Abstract
Damage to endothelial cells is the common feature of vascular disorders associated with hematopoietic stem cell transplantation (HSCT). Elevated numbers of circulating endothelial cells reflect the extent of endothelial damage in a variety of disorders but their use in HSCT has not been investigated so far. We studied 39 patients undergoing allogeneic HSCT with different conditioning regimens and 22 healthy controls. Circulating endothelial cells were enumerated with immunomagnetic isolation during the course of HSCT. After conditioning, cell numbers were significantly elevated (median 44 cells/mL) compared with baseline (median 16 cells/mL) and controls (median 8 cells/mL). Patients who received radiation had an earlier peak when compared with patients who received chemotherapy. Patients who received reduced-intensity conditioning had significantly lower cell numbers (median 24 cells/mL) than those who received standard conditioning. These observations provide a novel marker to investigate microvascular endothelial damage and the effects of different conditioning regimens in patients undergoing HSCT. (Blood. 2004;103:3603-3605)
Introduction
Veno-occlusive disease (VOD),1 pulmonary vasculopathy,2 thrombotic microangiopathy,3 and capillary leak syndrome (CLS)4 are vascular disorders that occur during the course of hematopoietic stem cell transplantation (HSCT). These disorders are poorly understood and remain a cause of morbidity and mortality although novel therapeutic options have recently become available.5 Damage to endothelial cells is regarded as the common feature of these disorders. More recently, endothelial damage, perpetuated by cytotoxic lymphocytes, has been linked to chronic graft-versus-host disease (GVHD).6
Diagnosing vascular complications in patients undergoing HSCT is challenging, particularly since few laboratory markers of endothelial damage are available. Soluble markers, such as thrombomodulin, have been studied,7-9 although these proteins may indicate endothelial stimulation as opposed to damage and also depend on renal function. New markers of ongoing endothelial damage are therefore eagerly awaited. Circulating endothelial cells are specific and sensitive markers of endothelial damage.10 They have recently gained interest in vasculitis11 associated with antineutrophil cytoplasmic antibodies and renal transplantation,12,13 whereas their use in patients undergoing HSCT has not been studied. The aim of the present study was to evaluate this marker during the conditioning and early posttrans-plantation period of HSCT.
Study design
We studied 39 patients (20 male, 19 female) undergoing allogeneic HSCT from HLA-matched related (n = 18) or unrelated (n = 11) donors. The study was approved by the institutional review board of Hannover Medical School and informed consent was obtained from all patients. In our study sample, 25 patients had acute leukemia, 10 had chronic myelogenous leukemia (CML), and 4 patients had other underlying disorders. There were 28 patients (median age 41 years) who received a conventional conditioning regimen with cyclophosphamide (120 mg/kg body weight) and either total body irradiation with 12 Gy (n = 14) or busulfan (16 mg/kg body weight; n = 14). There were 11 patients (median age 54 years) who underwent reduced-intensity conditioning with fludarabine (150 mg/m2) and busulfan (8 mg/kg body weight) or melphalane (120 mg/m2). We also studied 22 age-matched healthy controls.
Samples of peripheral blood were obtained before and after the conditioning regimen as well as 7, 14, and 21 days after transplantation. We were careful to perform nontraumatic venipuncture.11 Circulating endothelial cells were isolated with Pan-Mouse M-450 Dynabeads (Dynal, Oslo, Norway) coated with anti-CD146 antibody (Biocytex, Marseille, France) as described previously.11 CD146 is expressed by mature endothelial cells, although various tumor cell lines also express the antigen. In addition to immunomagnetic isolation, cells were therefore incubated with Ulex europaeus lectin-1 (UEA-1; Linaris, Wertheim, Germany; 2 mg/mL) for 1 hour in darkness. This second step was included in order to augment the specificity of the technique and facilitate enumeration.14 The sample was washed in the magnet and the cells finally suspended in buffer. Cells were counted with fluorescence microscopy and a Nageotte counting chamber. To exclude that our technique also detects endothelial progenitor cells (EPCs), isolated cells were stained with murine anti-human AC-133 antibody (Miltenyi, Bergisch Gladbach, Germany) and alkaline phosphatase/anti-alkaline phosphatase (APAAP) technique (Dako, Hamburg, Germany) while endothelial progenitor cells (donated by Dr F.H. Bahlmann, Hannover, Germany) were used as positive controls. These stains were uniformly negative (Figure 1). Likewise, stains for alpha smooth-muscle actin were carried out to exclude pericytes. Specifically, cells were stained with murine anti-human alpha smooth-muscle actin antibody (Cymbus Biotechnology, Chandlers Ford, United Kingdom) and APAAP technique (Dako) with human aortic smooth muscle cells as positive controls (donated by Dr I. Dumler, Hannover, Germany). These stains were also negative (data not shown).
AC-133 immunocytochemistry of circulating endothelial cells. Note that circulating endothelial cells from a patient after allogeneic hematopoietic stem cell transplantation were AC-133 negative (A-C), whereas human endothelial progenitor cells served as positive controls (panel D; donated by Dr F.H. Bahlmann); we used murine anti-human AC-133 antibody (Miltenyi), and APAAP technique (Dako).
AC-133 immunocytochemistry of circulating endothelial cells. Note that circulating endothelial cells from a patient after allogeneic hematopoietic stem cell transplantation were AC-133 negative (A-C), whereas human endothelial progenitor cells served as positive controls (panel D; donated by Dr F.H. Bahlmann); we used murine anti-human AC-133 antibody (Miltenyi), and APAAP technique (Dako).
Cell numbers were compared with the Mann-Whitney U test, paired Wilcoxon test, and the Friedman test for comparison of cell numbers at different points in time, respectively.
Results and discussion
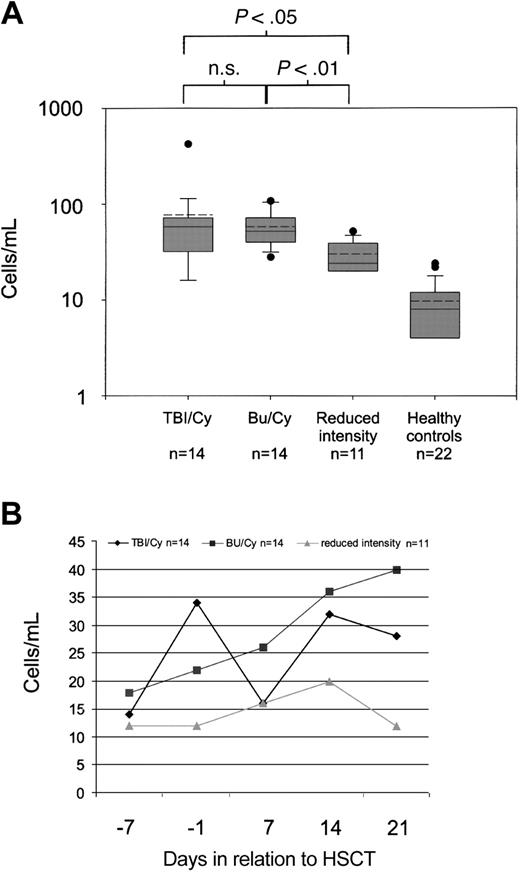
Low numbers of circulating endothelial cells were observed in healthy controls (range, 4-24 cells/mL; median, 8 cells/mL). In contrast, cell numbers were significantly elevated at baseline, that is, before the conditioning regimen (median, 16 cells/mL, P < .0001 when compared with healthy controls). Cells had the characteristic morphology of circulating endothelial cells (Figure 2) as described elsewhere.10 Briefly, they were oval-shaped, between 10 μm and 100 μm in length, and had a well-delineated cytoplasm. There was a significant increase in cell numbers after conditioning (maximum increase: range, 16-424 cells/mL; median, 44 cells/mL, P < .0001 when compared with baseline). Patients who received total body irradiation had similar cell numbers (maximum increase: range, 16-424 cells/mL; median, 58 cells/mL) when compared with those who received chemotherapy only (maximum increase: range, 28-108 cells/mL; median, 52 cells/mL; not significant). Patients who received reduced-intensity conditioning had significantly lower cell numbers (maximum increase: range, 20-52 cells/mL; median, 24 cells/mL) than their counterparts who underwent conventional conditioning with total body irradiation and cyclophosphamide (P < .05) or chemotherapy only with busulfan/cyclophosphamide (P < .01; Figure 3A). However, patients who received total body irradiation had an early peak of elevated cell numbers, whereas patients who received chemotherapy had a more protean elevation of cell numbers (Figure 3B).
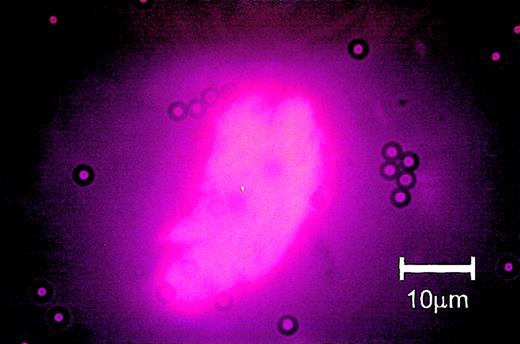
Circulating endothelial cell from a patient undergoing hematopoietic stem cell transplantation: immunomagnetic isolation and subsequent rhodamine-coupled UEA-1 stain. Note that several Dynabeads are attached to the cell and that the cytoplasm shows homogenous staining for UEA-1.
Circulating endothelial cell from a patient undergoing hematopoietic stem cell transplantation: immunomagnetic isolation and subsequent rhodamine-coupled UEA-1 stain. Note that several Dynabeads are attached to the cell and that the cytoplasm shows homogenous staining for UEA-1.
Numbers of circulating endothelial cells during the course of hematopoietic stem cell transplantation. (A) Maximal numbers of circulating endothelial cells according to different conditioning regimens until day 21 and cell numbers in healthy controls. Shaded boxes are interquartile ranges and horizontal lines indicate the median. Dashed lines indicate the mean. TBI denotes total body irradiation, Bu/Cy denotes busulfan/cyclophosphamide. (B) Cell numbers (median) during the course of hematopoietic stem cell transplantation.
Numbers of circulating endothelial cells during the course of hematopoietic stem cell transplantation. (A) Maximal numbers of circulating endothelial cells according to different conditioning regimens until day 21 and cell numbers in healthy controls. Shaded boxes are interquartile ranges and horizontal lines indicate the median. Dashed lines indicate the mean. TBI denotes total body irradiation, Bu/Cy denotes busulfan/cyclophosphamide. (B) Cell numbers (median) during the course of hematopoietic stem cell transplantation.
Damage to microvascular endothelial cells is a well-recognized complication of HSCT. Mechanisms of this disorder remain poorly understood, although both radiation and myeloablative chemotherapy have been implicated. Graft-versus-host prophylaxis with calcineurine inhibitors may also be involved, more so since these drugs have a propensity to damage endothelial cells.13 Elevation of soluble markers, such as sVWF and thrombomodulin, has been described during HSCT.7-9 Nurnberger and colleagues15 demonstrated an increase of thrombomodulin and plasminogen activator inhibitor type-1 (PAI-1) with the number of vascular complications (VOD, GVHD, CLS, sepsis) after bone marrow transplantation. Finally, endothelial damage has recently been linked to chronic GVHD.6
Here, we have demonstrated an increase in circulating endothelial cells as new markers of endothelial damage in conjunction with the conditioning phase of HSCT. Our findings also suggest the presence of a dose effect on the account that patients who underwent reduced-intensity conditioning had lower cell numbers than those who received conventional conditioning. We postulate that considerable endothelial damage occurs during the conditioning phase of HSCT. Surprisingly, patients already had slightly elevated cell numbers before conditioning. It is tempting to speculate that this finding may reflect prior chemotherapy. Alternatively, elevated cell numbers may also reflect the underlying disease, tissue repair, or ongoing angiogenesis. Significant differences between patients with CML and acute leukemia before transplantation were not observed. In this regard, slightly elevated numbers of circulating endothelial cells have been reported in patients with nonhematologic malignancies.16
Interestingly, patients who received total body irradiation had a shorter time from the end of the conditioning phase to their peak in cell numbers than those who received myeloablative chemotherapy alone. Much has been learned about the effects of ionizing radiation of vascular endothelial cells; induction of apoptosis is well documented.17,18 Moreover, endothelial damage is an important pathway for the development of radiation-induced enteritis.19 Our findings are in keeping with these findings and suggest endothelial damage, indicated by a rise in circulating endothelial cells, as a sequel to total body irradiation. Thus, our results are in contrast with a study by Takatsuka and coworkers,20 who demonstrated endothelial stimulation, but not injury, in patients undergoing total body irradiation. In summary, our findings suggest that total body irradiation initiates rapid endothelial injury while chemotherapy causes more protean endothelial damage. Both pathways, however, eventually culminate in detachment of endothelial cells from the basement membrane. The fate of these endothelial cells in blood is still unclear. It is conceivable that circulating endothelial cells may interact with other cell populations and initiate inflammatory mechanisms. Moreover, it is reasonable to assume extensive repair mechanisms21,22 as a sequel to widespread endothelial damage in these patients. The time frame of these events, however, has not been investigated so far. Neither have endothelial progenitor cells been documented extensively after HSCT, although recent evidence suggests that endothelial cells of donor origin occur at a later stage.23
In summary, circulating endothelial cells are a promising new marker to monitor microvascular endothelial damage in patients undergoing HSCT. Further studies should now corroborate our findings in larger numbers of patients, provide longer follow-up, and evaluate whether higher numbers of circulating endothelial cells indicate the development of vascular complications or GVHD.
Prepublished online as Blood First Edition Paper, January 8, 2004; DOI 10.1182/blood-2003-10-3479.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to Mrs Heide Regelsberger for expert technical assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal