Abstract
We have previously demonstrated that an anti-CD19 monoclonal antibody (MAb; HD37) inhibits the function of the P-glycoprotein (P-gp) pump in a multidrug-resistant (MDR) B-lymphoma cell line, Namalwa/MDR1, and that this effect is not due to the recognition of a cross-reactive epitope on P-gp. In this study, we have used the same cell line to define the mechanisms responsible for the effect of HD37 on the P-gp pump. Using fluorescence resonance energy transfer (FRET), we show that CD19 and P-gp are constitutively associated in cells. In the absence of treatment with anti-CD19, 40% of P-gp molecules expressed by Namalwa/MDR1 cells reside in the low-density lipid (ie, cholesterol-rich) microdomains (lipid rafts). Following treatment of the cells with HD37 and disruption of the interactions between P-gp and CD19, P-gp translocated out of lipid rafts and CD19 translocated into lipid rafts. The effect of chemosensitization on Namalwa/MDR1 cells was specific for CD19; an anti-CD22 MAb had no such effect, although the cells express CD22. These results suggest that anti-CD19 might chemosensitize P-gp+ cells by interfering with interactions between CD19 and P-gp, rapidly resulting in the translocation of P-gp into a compartment on the plasma membrane where it is no longer active.
Introduction
Following chemotherapy, multidrug-resistant (MDR) tumors often emerge; patients with such tumors have a poor prognosis.1 A variety of agents can prevent MDR but all have side effects. These agents include analogs of drug transporter substrates (verapamil) and inhibitors of adenosine triphosphate (ATP) binding or utilization.2,3 More recently, monoclonal antibodies (MAbs) against P-glycoprotein (P-gp; MRK16 and UIC2) have been used to inhibit the P-gp transporter.4-6 However, P-gp is expressed on cells in many normal tissues (eg, kidney, brain, gut, and liver), and by targeting MDR tumors, those organs are targeted as well, resulting in potentially undesirable side effects.7 Therefore, studies designed to find alternative nontoxic strategies to chemosensitize MDR tumors are needed.
Our previous studies have shown that an anti-CD19 MAb, HD37, induces cell cycle arrest in human B-lymphoma cell lines in vitro and has significant antitumor activity in severe combined immunodeficient (SCID) mice with human lymphoma xenografts.8 More recently, we demonstrated that HD37 can chemosensitize a P-gp+ lymphoma cell line in vitro and that this effect is due to its ability to inhibit the functional activity of P-gp.9 P-gp is an ATP-driven pump expressed on many MDR tumors,2,3 and several studies have suggested that the phosphorylation of P-gp can regulate drug efflux.10,11 HD37 does not alter ATP levels and does not cross-react with P-gp.9
P-gp is highly sensitive to its lipid environment and actively mediates cholesterol redistribution in the cell membrane.12 Forty percent of P-gp resides in the low-density lipid (ie, cholesterolrich) microdomains of the plasma membrane (lipid rafts),13,14 and this proportion increases when the cells are cultured with drug substrates,15 suggesting that it is active in lipid rafts. Moreover, it has been suggested that caveolae themselves might play a role in the acquisition of MDR in tumor cells.14,15
In this study, we determined whether CD19 and P-gp interact on Namalwa/MDR1 cells and, if so, whether HD37 inhibited this interaction and caused P-gp to translocate out of lipid rafts. This translocation would likely result in the inactivation of P-gp.
Materials and methods
Cell lines
The human Burkitt lymphoma cell line, Namalwa, was infected with a human MDR1 gene–containing retrovirus (Namalwa/MDR1) and was a gift from Dr R. O'Connor at ImmunoGen (Boston, MA).16 Both the parental P-gp– Namalwa cells and the P-gp+ Namalwa/MDR1 cells were maintained in culture in complete RPMI-1640 media (CM) containing 10% heat-inactivated fetal bovine serum (FBS) supplemented with 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 100 units/mL penicillin, 100 μg/mL streptomycin, and 100 mM l-glutamine. Cells were grown in a humidified atmosphere of 5% CO2 and air, and viability was determined by trypan blue exclusion. Cell lines were maintained in culture for 6 weeks and were then discarded and replaced with frozen stock. The expression of CD19, CD22, and P-gp was determined by flow cytometry.
Antibodies and reagents
The following MAbs or hybridomas were used: anti-CD19 (HD37) obtained from Dr D. Dorken, Germany, was grown and purified in our laboratory; anti–P-gp (UIC2; Immunotech, Miami, FL); anti-CD22 (RFB4) from Dr G. Janossy (Royal Free Hospital, London, United Kingdom); fluorescein isothiocyanate (FITC) goat anti–mouse immunoglobulin G (GAMIgG) (Kirkegaard & Perry Laboratory, Gaithersburg, MD); rabbit anti-CD19 was purchased from Cell Signaling Technology (Beverly, MA); and rabbit anti-MDR was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-human CD45 (clone 9.4) was prepared in our laboratory. Verapamil, rhodamine 123, methyl-β cyclodextrin (MBCD), and FITC- or horseradish peroxidase (HRP)–coupled cholera toxin B subunit (ChB) were purchased from Sigma (St Louis, MO).
Chemotherapeutic drugs
Doxorubicin (hydrochloride injection) was purchased from Gensia Sicor Pharmaceuticals (Irvine, CA) and vincristine sulfate (injection) was purchased from Faulding Pharmaceutical (a Mayne Group Company, Paramus, NJ).
Flow cytometry
Indirect immunofluorescence assays were carried out to evaluate the binding of anti–P-gp and anti-CD19 MAbs to both the Namalwa and the Namalwa/MDR1 cell lines as previously described.9 The cells were analyzed on a FACScan (Becton Dickinson, Mountain View, CA).
The expression of P-gp on Namalwa/MDR1 cells was also detected by direct staining of cells with phycoerythrin (PE)–labeled UIC2 (Beckman Coulter, Miami, FL). The levels of lipid rafts on the cell membranes were estimated from the levels of GM1 ganglioside as determined by the binding of FITC-ChB.
Cellular efflux assay
The activity of the P-gp pump was investigated by using a rhodamine 123 efflux assay as previously described.9 Verapamil, MAbs, or MBCD was constantly present in the serum-free medium. Following incubation, cells were washed and analyzed on the FACScan.
Cytotoxicity assays
The cytotoxicities of doxorubicin, vincristine, HD37, and RFB4, either alone or in combination, were evaluated using a 3H-thymidine incorporation assay as previously described.9 Cells were treated at 1.2 × 105/well in 96-well microtiter plates (Becton Dickinson Labware, Franklin Lakes, NJ) for 72 hours at 37° C, followed by a pulse with [3H]-thymidine for 18 hours as described by O'Connor et al.16
Flow cytometric detection of fluorescence resonance energy transfer (FRET)
Cells (106) were sequentially incubated with a saturating concentration (1 μg/mL) of PE anti–P-gp and cyanin 5 (Cy5) anti-CD19 or Cy5 anti-CD22 for 30 minutes on ice in the dark. The excess MAb was removed by centrifugation and washing, and cells were either used immediately or were fixed in 1% paraformaldehyde. The dual laser excitation at 488 and 635 nm was used to determine the efficiency of energy transfer. PE-labeled anti–P-gp served as the energy donor, and Cy5-labeled anti-CD19 served as the acceptor fluorochrome. In each experiment, 4 samples were included: (1) unlabeled cells, (2) donor dye-labeled cells, (3) acceptor dye-labeled cells, and (4) donor and acceptor dye-labeled cells. Forward angle light scatter was used to gate out debris. In each sample, 3 fluorescence parameters were detected simultaneously: (1) specific PE fluorescence in FL2 at an excitation of 488 nm, (2) direct Cy5 emission in FL4 at an excitation of 635 nm, and (3) the signal derived from PE and Cy5 emission in FL3. Interaction/steric proximity of the donor and acceptor dye in the nanometer range causes energy transfer between these fluorochromes, and its efficiency (E) was calculated with the following equation: E = 1 – FL2PE-Cy5/FL2-PE, where FL2PE-Cy5 and FL2-PE were the quenched and the unquenched donor dye fluorescence intensity, respectively.
Isolation and analysis of lipid rafts
In order to fractionate the cholesterol- and sphingolipid-rich membrane lipid rafts, 108 cells were washed twice with phosphate-buffered saline (PBS), pelleted, and lysed in 1 mL lysis buffer (20 mM Tris, pH 7.5, 1 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/mL leupeptin and aprotinin, and 1% Triton X-100). The cells were thoroughly vortexed, incubated on ice for 30 minutes, and then centrifuged for 10 minutes at 900g to pellet nuclei and particulate debris. The supernatant was mixed 1:1 with 85% sucrose in 20 mM Tris buffer and overlayered with 6 mL of 35% sucrose and 3.5 mL of 5% sucrose in the same buffer in a 12-mL ultracentrifuge tube. The tubes were centrifuged for 16 hours at 4° C in a Beckman ultracentrifuge L8-M at 35 000 rpm using a SW 41 Ti rotor. From the top to the bottom of the tube, 12 fractions of 1 mL each were collected and analyzed for the presence of target proteins by Western blotting. Each 1-mL fraction collected from the ultracentrifuge tube was diluted to less than 5% sucrose in 10 mL sucrose-free buffer (25 mM morpholinoethanesulfonic acid [MES], pH 6.5, 150 mM NaCl, and 1% Triton X-100). The diluted fractions were ultracentrifuged for 2 hours at 35 000 rpm, and the pellet was dissolved in 50 μL 2 × sodium dodecyl sulfate (SDS) loading buffer and analyzed by Western blotting.
Immunoprecipitation and Western blotting
Cell lysates (1 mL) were incubated overnight at 4° C with 20 μL protein G–agarose beads (Santa Cruz Biotechnology, CA) precoated with 1 μg rabbit IgG anti–P-gp, goat IgG anti-CD19, or mouse IgG anti-CD22 (RFB4). The immunoprecipitates were washed 3 times with lysis buffer and the proteins were recovered in 2 × SDS loading buffer containing β-mercaptoethanol. Samples were electrophoresed on either 6% (for the detection of P-gp and CD22) or 8% (for the detection of CD19) SDS–polyacrylamide gels (PAGEs), and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA). Membranes were blocked with 2% bovine serum albumin (BSA) in Tris-borate buffer containing 1% Tween-20 (TBST) for one hour at room temperature, and incubated overnight at 4° C with 1 μg/mL rabbit IgG anti–P-gp or rabbit IgG anti-CD19. Membranes were then incubated for one hour with the appropriate HRP-labeled secondary antibody (eg, HRP anti–rabbit IgG antibody; Amersham Pharmacia, United Kingdom) and visualized with a chemiluminescent substrate (Amersham Pharmacia, Piscataway, NJ).
Inhibition of cell growth
Namalwa/MDR1 or parental Namalwa cells (2 × 105 cells/mL) were grown for 7 days in CM supplemented daily with 15 μg/mL of either the HD37 or RFB4 MAbs. Cells were counted daily and viability was determined by trypan blue exclusion.
Results
Characterization of P-gp+ Namalwa/MDR1 cells
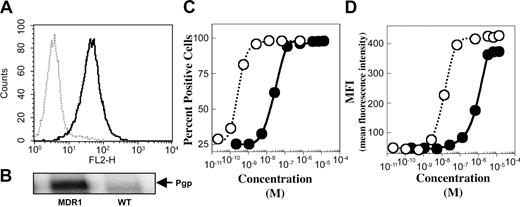
The levels of P-gp, CD19, and CD22 expressed on Namalwa/MDR1 and the parental cell lines were determined by indirect immunofluorescence staining. Both cell lines expressed equal numbers of CD19 and CD22 molecules on their cell surface as previously shown.9 P-gp was expressed on Namalwa/MDR1 cells but not on parental Namalwa cells as determined by flow cytometry (Figure 1A) or Western blotting of the whole cell lysate (Figure 1B). The relative affinities of the 2 antibodies (eg, the concentration needed to saturate 50% of cells) were determined by analysis of stained cells on a fluorescence-activated cell sorter (FACS) (Figure 1C-D). HD37 had a relative affinity of 1.8 × 10–10 M compared with 1.6 × 10–8 M for anti–P-gp (UIC2).
Expression of CD19 and P-gp as determined by the binding of anti-CD19 (HD37) and anti–P-gp (UIC2) MAbs to Namalwa/MDR1 cells. (A) Cell surface P-gp expression on Namalwa (dashed line) versus Namalwa/MDR1 (solid line), detected with PE-mouse anti–P-gp (UIC2) and analyzed on a FACScan, and (B) the corresponding total P-gp protein level, detected by Western blotting of the 1% Triton X-100 cell lysates (100 μg protein/lane). The binding affinities of HD37 (○) and UIC2 (•) for P-gp on Namalwa/MDR1 cells were calculated from the saturation curve expressed as (C) percent positive cells and (D) mean fluorescence intensity (MFI).
Expression of CD19 and P-gp as determined by the binding of anti-CD19 (HD37) and anti–P-gp (UIC2) MAbs to Namalwa/MDR1 cells. (A) Cell surface P-gp expression on Namalwa (dashed line) versus Namalwa/MDR1 (solid line), detected with PE-mouse anti–P-gp (UIC2) and analyzed on a FACScan, and (B) the corresponding total P-gp protein level, detected by Western blotting of the 1% Triton X-100 cell lysates (100 μg protein/lane). The binding affinities of HD37 (○) and UIC2 (•) for P-gp on Namalwa/MDR1 cells were calculated from the saturation curve expressed as (C) percent positive cells and (D) mean fluorescence intensity (MFI).
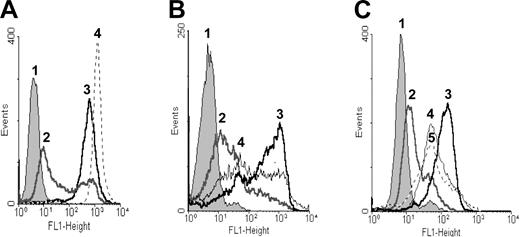
To determine whether P-gp was active, the cells were incubated with rhodamine 123 and the efflux of the dye was determined. Namalwa/MDR1 cells effluxed rhodamine 123, whereas the parental Namalwa cells retained the dye. When Namalwa/MDR1 cells were treated with verapamil, which is a well-known P-gp inhibitor, P-gp activity was blocked and the cells retained dye (Figure 2A). When the cells were treated for 10 minutes with 10 mM MBCD, P-gp activity was partially inhibited and cells retained rhodamine 123 (Figure 2B). When Namalwa/MDR1 cells were treated with HD37 or with UIC2 MAbs, the efflux of dye was inhibited by both MAbs (Figure 2C).
Rhodamine 123 efflux as a functional test for the P-gp pump. Namalwa/MDR1 cells at 5 × 105 per milliliter were treated for one hour at 37°C with 1.3 μM rhodamine 123 alone or in combination with 10 μM verapamil, 10–8 M HD37, or UIC2. Then cells were washed twice with RPMI 1640 without FBS, resuspended in FBS-free medium, and incubated for 2 more hours at 37°C to measure efflux. Namalwa cells were used as a positive control for the retention of rhodamine 123. (A) Namalwa/MDR1 cells in media (1) without rhodamine 123, (2) with rhodamine 123, and (3) with verapamil and rhodamine 123; and (4) Namalwa cells with rhodamine 123. (B) Namalwa/MDR1 cells (1) without rhodamine 123, and with rhodamine 123 in the (2) absence and (3) presence of verapamil. (4) Cells were pretreated for 10 minutes at 37°C with 10 mM MBCD prior to conducting the rhodamine 123 assay. (C) Namalwa/MDR1 cells with media (1) without rhodamine 123, (2) with rhodamine 123, (3) treated with verapamil and rhodamine 123, (4) treated with anti-CD19 (HD37) and rhodamine 123, and (5) treated with anti–P-gp (UIC2) and rhodamine 123. The histograms in each panel depict 1 representative experiment of 3 performed.
Rhodamine 123 efflux as a functional test for the P-gp pump. Namalwa/MDR1 cells at 5 × 105 per milliliter were treated for one hour at 37°C with 1.3 μM rhodamine 123 alone or in combination with 10 μM verapamil, 10–8 M HD37, or UIC2. Then cells were washed twice with RPMI 1640 without FBS, resuspended in FBS-free medium, and incubated for 2 more hours at 37°C to measure efflux. Namalwa cells were used as a positive control for the retention of rhodamine 123. (A) Namalwa/MDR1 cells in media (1) without rhodamine 123, (2) with rhodamine 123, and (3) with verapamil and rhodamine 123; and (4) Namalwa cells with rhodamine 123. (B) Namalwa/MDR1 cells (1) without rhodamine 123, and with rhodamine 123 in the (2) absence and (3) presence of verapamil. (4) Cells were pretreated for 10 minutes at 37°C with 10 mM MBCD prior to conducting the rhodamine 123 assay. (C) Namalwa/MDR1 cells with media (1) without rhodamine 123, (2) with rhodamine 123, (3) treated with verapamil and rhodamine 123, (4) treated with anti-CD19 (HD37) and rhodamine 123, and (5) treated with anti–P-gp (UIC2) and rhodamine 123. The histograms in each panel depict 1 representative experiment of 3 performed.
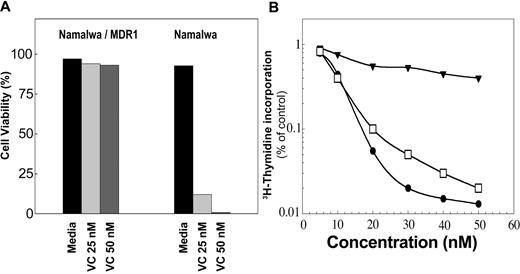
The activity of P-gp was also tested by monitoring the survival of cells in culture for 3 weeks in the presence of vincristine (25 and 50 nM). The cells' viability was determined by trypan blue exclusion. The viability of Namalwa/MDR1 cells was greater than 90% (Figure 3A), whereas the parental cells died after 3 days (Figure 3A). As shown in Figure 3B, the Namalwa/MDR1 cells were also resistant to doxorubicin under the same condition in which the parental Namalwa cells were killed with a 50% inhibitory concentration (IC50) of 9.5 nM doxorubicin. In the presence of HD37, Namalwa/MDR1 cells became as sensitive to doxorubicin as the parental Namalwa cells.
The chemoresistance of Namalwa/MDR1 cells can be overcome in the presence of anti-CD19. Cells were cultured in media ± vincristine (VC; 25 and 50 nM). Cells were counted daily and viability was determined by trypan blue exclusion. (A) Namalwa/MDR1 cells cultured with vincristine for 3 weeks survived, whereas parental Namalwa cells died after 3 days. This is 1 representative experiment of 3 performed. (B) The cytotoxic effect of doxorubicin in Namalwa/MDR1 cells in media (▾) or media supplemented with HD37 (15 μg/mL; □) versus parental Namalwa cells (•). Cells were incubated overnight with 15 μg/mL HD37, then plated in triplicates in 96-well plates and incubated for 72 hours in the presence of different concentrations (5-50 nM) of doxorubicin (triplicates were used for each concentration). The cells were then pulsed with 3H-thymidine for 18 hours, harvested, and counted. The radioactivity in treated cells versus control cells was then determined. This is a representative experiment of 5 performed.
The chemoresistance of Namalwa/MDR1 cells can be overcome in the presence of anti-CD19. Cells were cultured in media ± vincristine (VC; 25 and 50 nM). Cells were counted daily and viability was determined by trypan blue exclusion. (A) Namalwa/MDR1 cells cultured with vincristine for 3 weeks survived, whereas parental Namalwa cells died after 3 days. This is 1 representative experiment of 3 performed. (B) The cytotoxic effect of doxorubicin in Namalwa/MDR1 cells in media (▾) or media supplemented with HD37 (15 μg/mL; □) versus parental Namalwa cells (•). Cells were incubated overnight with 15 μg/mL HD37, then plated in triplicates in 96-well plates and incubated for 72 hours in the presence of different concentrations (5-50 nM) of doxorubicin (triplicates were used for each concentration). The cells were then pulsed with 3H-thymidine for 18 hours, harvested, and counted. The radioactivity in treated cells versus control cells was then determined. This is a representative experiment of 5 performed.
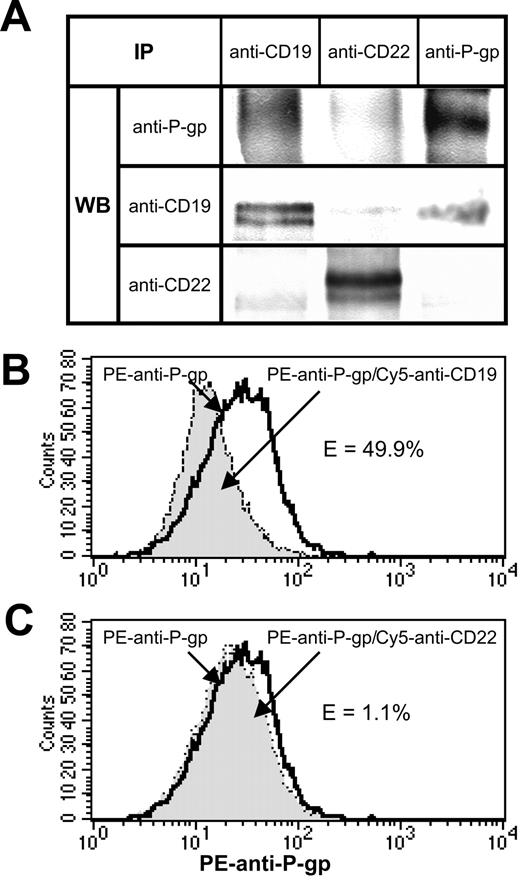
A portion of P-gp molecules constitutively associate with CD19 on the surface of Namalwa/MDR1 cells
One potential explanation for the chemosensitization induced by anti-CD19 is that it interferes with constitutive interactions between P-gp and CD19. To explore this possibility, 1% Triton X-100 lysates of Namalwa/MDR1 cells was immunoprecipitated with anti-CD19. (Figure 4A). We found that P-gp was coprecipitated even though we have shown previously that the HD37 does not recognize P-gp per se.9 This association was specific because P-gp was not coprecipitated when an anti-CD22 MAb was used. However, the association of CD19 and P-gp was disrupted in the presence of 1% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonic acid) or digitonin, indicating that it is a weak ionic interaction (data not shown). Moreover, this association was disrupted in less than an hour after stimulation with HD37 (data not shown). The constitutive association between these 2 molecules on the cell surface was confirmed by FRET using anti–P-gp (tagged with a donor dye, PE) and anti-CD19 or anti-CD22 (tagged with an acceptor dye, Cy5) (Figure 4B-C). The decrease in the donor dye intensity is indicative of molecular interactions.
Association of CD19 and P-gp in Namalwa/MDR1 cells. (A) Coprecipitation of CD19 and P-gp in 1% Triton X-100 lysates of Namalwa/MDR1 cells. Equal amounts of the total cell lysate proteins were immunoprecipitated with the indicated MAbs and analyzed by Western blotting for the presence of P-gp, CD19, and CD22. (B-C) Flow cytometric analysis of FRET between PE anti–P-gp and Cy5 anti-CD19 or Cy5 anti-CD22 in Namalwa/MDR1 cells. Cells (106) were incubated for 30 minutes on ice with 1 μg PE anti–P-gp, washed, and then incubated for another 30 minutes on ice in the absence or presence of 1 μg Cy5 anti-CD22 MAb or anti-CD19. The cells were washed, fixed with 1% paraformaldehyde, and analyzed on a FACS-Calibur flow cytometer. The bold line histogram represents background PE fluorescence in the absence of Cy5 anti-CD19 or anti-CD22, whereas filled histograms represent PE fluorescence in the presence of Cy5 anti-CD19 (B) or Cy5 anti-CD22 (C). PE fluorescence quenching in the presence of Cy5 anti-CD19 corresponds to the percent of energy transfer efficiency (E%) of 49.9%. Cy5 anti-CD22 did not induce fluorescence quenching (E = 1.1%). At least 3 independent experiments were performed. IP indicates immunoprecipitation.
Association of CD19 and P-gp in Namalwa/MDR1 cells. (A) Coprecipitation of CD19 and P-gp in 1% Triton X-100 lysates of Namalwa/MDR1 cells. Equal amounts of the total cell lysate proteins were immunoprecipitated with the indicated MAbs and analyzed by Western blotting for the presence of P-gp, CD19, and CD22. (B-C) Flow cytometric analysis of FRET between PE anti–P-gp and Cy5 anti-CD19 or Cy5 anti-CD22 in Namalwa/MDR1 cells. Cells (106) were incubated for 30 minutes on ice with 1 μg PE anti–P-gp, washed, and then incubated for another 30 minutes on ice in the absence or presence of 1 μg Cy5 anti-CD22 MAb or anti-CD19. The cells were washed, fixed with 1% paraformaldehyde, and analyzed on a FACS-Calibur flow cytometer. The bold line histogram represents background PE fluorescence in the absence of Cy5 anti-CD19 or anti-CD22, whereas filled histograms represent PE fluorescence in the presence of Cy5 anti-CD19 (B) or Cy5 anti-CD22 (C). PE fluorescence quenching in the presence of Cy5 anti-CD19 corresponds to the percent of energy transfer efficiency (E%) of 49.9%. Cy5 anti-CD22 did not induce fluorescence quenching (E = 1.1%). At least 3 independent experiments were performed. IP indicates immunoprecipitation.
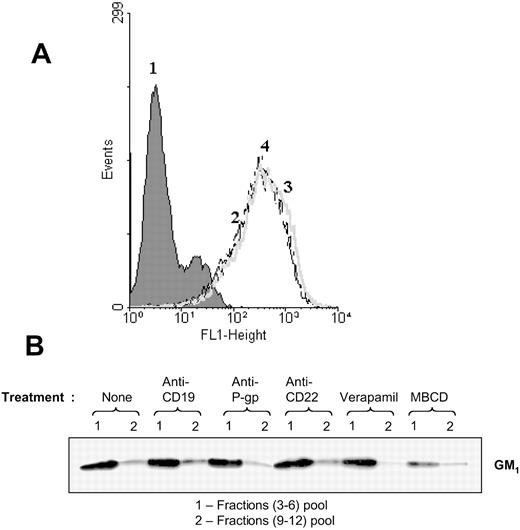
Anti-CD19 induces P-gp to translocate out of lipid rafts
Using FITC-ChB, we detected the levels of GM1 on the surface of Namalwa/MDR1 cells. The percent of GM1-positive cells and the mean fluorescence intensity (MFI) remained unchanged whether the cells were treated with anti-CD19 or anti–P-gp antibody prior to staining with FITC-ChB (Figure 5A). In addition, the presence of GM1 was determined in the fractions obtained after the ultracentrifugation of cell lysate on sucrose gradients (as described in “Materials and methods”). The GM1 distribution in lipid rafts remained unchanged after treatment with HD37 but did change after the treatment with 10 mM MBCD (Figure 5B), a compound that disrupts protein association with lipid rafts by extracting cholesterol from the plasma membrane.17
Detection of GM1 ganglioside as a marker for lipid rafts. (A) GM1 on intact cells. Cells were treated with FITC-ChB in a direct binding assay and the cells were analyzed on FACScan. Namalwa/MDR1 cells (1) in media, (2) stained with FITC-ChB, (3) pretreated with anti-CD19, or (4) pretreated with anti–P-gp prior to staining with FITC-ChB. (B) Distribution of GM1 ganglioside in the lipid fractions isolated by sucrose gradient ultracentrifugation of the Triton X-100 lysates of Namalwa/MDR1 cells. Cells were incubated for one hour at 37° C in the absence or presence of HD37, UIC2, or RFB4 (15 μg/106 cells), and 10 μM verapamil or 10 mM MBCD, lysed, and fractionated in a sucrose gradient. Combined raft fractions and soluble fractions from 5 × 108 cells were resolved (25 μL/lane) by 12% SDS-PAGE under nonreducing conditions and immunoblotted with ChB–horseradish peroxidase.
Detection of GM1 ganglioside as a marker for lipid rafts. (A) GM1 on intact cells. Cells were treated with FITC-ChB in a direct binding assay and the cells were analyzed on FACScan. Namalwa/MDR1 cells (1) in media, (2) stained with FITC-ChB, (3) pretreated with anti-CD19, or (4) pretreated with anti–P-gp prior to staining with FITC-ChB. (B) Distribution of GM1 ganglioside in the lipid fractions isolated by sucrose gradient ultracentrifugation of the Triton X-100 lysates of Namalwa/MDR1 cells. Cells were incubated for one hour at 37° C in the absence or presence of HD37, UIC2, or RFB4 (15 μg/106 cells), and 10 μM verapamil or 10 mM MBCD, lysed, and fractionated in a sucrose gradient. Combined raft fractions and soluble fractions from 5 × 108 cells were resolved (25 μL/lane) by 12% SDS-PAGE under nonreducing conditions and immunoblotted with ChB–horseradish peroxidase.
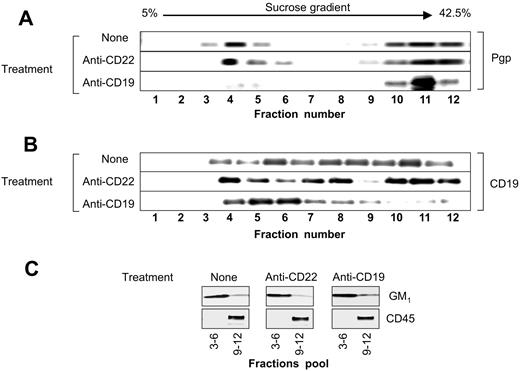
The levels of P-gp and CD19 were determined by Western blotting. Following sucrose gradient ultracentrifugation of the cell lysates prepared in 1% Triton X-100, the 12 fractions were collected and electrophoresed. As shown in Figure 6A, in untreated cells, 40% of the P-gp was present in the low-density detergent insoluble lipid rafts (fractions 3-6) and 60% was present in the soluble fraction (fractions 9-12). In untreated Namalwa/MDR1 cells, the majority of the CD19 molecules were located in the soluble membrane fraction; a minor proportion was located in lipid rafts (Figure 6B). Following treatment with HD37, CD19 translocated into lipid rafts, while P-gp translocated out of lipid rafts. Treatment with the anti-CD22 antibody did not change the distribution of P-gp. Treatment with the anti–P-gp MAb (UIC2) did not alter the localization of either CD19 or P-gp (data not shown). The localization of GM1 in lipid rafts and the localization of CD45 in soluble fraction remained unmodified (Figure 6C). The translocation of P-gp into the soluble fraction was not due to its internalization as shown in Figure 7.
Distribution of P-gp and CD19 in the lipid raft fractions of the plasma membrane. The 12 fractions isolated by sucrose gradient ultracentrifugation of the 1% Triton X-100 cell lysates from Namalwa/MDR1 cells before and after treatment with HD37 or RFB4 were analyzed for the presence of (A) P-gp and (B) CD19. The combined lipid raft fractions from the gradient (3-6) and soluble fractions from the gradient (9-12) were analyzed for the presence of GM1 and CD45, respectively (C). The proteins were electrophoresed on a 6% (P-gp), 7.5% (CD45), 8% (CD19), and 12% SDS-PAGE (GM1), and analyzed by Western blotting using either specific antibodies or HRP-ChB, as indicated in “Materials and methods.”
Distribution of P-gp and CD19 in the lipid raft fractions of the plasma membrane. The 12 fractions isolated by sucrose gradient ultracentrifugation of the 1% Triton X-100 cell lysates from Namalwa/MDR1 cells before and after treatment with HD37 or RFB4 were analyzed for the presence of (A) P-gp and (B) CD19. The combined lipid raft fractions from the gradient (3-6) and soluble fractions from the gradient (9-12) were analyzed for the presence of GM1 and CD45, respectively (C). The proteins were electrophoresed on a 6% (P-gp), 7.5% (CD45), 8% (CD19), and 12% SDS-PAGE (GM1), and analyzed by Western blotting using either specific antibodies or HRP-ChB, as indicated in “Materials and methods.”
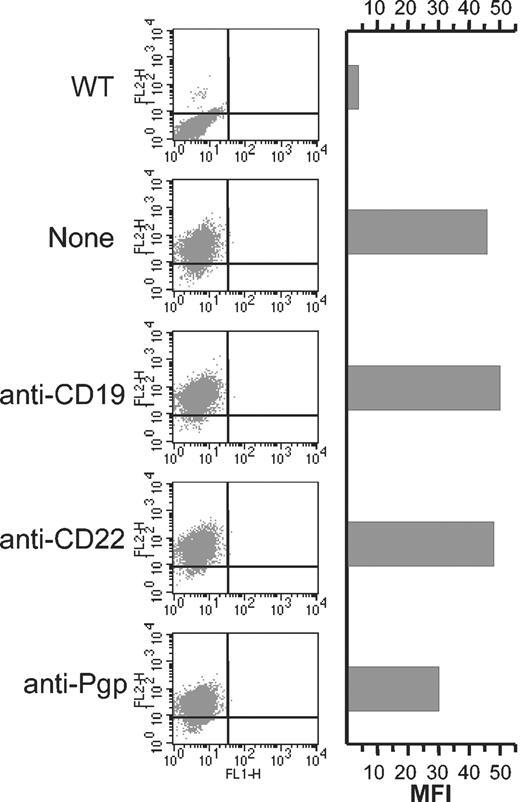
Anti-CD19 does not induce the internalization of P-gp. Cells (106) were incubated for 30 minutes on ice in the absence or presence of 15 μg anti-CD19, anti-CD22 (negative control), or anti–P-gp (positive control). The excess unbound antibody was removed by 2 washes in PBS, and then the cells were incubated at 37° C for 4 hours. The level of cell surface P-gp was then detected using PE-UIC2 and cells were analyzed by FACS. WT indicates wild type Namalwa cells.
Anti-CD19 does not induce the internalization of P-gp. Cells (106) were incubated for 30 minutes on ice in the absence or presence of 15 μg anti-CD19, anti-CD22 (negative control), or anti–P-gp (positive control). The excess unbound antibody was removed by 2 washes in PBS, and then the cells were incubated at 37° C for 4 hours. The level of cell surface P-gp was then detected using PE-UIC2 and cells were analyzed by FACS. WT indicates wild type Namalwa cells.
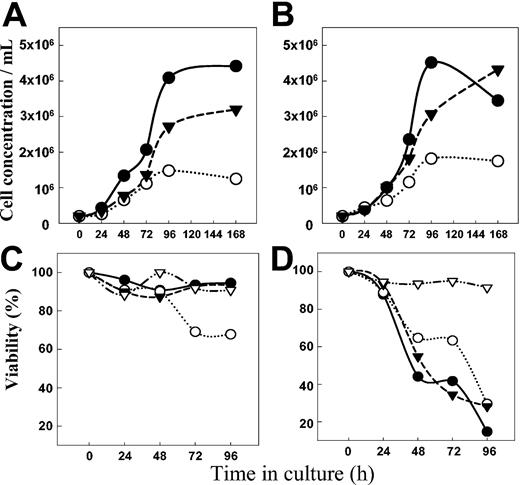
Anti-CD19 inhibits the growth of Namalwa/MDR1 and Namalwa cells in vitro
The growth of both Namalwa/MDR1 (Figure 8A) and parental Namalwa cells (Figure 8B) was inhibited by HD37 but not by RFB4. This cytostatic effect of HD37 required long incubation times. In contrast, HD37 chemosensitized cells very rapidly.9 While the growth of the Namalwa/MDR1 cells was inhibited by HD37, cell death occurred only when a nontoxic concentration of HD37 (but not RFB4) was combined with vincristine (Figure 8C). In contrast, the same concentration of vincristine alone was sufficient to kill the parental Namalwa cells regardless of the presence of the anti-CD19 MAb (Figure 8D).
Anti-CD19 inhibits cell growth. The number of cells (top panels) and the viability detected by trypan blue exclusion (bottom panels) are shown. Namalwa/MDR1 cells (A) and parental Namalwa cells (B) were cultured either in media (control) (•) or in the presence of 15 μg/mL of HD37 (○) and RFB4 (▾), and the number of cells was determined daily. The viability of Namalwa/MDR1 cells (C) and parental Namalwa cells (D) was also determined for cells grown in the presence of 25 nM vincristine in media only (•) or in media supplemented with 15 μg/mL of either HD37 (○) or RFB4 (▾). Viabilities of both cell lines treated with HD37 alone are also shown (▿).
Anti-CD19 inhibits cell growth. The number of cells (top panels) and the viability detected by trypan blue exclusion (bottom panels) are shown. Namalwa/MDR1 cells (A) and parental Namalwa cells (B) were cultured either in media (control) (•) or in the presence of 15 μg/mL of HD37 (○) and RFB4 (▾), and the number of cells was determined daily. The viability of Namalwa/MDR1 cells (C) and parental Namalwa cells (D) was also determined for cells grown in the presence of 25 nM vincristine in media only (•) or in media supplemented with 15 μg/mL of either HD37 (○) or RFB4 (▾). Viabilities of both cell lines treated with HD37 alone are also shown (▿).
Discussion
We have previously reported that anti-CD19 chemosensitized Namalwa/MDR1 cells and that it inhibited the activity of P-gp.9 The objective of this study was to explore the mechanisms underlining this inhibition. The major findings to emerge from these studies are as follows: (1) A portion of CD19 and P-gp molecules constitutively associates on intact MDR1+ cells. (2) Under normal conditions, 40% of the total P-gp and a small percentage of the CD19 molecules are present in lipid rafts on the plasma membrane of MDR+ cells. (3) Treatment of these MDR1 cells with HD37 under conditions in which P-gp is partially inactivated and the cells are chemosensitized disrupts the association between P-gp and CD19, and P-gp is removed from lipid rafts while CD19 translocates into the insoluble fraction of the plasma membrane. This effect was not observed using a control anti-CD22 MAb, which bound to the cells but did not reverse MDR. (4) A long period of exposure of MDR+ and MDR– cells to anti-CD19 results in the inhibition of the growth of both cell types. However, the Namalwa/MDR1 cells died when the anti-CD19 was added along with a chemotherapeutic drug (eg, vincristine), whereas the parental Namalwa cells were killed by drug alone.
Several mechanisms may contribute to MDR,18 of which 2 are best characterized: the decrease in the permeability of the plasma membrane to anti-cancer agents19 and the overexpression of P-gp. It has been suggested that P-gp can “control” drug permeation by modulating the properties of hydrophobic membrane regions.20
Not surprisingly, P-gp activity was highly sensitive to the lipid environment of the cell membrane. Indeed, there is evidence to suggest that P-gp recognizes its substrates in the lipid phase. Moreover, the affinity of vinblastine, doxorubicin, and verapamil for P-gp is dependent upon the amount of the drug in the lipid phase. It has also been suggested that caveolae themselves might play a role in the acquisition of MDR in tumor cells.13,14
In order to overcome MDR in tumor cells, different chemosensitizers or “reversal agents” have been used, but they all induce side effects. Work with these modulators has suggested that the lipid phase of the plasma membrane plays an important role in P-gp–mediated MDR.19 The relative hydrophobicity of the substrates and chemosensitizers allows them to enter cells by passive diffusion. They are then expelled by P-gp. We postulated that HD37 might alter the function of P-gp by changing its lipid environment (eg, inducing it to translocate from lipid rafts into the soluble fraction of the cell membranes). Our interpretation is consistent with the fact that reagents such as MBCD, which disrupt lipid rafts, also perturb the activity of P-gp. However, the dissociation/translocation of CD19/P-gp did not modify the distribution of GM1 in lipid rafts or the distribution of CD45 in the soluble fractions.
The present findings suggest that P-gp must localize in lipid rafts to be functionally active and that treatment with HD37 induces translocation of P-gp from the rafts such that cells become drug sensitive. This anti-CD19–mediated effect may result from the dissociation of those CD19 and P-gp molecules that constitutively interact on the cells, allowing CD19 to translocate into lipid rafts and P-gp to translocate out of rafts.
CD19, in association with CD21 (CR2), Leu-13, and CD81 (TAPA-1), is a general regulator of the signaling thresholds initiated by the B-cell receptor.21,22 Association with the tetraspan molecule, CD81, involves the extracellular domain of CD19.23,24 It is therefore possible that CD19 associates directly with the dodecaspan molecule of P-gp by a similar mechanism. Alternatively, CD19 might bind to P-gp indirectly via a CD81-mediated molecular complex. Indeed, association with CD81 is responsible for a series of effects triggered by CD19 cross-linking such as homotypic aggregation24 or calcium mobilization.25
HD37 therefore has 2 effects: One is immediate, that is, the loss of the P-gp from lipid rafts and the translocation of the CD19 from the soluble fractions into lipid rafts. The other occurs slowly and is cytostatic. The cytostatic effect can become cytotoxic when very low doses of drugs, which are not cytotoxic on their own, are added.
Since HD37 appears to partially reverse MDR in Namalwa/MDR1 cells, it will be important to determine if it has therapeutic efficacy in SCID mice carrying these tumors. It will also be critical to extend these studies to other CD19+ cell lines infected with the MDR1 gene, to chemoselected cells, and to MDR tumors from lymphoma patients.
Prepublished online as Blood First Edition Paper, March 4, 2004; DOI 10.1182/blood-2003-12-4255.
Supported by National Institutes of Health (NIH) grant CA-64679.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Tia Bowdler and Ms Linda Owens for administrative assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal