Abstract
Inhibition by all-trans retinoic acid (atRA) of the microvasculature formation in chicken chorioallantoic membrane (CAM) accompanied remarkably reduced numbers of endothelial cells (ECs) and increased numbers of mural cells (MCs) under the chorionic epithelial layer. Ro41-5253 (retinoid antagonist) exerted the opposite effect. Although atRA did not affect the differentiation of murine embryonic stem cell–derived vascular progenitor cells (VPCs) into ECs or MCs, atRA suppressed EC-MC interaction, leading to impaired branching. In both atRA-treated VPC cultures and CAM tissues underneath the chorionic epithelial layer, the expression of angiopoietin-2 (Ang-2; competitor for Ang-1) was enhanced, whereas that of Tie2 (a receptor for Angs) was reduced. Simultaneous treatment with Ang-1 partially blocked RA induction of EC-MC malinteraction and reduction in blood vessel formation. These results suggest that retinoid(s) may reduce EC-MC interaction by down-regulating Tie2 signaling as well as decreased EC numbers, which lead to impaired vascular remodeling.
Introduction
Vasculogenesis is the formation of primitive vascular networks mainly through differentiation of vascular progenitor cells (VPCs) into endothelial cells (ECs), while angiogenesis is the growth and sprouting of additional blood vessels from pre-existing blood vessels.1-6 In either case, formation of blood vessel networks is completed by branching (remodeling) of blood vessels through interaction between ECs and surrounding mural cells (MCs; smooth muscle cells or pericytes),1-4 and tightly regulated by various factors (eg, vascular endothelial growth factor [VEGF], fibroblast growth factor [FGF], angiostatin, endostatin, and angiopoietins [Ang-1 and Ang-2]).2,4,7,8 Ang-1 sustains interaction between ECs and MCs and stabilizes the vasculature through binding to Tie2 receptor and up-regulation of its phosphorylation, although underlying detailed mechanisms have not been elucidated. Normally, Ang-2 counteracts the action of Ang-1 by competitively inhibiting its binding to Tie2.2,8 Retinoids (vitamin A and its derivatives) are reported to exert a potent antiangiogenic activity in the chorioallantoic membranes (CAMs) of growing chick embryos, although underlying mechanisms have been unclear.9 Here, we analyzed this mechanism using chicken CAM and mouse embryonic stem (ES)–derived VPCs.
Study design
Materials
AtRA (pan retinoic acid receptor [RAR] agonist) was purchased from Sigma-Aldrich (St Louis, MO). RARα-selective antagonist Ro41-5253 was a generous gift from Dr M. Klaus (F. Hoffmann-La Roche, Basel, Switzerland).10 Recombinant human Ang-1 and Ang-2 were purchased from R&D Systems (Minneapolis, MN).
Chicken CAM assay
Chicken CAM was assessed as described.9 Angiogenic/antiangiogenic responses were quantitated using angiogenesis measuring software (KURABO Angiogenesis Image Analyzer version 1.0; Kurabo, Osaka, Japan). Data are expressed as the mean plus or minus the standard deviation (SD). Statistical significance was assessed with one-way analysis of variance followed by the Sheffe t test.
Cell culture and sorting
Maintenance, differentiation, and sorting of the CCE/nLacZ ES cell cultures were performed as described.6 The Flk1-positive (Flk1+) cells bound to the microbeads were collected using the autoMACS magnetic cell sorting system (Miltenyi Biotec, Auburn, CA) to yield purity of more than 95%. Human umbilical vein endothelial cells (HUVECs) were kindly provided by Professor K. Umezawa (Keio University, Yokohama, Japan) and cultured as described.11 Umbilical artery smooth muscle cells (UASMCs) were purchased from Cambrex Bio Science Walkersville (Walkersville, MD) and maintained according to the manufacturer's instructions.
Immunohistochemistry
CAM tissues were fixed with 4% paraformaldehyde and embedded in either Technovit 8100 synthetic resin (Heraeus Kulzer, Wehrheim, Germany) or Tissue-Tek OCT compound (Miles, Elkhart, IN).12 Vertical sections (5 μm) were stained with hematoxylin and eosin and frozen sections (10 μm) were immunostained with a combination of either a mouse anti–Quek-1 monoclonal antibody, which recognizes the chicken Flk1/KDR/VEGFR2, or a murine antiproliferating cell nuclear antigen (PCNA) monoclonal antibody (Dako A/S, Glostrup, Denmark), which reacts with eukaryotic antigens in early S phase without discrimination between ECs and MCs, plus a murine anti-α smooth muscle actin (αSMA) monoclonal antibody (Sigma-Aldrich) as described.12-15 Immunostaining of cultured cells with a combination of a goat anti–PECAM-1 polyclonal antibody (Pharmingen, San Diego, CA) and a murine anti-αSMA monoclonal antibody was performed as described.6 Immunostained sections and cultures were observed under a Leica model DM TRB microscope (Leica Microsystems, Heidelberg, Germany) using a phase-contrast lens (× 20 or × 40 objective). Photomicrographs were taken with a digital camera (DC 200; Leica Microsystems).
RNA extraction and reverse transcriptase–polymerase chain reaction (RT-PCR)
Total RNA was extracted from microvasculatures, which were dissected out from vertical sections (15 μm) of CAM tissues using a Laser Micro Dissection LMD system (Leica Microsystems), or from cell cultures using the RNeasy Mini Kit (Qiagen, Valencia, CA). RT-PCR was carried out using SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, CA), and sets of specific primers summarized in Table 1. Quantitative PCR was performed using Light Cycler quantitative PCR system (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instruction.
Primers for RT-PCR experiments
Gene . | . | Sequence . | Nucleotide no. . |
|---|---|---|---|
| Chicken GAPDH | Sense | CCTCTCTGGCAAAGTCCAAG | 63-82 |
| Antisense | CATCCACCGTCTTCTGTGTG | 543-562 | |
| Chicken Flk1 | Sense | CAAGTGGGAGTTTCCCAGAG | 6-25 |
| Antisense | TTTGTGCAGGCTCCAAGTAA | 232-251 | |
| Chicken αSMA | Sense | GAATCCCAAAGCCAATCGAG | 336-355 |
| Antisense | AGGAGGAAGAGGCAGCAGT | 691-709 | |
| Chicken Ang-2 | Sense | GAGCAGACCCGCAAATTAAC | 142-161 |
| Antisense | GAACTGTCCAACCTCCTCCA | 687-706 | |
| Mouse GAPDH | Sense | ACCTTGTCAGCCTTTGCACT | 779-798 |
| Antisense | GCTGAAATCAGCACCGTGTA | 1259-1278 | |
| Mouse Flk1 | Sense | GATCACCATTCATCGCCTCT | 371-390 |
| Antisense | AAACATCTTCGCCACAGTCC | 851-870 | |
| Mouse PECAM-1 | Sense | CACCTCGAAAAGCAGGTCTC | 159-178 |
| Antisense | TGCACGGTGACGTATTCACT | 637-656 | |
| Mouse Endoglin | Sense | CACAACAGGTCTCGCAGAAA | 63-82 |
| Antisense | TTCTGGCAAGCACAAGAATG | 542-561 | |
| Mouse αSMA | Sense | TGTGCTGGACTCTGGAGATG | 459-478 |
| Antisense | CTTCTGCATCCTGTCAGCAA | 932-951 | |
| Mouse Ang-1 | Sense | ACCTTGTCAGCCTTTGCACT | 779-798 |
| Antisense | GCTGAAATCAGCACCGTGTA | 1259-1278 | |
| Mouse Ang-2 | Sense | CAAGTCAGGACTCACCACCA | 864-883 |
| Antisense | AAGTTGGAAGGACCACATGC | 1345-1364 | |
| Mouse Tie2 | Sense | GTGAAGCCAGATGGGACAGT | 1156-1175 |
| Antisense | TTGGCAGGAGACTGAGACCT | 1635-1654 | |
| Human GAPDH | Sense | ACCCAGAAGACTGTGGATGG | 610-629 |
| Antisense | CCCTGTTGCTGTAGCCAAAT | 1011-1030 | |
| Human Flk1 | Sense | CAGCTTCCAAGTGGCTAAGG | 3024-3042 |
| Antisense | ATTTCCCAAATGTTCCACCA | 3467-3486 | |
| Human αSMA | Sense | CTGAGCGTGGCTATTCCTTC | 587-606 |
| Antisense | GCTGGAAGGTGGACAGAGAG | 1047-1066 | |
| Human Ang-2 | Sense | CCACAAATGGCATCTACACG | 878-897 |
| Antisense | AAGTTGGAAGGACCACATGC | 1345-1364 |
Gene . | . | Sequence . | Nucleotide no. . |
|---|---|---|---|
| Chicken GAPDH | Sense | CCTCTCTGGCAAAGTCCAAG | 63-82 |
| Antisense | CATCCACCGTCTTCTGTGTG | 543-562 | |
| Chicken Flk1 | Sense | CAAGTGGGAGTTTCCCAGAG | 6-25 |
| Antisense | TTTGTGCAGGCTCCAAGTAA | 232-251 | |
| Chicken αSMA | Sense | GAATCCCAAAGCCAATCGAG | 336-355 |
| Antisense | AGGAGGAAGAGGCAGCAGT | 691-709 | |
| Chicken Ang-2 | Sense | GAGCAGACCCGCAAATTAAC | 142-161 |
| Antisense | GAACTGTCCAACCTCCTCCA | 687-706 | |
| Mouse GAPDH | Sense | ACCTTGTCAGCCTTTGCACT | 779-798 |
| Antisense | GCTGAAATCAGCACCGTGTA | 1259-1278 | |
| Mouse Flk1 | Sense | GATCACCATTCATCGCCTCT | 371-390 |
| Antisense | AAACATCTTCGCCACAGTCC | 851-870 | |
| Mouse PECAM-1 | Sense | CACCTCGAAAAGCAGGTCTC | 159-178 |
| Antisense | TGCACGGTGACGTATTCACT | 637-656 | |
| Mouse Endoglin | Sense | CACAACAGGTCTCGCAGAAA | 63-82 |
| Antisense | TTCTGGCAAGCACAAGAATG | 542-561 | |
| Mouse αSMA | Sense | TGTGCTGGACTCTGGAGATG | 459-478 |
| Antisense | CTTCTGCATCCTGTCAGCAA | 932-951 | |
| Mouse Ang-1 | Sense | ACCTTGTCAGCCTTTGCACT | 779-798 |
| Antisense | GCTGAAATCAGCACCGTGTA | 1259-1278 | |
| Mouse Ang-2 | Sense | CAAGTCAGGACTCACCACCA | 864-883 |
| Antisense | AAGTTGGAAGGACCACATGC | 1345-1364 | |
| Mouse Tie2 | Sense | GTGAAGCCAGATGGGACAGT | 1156-1175 |
| Antisense | TTGGCAGGAGACTGAGACCT | 1635-1654 | |
| Human GAPDH | Sense | ACCCAGAAGACTGTGGATGG | 610-629 |
| Antisense | CCCTGTTGCTGTAGCCAAAT | 1011-1030 | |
| Human Flk1 | Sense | CAGCTTCCAAGTGGCTAAGG | 3024-3042 |
| Antisense | ATTTCCCAAATGTTCCACCA | 3467-3486 | |
| Human αSMA | Sense | CTGAGCGTGGCTATTCCTTC | 587-606 |
| Antisense | GCTGGAAGGTGGACAGAGAG | 1047-1066 | |
| Human Ang-2 | Sense | CCACAAATGGCATCTACACG | 878-897 |
| Antisense | AAGTTGGAAGGACCACATGC | 1345-1364 |
Results and discussion
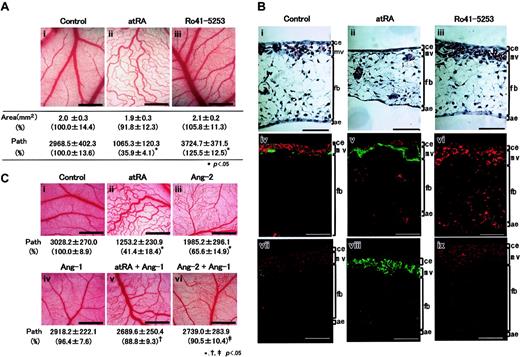
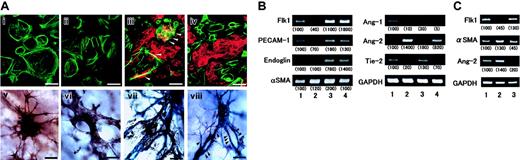
Compared with control CAM (Figure 1Ai), atRA inhibited the formation of fine vascular networks and, instead, formed weaving networks of relatively larger vessels (Figure 1Aii), accompanying reduced number of paths, while Ro41-5253 stimulated the fine capillary formation (Figure 1Aiii) accompanying a slightly increased number of paths. In control CAM tissue, many capillaries, which were mainly composed of Flk1-expressing ECs and some αSMA-expressing MCs, developed underneath the chorionic epithelium (Figure 1Bi,iv). AtRA remarkably reduced the number of capillaries accompanying a loss of ECs and, instead, a strong expression of αSMA in a decapillarized layer (Figure 1Bii,v), and made several vessels scattered within the mesenchymal layer (arrowheads), whereas Ro41-5253 stimulated microvasculature formation with increased Flk1 signals and reduced αSMA signals (Figure 1Biii,vi). Upon atRA treatment the number of PCNA-positive ECs reduced, but an increase in PCNA-positive MCs was not observed (Figure 1Bviii). Ro41-5253 augmented the number of PCNA-positive ECs in the microvasculatures (Figure 1Bix). These data suggested that exogenous and endogenous retinoid might affect the vascular remodeling in addition to reducing EC numbers. Mouse embryos lacking Ang-1 or Tie2 exhibit a defect in remodeling function,16-18 which leads to interference with normal proliferation of newly differentiated ECs.19 Therefore, we examined an involvement of Ang-1 and Ang-2 in the antiangiogenic action of atRA (Figure 1C). Although Ang-1 alone did not affect blood vessel formation (Figure 1Civ), simultaneous treatment with atRA and Ang-1 partially reverted the suppressive effect of atRA (compare Figure 1Cii,v). In contrast, Ang-2 alone displayed a similar antiangiogenic action (Figure 1Ciii). We then examined whether atRA might affect the expression of angiopoietins and/or Tie2 mRNA in HUVECs or UASMCs, but the answer was no (data not shown). Therefore, we hypothesized that retinoid might act on VPCs rather than mature vascular cells. To explore this idea, we first examined the effect of atRA on ES cell–derived Flk1+ cells, which function as VPCs.6 Almost all Flk1+ cells differentiated into MCs in the absence of VEGF (Figure 2Ai,v). AtRA did not affect this (Figure 2Aii,vi). VEGF induced the differentiation of Flk1+ cells into ECs, whose surface was covered by several MCs (white arrowheads in Figure 2Aiii), and enhanced the formation of vascular tree–like structures composed of ECs and MCs (Figure 2Avii). Simultaneous stimulation of atRA and VEGF resulted in a separation of VEGF-induced ECs from MCs (Figure 2Aiv), and inhibited the branching of endothelial tubes and the attachment of MCs to ECs (black arrowheads in Figure 2Aviii). A similar result was obtained by a combination of VEGF plus Ang-2 (data not shown). As seen in Figure 2B, VEGF enhanced the mRNA expression of Flk1, PECAM-1, and endoglin (lane 3). Either alone (lane 2) or in conjunction with VEGF (lane 4), atRA up-regulated Ang-2 mRNA expression 14.0-fold and 8.2-fold, respectively, and down-regulated the expression of Ang-1 and Tie2 mRNAs (10% and 5%, 20%, and 70%, respectively). A similar result was also observed underneath the chorionic epithelium in CAM (Figure 2C). AtRA decreased mRNA levels of Flk1 by 45% and increased those of αSMA and Ang-2 1.3-fold and 1.4-fold, respectively, while an antagonist decreased them to 45% and 20%, respectively. These results suggest that atRA may inhibit Tie2 signaling in VPCs by modulating the expression of Ang-2 and Tie2 mRNAs in opposite directions, leading to inhibition of EC-MC interaction and, subsequently, suppression of normal remodeling of blood vessels and reduction in EC cell numbers. Although impaired remodeling during vascular development might cause the dynamic change in the numbers of ECs and MCs underneath the chorionic epithelium (Figure 1B), other possibilities cannot be excluded. Especially, as transforming growth factor β (TGF-β) signals are essential for both formation and maturation of blood vessels as well as for reduction in EC cell number,20-23 we are now examining a role of TGF-β as a mediator of retinoid action, and a relationship between Tie2 and TGF-β signal pathway.
Impaired vascular remodeling by retinoid induced in CAM. The 4.5-day-old CAMs were implanted with a silicon ring and charged with different amounts of atRA or Ro41-5253. (A) Effect of retinoids on blood vessel formation within CAM. After 48 hours, developing new blood vessels were observed under a microscope and photographed. i, vehicle (1% ethanol); ii, 100 ng atRA; iii, 1 μg Ro41-5253. Original magnification, ×35; scale bar, 1 mm. The total area and the number of paths of blood vessels were analyzed using angiogenesis measuring software. Their relative changes are shown in parentheses. Each set of data represents the average ± SD (n = 3). Asterisks represent significant difference (P < .05) from control. (B) Structural changes induced in CAM tissues following treatment with retinoids. Vertical sections (5 μm) were stained with hematoxylin and eosin (i-iii), vertical sections (10 μm) were prepared and double-stained with a combination of either anti–Queck-1 antibody (for ECs, red) and anti-αSMA antibody (for MCs, green) (iv-vi), or anti-PCNA antibody (red) and anti-αSMA antibody (green) (vii-ix). The 6.5-day-old CAM was composed of 4 different layers, including a thin chorionic epithelium (ce), microvasculatures (mv), a thick mesenchymal layer (fb) consisting of sparsely distributed fibroblasts and a few small blood vessels (bv), and a thin allantoic epithelium (ae). Original magnification, × 200. Scale bar, 50 μm. (C) Effect of simultaneous treatment with atRA, Ang-1, and Ang-2 on blood vessel formation. The 4.5-day-old CAMs were treated with vehicle (1% ethanol; i), 100 ng atRA (ii), 300 ng human recombinant Ang-2 (iii), 300 ng human recombinant Ang-1 (iv), and combinations of atRA and Ang-1 (v), or Ang-2 and Ang-1 (vi). After 48 hours, developing new blood vessels were observed under a microscope and photographed. Original magnification, ×35; scale bar, 1 mm. The number of paths of blood vessels was analyzed as described above and presented underneath each picture with relative changes in parenthesis. Symbols *, †, and ‡ represent significant difference (P < .05) obtained by comparing to control samples (i), samples treated with atRA (ii), and samples treated with Ang-2 (iii), respectively. For panels A to C, a total of 15 eggs (n = 5 × 3 times) for each experimental group were evaluated and representative results are shown.
Impaired vascular remodeling by retinoid induced in CAM. The 4.5-day-old CAMs were implanted with a silicon ring and charged with different amounts of atRA or Ro41-5253. (A) Effect of retinoids on blood vessel formation within CAM. After 48 hours, developing new blood vessels were observed under a microscope and photographed. i, vehicle (1% ethanol); ii, 100 ng atRA; iii, 1 μg Ro41-5253. Original magnification, ×35; scale bar, 1 mm. The total area and the number of paths of blood vessels were analyzed using angiogenesis measuring software. Their relative changes are shown in parentheses. Each set of data represents the average ± SD (n = 3). Asterisks represent significant difference (P < .05) from control. (B) Structural changes induced in CAM tissues following treatment with retinoids. Vertical sections (5 μm) were stained with hematoxylin and eosin (i-iii), vertical sections (10 μm) were prepared and double-stained with a combination of either anti–Queck-1 antibody (for ECs, red) and anti-αSMA antibody (for MCs, green) (iv-vi), or anti-PCNA antibody (red) and anti-αSMA antibody (green) (vii-ix). The 6.5-day-old CAM was composed of 4 different layers, including a thin chorionic epithelium (ce), microvasculatures (mv), a thick mesenchymal layer (fb) consisting of sparsely distributed fibroblasts and a few small blood vessels (bv), and a thin allantoic epithelium (ae). Original magnification, × 200. Scale bar, 50 μm. (C) Effect of simultaneous treatment with atRA, Ang-1, and Ang-2 on blood vessel formation. The 4.5-day-old CAMs were treated with vehicle (1% ethanol; i), 100 ng atRA (ii), 300 ng human recombinant Ang-2 (iii), 300 ng human recombinant Ang-1 (iv), and combinations of atRA and Ang-1 (v), or Ang-2 and Ang-1 (vi). After 48 hours, developing new blood vessels were observed under a microscope and photographed. Original magnification, ×35; scale bar, 1 mm. The number of paths of blood vessels was analyzed as described above and presented underneath each picture with relative changes in parenthesis. Symbols *, †, and ‡ represent significant difference (P < .05) obtained by comparing to control samples (i), samples treated with atRA (ii), and samples treated with Ang-2 (iii), respectively. For panels A to C, a total of 15 eggs (n = 5 × 3 times) for each experimental group were evaluated and representative results are shown.
Changes in angiopoietins and Tie2 expressions following treatment with retinoid. (A) Effect of atRA and VEGF on differentiation of Flk1+ cells into ECs/MCs and formation of vascular tree–like structures. Flk1+ cells were cultured on collagen type 1–coated dishes for 5 days or spheroids formed by Flk1+ cells were cultured for 5 days in collagen gels in the absence and presence of atRA and VEGF. In 2D cultures, ECs and MCs were immunostained with anti–PECAM-1 antibody (red) and anti-αSMA antibody (green), respectively (i-iv). White arrowheads indicate margins of MC-EC interaction. In 3D cultures, ECs and MCs were immunostained with anti–PECAM-1 antibody (purple) and anti-αSMA antibody (brown), respectively (v-viii). Black arrowheads indicate the points showing dissociation of ECs from MCs. i, vehicle (0.5% ethanol); ii, 1 μM atRA; iii, 50 ng/mL VEGF; iv, 1 μM atRA plus 50 ng/mL VEGF. Original magnification, × 400. Scale bar, 10 μm. Representative micrographs from 3 different fields (n = 3) are presented. (B) Changes in angiopoietins and Tie2 expressions. Spheroids formed by Flk1+ cells were cultured for 5 days in collagen gels. Flk1+ cells were treated with vehicle (0.5% ethanol; lane 1), 1 μM atRA (lane 2), 50 ng/mL VEGF (lane 3), or 1 μM atRA plus 50 ng/mL VEGF (lane 4). Total RNA was isolated and mRNA levels of each of the indicated factors were assessed by RT-PCR and quantified by quantitative PCR as described in “Study design.” Representative data from 3 different experiments with similar results are presented. Relative changes were calculated after normalization to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA levels and presented in parentheses under each corresponding band. (C) Effect of atRA treatment on Ang-2 mRNA levels in microvasculature layer within CAM. The 4.5-day-old CAMs were treated with vehicle (1% ethanol; lane 1), 100 ng atRA (lane 2), or 1 μg Ro41-5253 (lane 3) for 48 hours. CAM tissue was harvested from embryos, and vertical sections (15 μm) were prepared. Regions underneath the chorionic epithelium (50 pieces for one sample; each piece corresponded to about 25 μm × 500 μm) were collected using the microdissection system and total RNA was isolated. Changes in mRNA levels of the indicated factors were assessed by RT-PCR and quantitated as described in “Study design.” Relative changes were calculated after normalization to GAPDH mRNA levels and presented in parentheses under each corresponding band.
Changes in angiopoietins and Tie2 expressions following treatment with retinoid. (A) Effect of atRA and VEGF on differentiation of Flk1+ cells into ECs/MCs and formation of vascular tree–like structures. Flk1+ cells were cultured on collagen type 1–coated dishes for 5 days or spheroids formed by Flk1+ cells were cultured for 5 days in collagen gels in the absence and presence of atRA and VEGF. In 2D cultures, ECs and MCs were immunostained with anti–PECAM-1 antibody (red) and anti-αSMA antibody (green), respectively (i-iv). White arrowheads indicate margins of MC-EC interaction. In 3D cultures, ECs and MCs were immunostained with anti–PECAM-1 antibody (purple) and anti-αSMA antibody (brown), respectively (v-viii). Black arrowheads indicate the points showing dissociation of ECs from MCs. i, vehicle (0.5% ethanol); ii, 1 μM atRA; iii, 50 ng/mL VEGF; iv, 1 μM atRA plus 50 ng/mL VEGF. Original magnification, × 400. Scale bar, 10 μm. Representative micrographs from 3 different fields (n = 3) are presented. (B) Changes in angiopoietins and Tie2 expressions. Spheroids formed by Flk1+ cells were cultured for 5 days in collagen gels. Flk1+ cells were treated with vehicle (0.5% ethanol; lane 1), 1 μM atRA (lane 2), 50 ng/mL VEGF (lane 3), or 1 μM atRA plus 50 ng/mL VEGF (lane 4). Total RNA was isolated and mRNA levels of each of the indicated factors were assessed by RT-PCR and quantified by quantitative PCR as described in “Study design.” Representative data from 3 different experiments with similar results are presented. Relative changes were calculated after normalization to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA levels and presented in parentheses under each corresponding band. (C) Effect of atRA treatment on Ang-2 mRNA levels in microvasculature layer within CAM. The 4.5-day-old CAMs were treated with vehicle (1% ethanol; lane 1), 100 ng atRA (lane 2), or 1 μg Ro41-5253 (lane 3) for 48 hours. CAM tissue was harvested from embryos, and vertical sections (15 μm) were prepared. Regions underneath the chorionic epithelium (50 pieces for one sample; each piece corresponded to about 25 μm × 500 μm) were collected using the microdissection system and total RNA was isolated. Changes in mRNA levels of the indicated factors were assessed by RT-PCR and quantitated as described in “Study design.” Relative changes were calculated after normalization to GAPDH mRNA levels and presented in parentheses under each corresponding band.
Prepublished online as Blood First Edition Paper, March 16, 2004; DOI 10.1182/blood-2003-09-3293.
Supported in part by the Grants-in-Aids for Young Scientists (B) (Y.S.), for Scientific Research (A) (A.Y.), and the Special Coordination Fund for the promotion of Science and Technology (S.K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, as well as the Chemical Biology Research Project (S.K.) and Special Postdoctoral Research Program (Y.S.) from RIKEN.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank A. Uemura (Kyoto University, Japan) for his critical advice, and K. Umezawa and O. Ohno (Keio University, Yokohama, Japan) for a provision of HUVECs.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal