source was bone marrow for all patients.
ANC indicates absolute neutrophil count (day of recovery after transplantation); PLT, platelets (day of recovery after transplantation); HLH, hemophagocytic lymphohistiocytosis; UD, unrelated donor; BU, busulfan, TT, thiotepa; FLU, fludarabine; ALL, acute lymphoblastic leukemia; TBI, total body irradiation; CY, cyclophosphamide; Sibl, sibling; AML, acute myelogenous leukemia; and L-PAM, melphalan.
Monoclonal antibodies and reagents
The following fluorescein isothiocyanate (FITC), phycoerythrin (PE), or phycoerythrin cyanin 5.1 (PC-5) directly conjugated monoclonal antibodies (mAbs) were used to perform the cell surface staining: Anti-CD14 FITC (M5E2, immunoglobulin G2a [IgG2a]), anti-CD16 FITC and PE (3G8, IgG1), anti-CD2 PE (RPA-2.10, IgG1), anti-CD4 Cychrome (RPA-T4, IgG1), anti–HLA-DR FITC (G46-6, IgG2a), and anti-CD123 PE (9F5, IgG1) were purchased from Pharmingen (Becton Dickinson Immunocytometry System [BDIS]; San Diego, CA); anti-CD33 PC-5 (D3HL60.251, IgG1) was purchased from Coulter-Immunotech (Hialeah, FL); and anti–BDCA-2 FITC (AC144, IgG1) was purchased from Miltenyi Biotec (Bergish Gladbach, Germany).
To perform intracellular cytokine staining, the following mAbs and reagents were used. Anti–TNF-α PE (Mab11, IgG1), anti–IL-12 PE p40/p70 (C11.5, IgG1), and rat anti–mouse Igκ light-chain PE (X36) were purchased from Pharmingen; unconjugated anti–IFN-α (MMHA-11, IgG1k) was purchased from PBL Biomedical Laboratories (New Brunswick, NJ); and the Fix and Perm kit was from Caltag Laboratories (San Francisco, CA).
The following stimuli were used to perform the functional assays: Lipopolysaccharide (LPS, from Escherichia coli, serotype 055:B5; Sigma, St Louis, MO), brefeldin A (BFA; Sigma), and the CpG-oligodeoxynucleotide (CpG-ODN) 221629 5′-ggGGGACGATCGTCgggggG-3′ were purchased from Eurogentec (Seraing, Belgium).
Cell surface staining
Myeloid reconstitution was evaluated daily during the first month after Allo-HSCT; afterward, patients were evaluated at 40 and 50 days after transplantation.
Cells were prepared as previously described.20 Briefly, at least 100 μL EDTA (ethylenediaminetetraacetic acid) containing freshly drawn blood was used for each staining tube. Cells were incubated for 20 minutes on ice with mAbs (1 μg per million leukocytes). Red cells lysis was obtained by adding 2 mL of fluorescence-activated cell sorter (FACS) lysing solution (BDIS) to samples and leaving them 10 minutes at room temperature in the dark, according to the manufacturer's instructions. After 2 passages in washing buffer, cells were resuspended in FACS flow (BDIS) and acquired by flow cytometry.
Cell culture and intracellular cytokine staining
Peripheral blood mononuclear cells (PBMCs) were obtained from 1 to 2 mL of whole blood collected in EDTA and processed by standard Ficoll-Paque (AmershamPharmacia Biotech AB, Uppsala, Sweden) density gradient centrifugation. PBMCs were suspended in RPMI 1640 with stable l-glutamine culture medium (Euroclone, Wetherby, United Kingdom), supplemented with 10% fetal calf serum (FCS; Euroclone), 100 U/mL penicillin, and 100 μg/mL streptomycin (Euroclone) and incubated at 37° C in a humidified 5% CO2-containing atmosphere with LPS (100 ng/mL) and BFA (1 μg/mL) or with CpG–ODN 2216 (5 μg/mL). PBMC stimulation lasted overnight for LPS (16-20 hours) and 5 hours for ODN. After stimulation, cells were harvested, washed, suspended in PBS containing 1% FCS, 1% human serum, 10% mouse serum, and 0.01% sodium-azide, and differently stained to detect distinct intracellular cytokine production. To identify cytokine secretion by LPS-stimulated My-DCs, a double-surface staining with anti-CD14 FITC and anti-CD33 PC-5 mAbs was performed, and the TNF-α and IL-12 production was then evaluated. After a 20-minute incubation on ice, cells were washed with washing buffer and fixed and permeabilized using a Fix and Perm kit, according to the manufacturer's instructions. For intracellular cytokine detection, either anti–TNF-α or anti–IL-12 mAbs were added.
To identify cytokine secretion by ODN-stimulated PDCs, first we verified that HLA-DR+/CD123++ cells were also BDCA-2+, and then intracellular IFN-α production was analyzed in BDCA-2 positively labeled cells. For detection of IFN-α, cells were again suspended in 100 μL permeabilization buffer and stained with rat anti–mouse Igκ light chain. Finally, cells were suspended in FACS flow and acquired.
Flow cytometry
Flow cytometry was performed using a FACScalibur flow cytometer (BDIS) equipped with a 488-nm argon laser and operated with CellQuest software (BDIS). Instrument setting was established as previously described20 : First unstained leukocytes were acquired, and then the limits of negative controls were defined by leukocytes stained with IgG of irrelevant specificity. For cell surface staining, compensation was adjusted by acquiring cells stained with each brightly positive single fluorochrome-conjugated mAb and then by running the same single-stained sample mixed altogether.
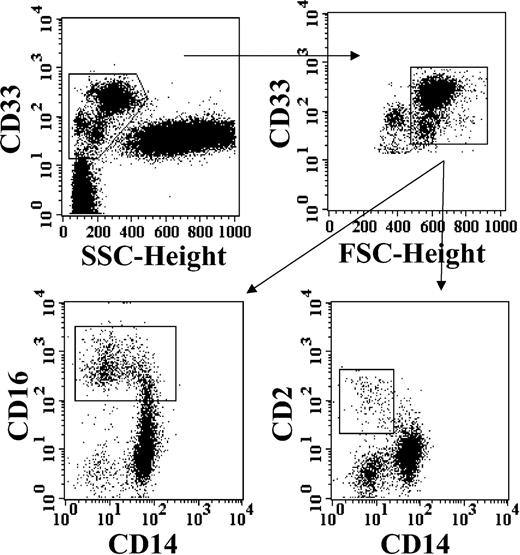
For kinetics description, at least 1 × 105 viable nucleated total cells was acquired according to linear forward light scatter (FSC) versus linear side light scatter (SSC). My-DCs were identified as CD33+ cells with a low SSC and a high FSC, by sequential gating analysis (Figure 1). Then, among My-DCs, 3 cell populations were identified by their different expression of CD14, CD16, and CD2 surface antigens (Figure 1). PDCs were identified as mononuclear cells (according to SSC and FSC features), which were HLA-DR+, CD4+, and brightly positive (++) for expression of CD123.
Identification of distinct subsets of DC precursors from pediatric patients recovering from Allo-HSCT. Top plots: sequential progressive gating of a population of large mononuclear myeloid cells, by combination of CD33 expression with light scatter parameters (SSC and FSC, as described in “Patients, materials, and methods”). Bottom plots: analysis of CD33+ mononuclear large cells from the gate selected in the top right plot, by 3-color counterstaining with CD14, CD16, and CD2.
Identification of distinct subsets of DC precursors from pediatric patients recovering from Allo-HSCT. Top plots: sequential progressive gating of a population of large mononuclear myeloid cells, by combination of CD33 expression with light scatter parameters (SSC and FSC, as described in “Patients, materials, and methods”). Bottom plots: analysis of CD33+ mononuclear large cells from the gate selected in the top right plot, by 3-color counterstaining with CD14, CD16, and CD2.
DC morphology
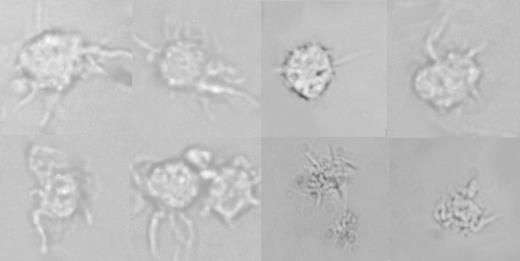
PBMCs were isolated from peripheral blood by Ficoll-Paque gradient separation, suspended in complete culture medium, and left overnight at 37° C in 5% CO2-containing atmosphere. On the following day, cells were overlayered onto 2 mL of 14% (wt/vol) metrizamide (Sigma) in 10% human serum RPMI and centrifuged at 400g for 15 minutes at room temperature, as previously described.22,30,31 Cells in the low-density layer (DC enriched) were washed twice, resuspended in RPMI–10% FCS, and plated in 96-well plates at 37° C in 5% CO2-containing atmosphere. After maturation, cell morphology was directly observed by optical microscopy (Figure 2). Direct optical microscopy visualization was obtained by a Leitz Wetzlar Ortholux microscope (Wetzlar, Germany); original magnification, × 45. Imaging medium was RPMI 1640 with 10% FCS as described. Pictures were taken with a Leica camera (WILD MPS52; Heerburg, Switzerland), and were acquired by a Canberra Packard scanner (Milan, Italy) made in Taiwan (Umaxdata Systems).
Direct visualization of cells with dendritic morphology from patients recovering from Allo-HSCT. Pictures were taken by direct optical microscopy of viable cells obtained from the low-density layer after centrifugation over hypertonic gradient following overnight culture of PBMCs isolated from 1 to 2 mL of peripheral blood. Original magnification, × 45.
Direct visualization of cells with dendritic morphology from patients recovering from Allo-HSCT. Pictures were taken by direct optical microscopy of viable cells obtained from the low-density layer after centrifugation over hypertonic gradient following overnight culture of PBMCs isolated from 1 to 2 mL of peripheral blood. Original magnification, × 45.
Statistical analysis
Data are shown throughout as median values with interquartiles. Data from patients treated or not with G-CSF were compared by nonparametric Mann-Whitney test. To compare results from patients observed before and after the onset of GVHD, paired analysis was performed using Student t test. P values less than .05 were considered significant and marked with asterisks in Figures 4 and 5.
Dynamics of percentages of distinct plasmacytoid and myeloid DC precursors in patients treated or untreated with G-CSF after Allo-HSCT. Median values with interquartiles (error bars) were obtained from patients without evidence of GVHD or any other major complication. Data from a group of 12 patients treated with G-CSF (•) and from a group of 14 untreated patients (○) are shown. Data were compared by nonparametric Mann-Whitney test. *P < .05 was considered. Also, data from 10 age-matched healthy subjects and from 15 patients of each group obtained before Allo-HSCT are shown as □ and ▪ on the left side of each graph, respectively.
Dynamics of percentages of distinct plasmacytoid and myeloid DC precursors in patients treated or untreated with G-CSF after Allo-HSCT. Median values with interquartiles (error bars) were obtained from patients without evidence of GVHD or any other major complication. Data from a group of 12 patients treated with G-CSF (•) and from a group of 14 untreated patients (○) are shown. Data were compared by nonparametric Mann-Whitney test. *P < .05 was considered. Also, data from 10 age-matched healthy subjects and from 15 patients of each group obtained before Allo-HSCT are shown as □ and ▪ on the left side of each graph, respectively.
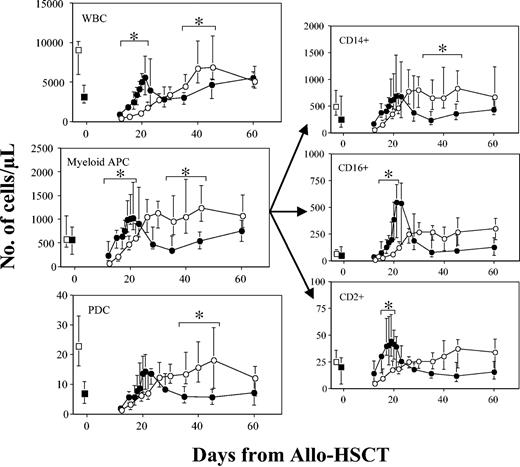
Dynamics of absolute values of WBCs and distinct plasmacytoid and myeloid DC precursors in patients treated or untreated with G-CSF after Allo-HSCT. Median values with interquartiles (error bars) were obtained from patients without evidence of GVHD or any other major complication. Data from a group of 12 patients treated with G-CSF (•) and from a group of 14 untreated patients (○) are shown. Data were compared by nonparametric Mann-Whitney test. *P < .05 was considered significant. Also, data from 10 age-matched healthy subjects and from 15 patients of each group obtained before Allo-HSCT are shown as □ and ▪ on the left side of each graph, respectively.
Dynamics of absolute values of WBCs and distinct plasmacytoid and myeloid DC precursors in patients treated or untreated with G-CSF after Allo-HSCT. Median values with interquartiles (error bars) were obtained from patients without evidence of GVHD or any other major complication. Data from a group of 12 patients treated with G-CSF (•) and from a group of 14 untreated patients (○) are shown. Data were compared by nonparametric Mann-Whitney test. *P < .05 was considered significant. Also, data from 10 age-matched healthy subjects and from 15 patients of each group obtained before Allo-HSCT are shown as □ and ▪ on the left side of each graph, respectively.
Results
Identification and classification of peripheral blood DC precursors after Allo-HSCT
We distinguished a unique population of PDC precursors as mononuclear cells lacking lineage markers and bearing very high levels of CD123 along with CD4 (Figure 3), HLA-DR, and BDCA-2 (not shown). The identification of My-DC precursors was more elaborate. As already mentioned, distinct myeloid mononuclear cells bearing either CD14, CD16, or CD2 surface antigen have been reported to give rise to DCs. Therefore, we preliminarily classified all of these DC precursors as a population consisting of large mononuclear cells, expressing various myeloid antigens such as CD33, CD4, CD11c, as well as HLA-DR.20 As shown in Figure 1, 3 distinct subsets could be identified on the basis of the expression of CD14, CD16, and CD2. The largest subset was constituted by CD14+ monocytes and by 2 other subpopulations, expressing gradually lower levels of CD14 surface antigen, along with increasing levels of CD16 and CD2. It should be noted that expression of CD16 and CD2 was reciprocally exclusive (not shown), while the small subset usually described for its lineage marker defect was equivalent to CD2+CD14– cells.20
Progressive appearance of distinct plasmacytoid and myeloid DC precursors in whole peripheral blood after Allo-HSCT. Dot plots were obtained as reported in “Patients, materials, and methods” at the indicated time points from a patient treated with Allo-HSCT for severe aplastic anemia. Before Allo-HSCT, this patient had a significant number of lymphocytes but was totally deficient in myeloid and plasmacytoid DC precursors. After Allo-HSCT, there was a gradual and progressive appearance of myeloid and plasmacytoid mononuclear cells. The percentages of DC subtypes are referred to WBC.
Progressive appearance of distinct plasmacytoid and myeloid DC precursors in whole peripheral blood after Allo-HSCT. Dot plots were obtained as reported in “Patients, materials, and methods” at the indicated time points from a patient treated with Allo-HSCT for severe aplastic anemia. Before Allo-HSCT, this patient had a significant number of lymphocytes but was totally deficient in myeloid and plasmacytoid DC precursors. After Allo-HSCT, there was a gradual and progressive appearance of myeloid and plasmacytoid mononuclear cells. The percentages of DC subtypes are referred to WBC.
To confirm that DC precursors of patients treated with Allo-HSCT could differentiate without addition of exogenous cytokine into typical DCs, a small amount of PBMCs recovered from 1 to 2 mL freshly drawn peripheral blood was cultured for 16 to 20 hours without addition of exogenous cytokines, as previously described elsewhere.30 As shown in Figure 2, a small fraction of cells spontaneously developed the morphology of veiled DCs not adherent to plastic. Large low buoyant cells, including cells with dendritic morphology, could be further enriched by centrifugation over hypertonic gradient and confirmed to express high levels of HLA-DR and accessory molecules, but not lymphoid markers (details not shown). Similarly, when plastic adherent PBMCs (most of which express CD14) were cultured for 6 days in complete medium with addition of exogenous granulocyte-macrophage–CSF and IL-4,31 immature monocyte-derived DCs could be obtained (not shown).
Kinetics of appearance of DCs in peripheral blood after Allo-HSCT
To determine the kinetics of DC engraftment, white blood cell (WBC) counts were monitored daily after Allo-HSCT, while flow cytometry analysis of My-DCs and PDCs from freshly drawn peripheral blood was performed when WBCs were at least .3 × 109/L (300/μL). The quantitative evaluation of PDC and My-DC release was performed routinely in a series of 43 pediatric patients who did (n = 25) or did not (n = 18) receive G-CSF after Allo-HSCT. For comparison, we also evaluated My-DCs and PDCs in 10 age-matched healthy subjects and, before transplantation, in 15 of the 43 patients. We could identify mononuclear cells with the typical surface phenotype of PDCs and My-DCs in all of the reconstitution phases considered in our study (Figure 3), regardless of treatment with G-CSF. Since some patients developed symptoms and/or signs of acute GVHD and, accordingly, were treated with steroids, the physiology of DC recovery after Allo-HSCT was investigated only in patients exempt from acute GVHD at the time of evaluation.
When we examined DC percentages (Figure 4), we observed that whole My-DCs reached their highest proportion among WBCs during the earliest phase of recovery. In patients given G-CSF, My-DCs represented initially about 25% of WBCs and then gradually decreased. In patients who did not receive G-CSF, My-DCs rose further, reaching a peak about 20 days after transplantation, in some cases My-DCs eventually representing even more than 50% of WBCs. The difference in the percentage of My-DCs between the 2 groups at day +20 is statistically significant (see also Figure 4). Among My-DC subsets, CD14+ and CD2+ were better represented initially (before day +20), while the highest percentages of CD16+ cells were found in a slightly later phase (between day +20 and +30). At day +20, the difference in the percentage of CD14+ My-DCs between patients who did or did not receive G-CSF is also statistically significant (Figure 4). As far as PDCs are concerned, their proportion appeared not to be influenced by administration of G-CSF. Notably, in some untreated patients, percentages of PDCs tended to be higher around 20 days after transplantation. On the whole, with few notable exceptions, we did not observe significant differences related to G-CSF treatment. The most consistent finding was represented by the initial tendency for high proportions of My-DCs and their CD14+ subset in peripheral blood. After such an initial expansion, DC percentages appeared to decrease gradually, and returned to baseline values, regardless of prior administration of G-CSF.
Looking at the absolute numbers, the kinetics of total WBCs appeared to be substantially different between patients treated or not with G-CSF (Figure 5). As expected, administration of G-CSF induced more rapid recovery of leukocyte counts, the median time for myeloid engraftment of patients treated or untreated with G-CSF being 15 (range, 9-18 days) and 17 days (range, 12-22 days), respectively. Subsequently, G-CSF withdrawal was followed by significant decrease of total leukocyte count, which reached stable values about 45 to 60 days after transplantation. In untreated patients, WBC counts recovered more slowly and DCs reached peak values about 40 days after transplantation, eventually achieving stable values similar to those seen in patients who had been treated with G-CSF. These G-CSF–related differences in WBC counts also appeared to influence the kinetics of recovery for both My-DCs and PDCs (see also Figure 5). In fact, irrespective of G-CSF administration, each DC population showed a kinetics pattern closely correlated with WBC counts and therefore very similar to each other. In particular, in the group of patients treated with G-CSF, the absolute counts of both My-DCs and PDCs grew very rapidly between day +10 and +20 after Allo-HSCT. In this period, the number of whole My-DCs, as well as that of the CD16+ and CD2+ subsets, was significantly higher in patients receiving G-CSF compared with children who did not receive this cytokine (see also Figure 5). Subsequently, G-CSF therapy withdrawal was accompanied by a consistent reduction of My-DC and PDC absolute counts that persisted during the following 20 days. By contrast, although G-CSF–untreated patients recovered initially more slowly after the allograft, they showed a more prolonged and stable increase of both DC populations. In particular, between days +25 and +45, the number of PDCs, of whole My-DCs, as well as of their CD14+ subset was significantly higher in patients who did not receive G-CSF (see also Figure 5). Compared with healthy children, Allo-HSCT recipients had a similar number of My-DCs already in the early posttransplantation phase, whereas the absolute number of PDCs was lower, particularly when G-CSF was used after grafting.
On a strictly quantitative basis, the DC reconstitution kinetics we observed may be described as follows: (1) Both My-DCs and PDCs were largely released during the initial phase of hematopoietic recovery after Allo-HSCT, and both populations usually reached high absolute values; (2) the time for recovery after transplantation did not differ significantly between My-DC and PDC subsets; (3) G-CSF treatment affected the release kinetics of whole WBCs, including both DC populations, but it induced very little alteration between DC proportions; and (4) although G-CSF–untreated patients recovered initially more slowly after the allograft, they showed a more prolonged and stable increase of both DC populations.
Cytokine-secreting potential of DC precursors after Allo-HSCT
The cytokine-secreting potential was tested for My-DCs by shortterm in vitro stimulation of whole PBMCs with LPS and for PDCs by short-term in vitro stimulation with CpG (Figure 6). Intracellular IFN-α (and IFN-β, not shown) was consistently detectable in almost all cells bearing the BDCA-2 plasmacytoid marker, after stimulation with a specific CpG-containing oligodeoxynucleotide. Although we did not pursue an extensive study of this secreting activity, these results provide evidence that PDC precursors are competent for type I IFN production in response to specific stimulation both in patients who did and in those who did not receive G-CSF. Similarly, TNF-α and IL-12 were detectable at the intracellular level in a substantial proportion of My-DCs after stimulation for 16 to 20 hours with LPS. In particular, we observed that the proportion of TNF-α–secreting cells was quite high, although variable, but, apparently, did not differ significantly between patients treated or not with G-CSF (Table 3). By contrast, we found that patients treated with G-CSF had a significantly lower proportion of IL-12–secreting My-DCs compared with untreated patients (Table 3). These results indicate that, as already reported by others,32 at least under circumstances of hematopoietic reconstitution, G-CSF treatment reduces the proportion of cells producing IL-12.
Cytokine-secreting activity of myeloid and plasmacytoid DC precursors released in peripheral blood after Allo-HSCT. Whole PBMCs obtained from patients at the WBC zenith point were cultured with complete medium and stimulated with either LPS or a specific CpG oligodeoxynucleotide, as described in “Patients, materials, and methods,” and stained for CD33+ surface antigen expression and intracellular TNF-α, IL-12 p40/p70 heterodimer, and IFN-α. Percentages refer to myeloid CD33+ mononuclear cells and to cultured PBMCs. The experiment shown for IFN-α production following stimulation with CpG oligodeoxynucleotide is representative of at least 3 others similarly performed. The experiment shown for TNF-α and IL-12 following stimulation with LPS is representative of 10 experiments (5 in G-CSF–treated and 5 in untreated patients). In the bottom plots, representative intracellular IL-12 staining, from a patient treated with G-CSF and from another patient not treated, is shown.
Cytokine-secreting activity of myeloid and plasmacytoid DC precursors released in peripheral blood after Allo-HSCT. Whole PBMCs obtained from patients at the WBC zenith point were cultured with complete medium and stimulated with either LPS or a specific CpG oligodeoxynucleotide, as described in “Patients, materials, and methods,” and stained for CD33+ surface antigen expression and intracellular TNF-α, IL-12 p40/p70 heterodimer, and IFN-α. Percentages refer to myeloid CD33+ mononuclear cells and to cultured PBMCs. The experiment shown for IFN-α production following stimulation with CpG oligodeoxynucleotide is representative of at least 3 others similarly performed. The experiment shown for TNF-α and IL-12 following stimulation with LPS is representative of 10 experiments (5 in G-CSF–treated and 5 in untreated patients). In the bottom plots, representative intracellular IL-12 staining, from a patient treated with G-CSF and from another patient not treated, is shown.
Comparison of intracellular TNF-α and IL-12-secreting activities of CD33+ PBMCs after stimulation with LPS in patients treated (n = 5) or not (n = 5) with G-CSF
. | Median % of CD33+ PBMC (range) . | . | . | |
|---|---|---|---|---|
. | NIL . | G-CSF . | P . | |
| TNF-α | 62.42 (82.7-27.1) | 48.35 (41.8-78.6) | NS | |
| IL-12 | 38.55 (12.8-54.3) | 9.07 (0.1-12.9) | .02 | |
. | Median % of CD33+ PBMC (range) . | . | . | |
|---|---|---|---|---|
. | NIL . | G-CSF . | P . | |
| TNF-α | 62.42 (82.7-27.1) | 48.35 (41.8-78.6) | NS | |
| IL-12 | 38.55 (12.8-54.3) | 9.07 (0.1-12.9) | .02 | |
Whole PBMCs obtained from patients at the WBC zenith point were cultured with complete medium and stimulated with LPS, as described in “Patients, materials, and methods,” and stained for CD33+ surface antigen expression and intracellular TNF-α and IL-12 p40/p70 heterodimer. Data refer to percentages of myeloid CD33+ mononuclear cells, as shown in Figure 6. Data were compared by nonparametric Mann-Whitney test.
NS indicates not significant.
Quantitative alterations of DC precursors occurring with GVHD onset and following its treatment
We analyzed separately data from patients who developed acute GVHD and were treated accordingly. In most patients with GVHD, we found that numbers of circulating DCs did not follow any definite kinetics pattern, but varied broadly and apparently rather unpredictably. Therefore, we further confined our analysis to some of the most critical time intervals. Although in a limited number of patients (n = 6), we had the opportunity to measure the number of DCs during the window interval between GVHD onset and the beginning of treatment with steroids. As shown in Figure 7A, in 4 of 5 patients in whom DC precursors were measurable, numbers of PDCs and My-DCs were higher than those registered in patients who did not develop GVHD during the corresponding time period after Allo-HSCT. These data may suggest that DCs could be increased at the onset of GVHD. However, the sixth patient showed an opposite pattern with no measurable DC precursors at the onset of acute GVHD.
Plasmacytoid and myeloid DC precursors observed at the onset of GVHD and after starting treatment with steroids. (A) Absolute values obtained from 5 patients observed on the same day of GVHD onset are shown as ♦. Median values with interquartiles (error bars) of uncomplicated patients observed in the corresponding period are shown for comparison as □. (B) Median absolute values with interquartiles (error bars) were obtained from 5 patients observed both before (left side in each graph) and after (right side in each graph) starting GVHD treatment with steroids. Statistical analysis was obtained by paired Student t test. *P < .05 was considered significant. The P value for PDCs is .012.
Plasmacytoid and myeloid DC precursors observed at the onset of GVHD and after starting treatment with steroids. (A) Absolute values obtained from 5 patients observed on the same day of GVHD onset are shown as ♦. Median values with interquartiles (error bars) of uncomplicated patients observed in the corresponding period are shown for comparison as □. (B) Median absolute values with interquartiles (error bars) were obtained from 5 patients observed both before (left side in each graph) and after (right side in each graph) starting GVHD treatment with steroids. Statistical analysis was obtained by paired Student t test. *P < .05 was considered significant. The P value for PDCs is .012.
In the same limited cohort of patients, we had the opportunity to directly compare numbers of DCs immediately before and after the beginning of treatment with steroids. As shown in Figure 7B, there was quite a large variability in WBC and My-DC counts. By contrast, the number of PDCs was consistently and significantly reduced soon after the beginning of therapy with steroids. Although preliminary, these data suggest that steroid treatment of GVHD may decrease quite rapidly the number of PDC precursors.
Discussion
A correct representation of the peripheral DC pool, consisting of the appropriate mixture of various DC subtypes, may be important for adequate immune competence against pathogens and for maintaining peripheral T-cell tolerance.33 Homeostasis and renewal of DCs is regulated both from within peripheral tissues34 and by central hematopoiesis through release of DC precursors. To get insights into the human regenerative potential after acute and maximal depletion of peripheral blood DCs, we monitored the dynamics of DC composition in pediatric patients given myeloablative therapy followed by Allo-HSCT.
The most informative aspect of this study is represented by the detailed analysis of DC dynamics during the critical period that follows bone marrow graft. Such analysis emphasizes that DC precursors can recover quickly and represent bone marrow recent emigrants, since they reached their highest proportions among WBCs during the earliest phase of reconstitution (Figure 4). Accordingly, the recovery of DC absolute numbers in uncomplicated patients after myeloablation was consistently characterized by a short period of My-DC precursor overrepresentation (Figure 5). By contrast, compared with healthy children, we found that the absolute number of PDCs was lower, particularly when G-CSF was used after grafting. Our results, obtained through repeated serial determinations at various time points after transplantation, are in agreement with previous studies, which occasionally reported similar data on DC precursors obtained at early, although isolated, time points after either Allo-HSCT35,36 or autologous HSCT.37 Such overrepresentation inevitably preceded normalization of DC levels and it is suggestive of a homeostatic positive regulation, perhaps exerted by acute release of DC-differentiating or mobilizing growth factors such as Flt 3 ligand (Flt-3L).38 Furthermore, the efficient recovery of DCs supports the concept that they may contribute to reduce the risk of infectious complications in the immediate posttransplantation period.
In spite of profound alterations of DC levels, the reconstitution patterns of both PDCs and My-DCs were surprisingly similar under regenerative circumstances (Figures 4, 5). Thus, our findings do not seem to confirm the concept that PDCs may be of lymphoid origin, but rather suggest that PDCs may also derive from myeloid progenitors.
Likewise, when we compared data from patients treated with G-CSF versus G-CSF–untreated patients, we found that the reconstitution patterns of PDC and My-DC precursors remained quite similar. Our results, which are probably the first reported on the effects, at the quantitative level, of G-CSF administration on DC reconstitution after transplantation, were unexpected since G-CSF increases preferentially the mobilization of PDCs, both in healthy donors10 and in cancer patients,39 when tested outside regenerative circumstances. The observation that G-CSF administration could slightly anticipate recovery of My-DCs may be explained by the effect exerted by the growth factor on recovery of WBCs (Figure 5).
Another intriguing effect induced by G-CSF therapy was observed when patients were tested for cytokine secretion potential: IL-12 production was found to be profoundly impaired in all the patients treated with G-CSF we tested, compared with untreated patients (see also Figure 6 and Table 3). This finding confirms the data reported by Volpi et al32 in patients given T-cell–depleted HSC transplants from a haploidentical donor and suggests that postgrafting administration of G-CSF, resulting in impaired production of IL-12 by DCs, could explain, at least in part, the promoting activity exerted in vivo by this cytokine toward polarization of T-cell response in the T helper 2 (Th2) direction. Several studies in human volunteers and animals also showed that G-CSF increases monocyte production of IL-10,40 which is known to inhibit both the ability to produce IL-12 and the stimulatory capacity of DCs, and to promote Th2 immune deviation.41 Experimental animal models of infections have demonstrated that Th1 immune response is responsible for resistance to pathogens, while Th2 reactivity is associated with susceptibility.42,43 Since a high level of production of IL-12 by DCs is a key factor in the initiation of protective Th1 immunity against fungi, bacteria, and viruses,44,45 our results, supporting a negative effect displayed by G-CSF on IL-12 production, raise some theoretical concerns that G-CSF administration after transplantation may, at least partly, contribute to the postgrafting immune deficiency syndrome. It can be also hypothesized that this biologic property of G-CSF may account for the GVHD-preventing activity attributed to this cytokine in several instances.46 In view of our data, we can conclude that, at least during hematopoietic regeneration, G-CSF also affects whole peripheral availability of DC precursors, and this, in turn, may influence systemic immune reactivity, including susceptibility to GVHD.
There is a persuasive experimental and conceptual basis supporting several roles for DCs of various types and origins in the pathogenesis of GVHD. Host DCs resident in peripheral tissues are thought to directly trigger GVHD,8 whereas PDCs transferred with the graft are thought to prevent GVHD10 and, in one study, have been correlated with significantly lower incidence of chronic GVHD after Allo-HSCT.11 On the other hand, neogenerated DCs could also play a significant role in this regard, since administration of Flt-3L to recipients can alter GVHD severity in animal models.14,47 In humans, it has been proposed that an anti-CD52 monoclonal antibody can prevent GVHD, not only by inducing T-cell depletion but also by removing My-DC precursors from peripheral blood.48 Unfortunately, our study is too limited to provide conclusive results about possible relationships between DC reconstitution and GVHD incidence and severity. However, we documented that many patients developing GVHD had substantial increase of DC precursors. Assuming that these data may be further confirmed, we suggest that DC precursors are affected by GVHD and/or participate in its pathogenesis. In light of this hypothesis, our findings may also contribute to explain the previously reported observation of Fearnley et al that patients suffering from acute GVHD have reduced levels of DCs.49 In that regard, since immunosuppressive therapy is usually administered whenever symptoms and signs of GVHD occur, we may suppose that these authors have tested patients during steroid treatment. If that was the case, the low levels of DCs were probably due to this therapy rather than to GVHD itself. Such interpretation may be further supported by a recently published report50 that suggests that steroid treatment of GVHD decreases significantly the number of PDC precursors. The observation that steroid therapy can reduce peripheral blood CD123+ DC levels is also in accordance with a study performed in healthy donors.51
We conclude that therapeutic strategies targeting DCs after Allo-HSCT may influence the capability to effectively mount an immune response toward common pathogens and suggest that disruption of DC homeostasis may play a role in the pathogenesis of GVHD. Knowledge of the kinetics of recovery of DCs may be important to assess the risk of developing both GVHD and infectious complications and also to guide strategies for active immunotherapy in the setting of Allo-HSCT.
Prepublished online as Blood First Edition Paper, March 9, 2004; DOI 10.1182/blood-2003-07-2443.
Supported by grants from AIRC (Associazione Italiana Ricerca sul Cancro), CNR (Consiglio Nazionale delle Ricerche), MURST (Ministero dell'Università e della Ricerca Scientifica e Tecnologica), European Community (AlloStem FP6 Program), and IRCCS (Istituto di Ricovero e Cura a Carattere Scientifico)
Policlinico S. Matteo, Ricerca Finalizzata to F.L, from AIRC and IRCCS Policlinico S. Matteo, Ricerca Finalizzata to R.M., and from IRCCS Policlinico S. Matteo, Ricerca Finalizzata to G.G.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal