Abstract
Notch receptors expressed on hematopoietic stem cells interact with their ligands on bone marrow stromal cells and thereby control cell fate decisions and survival. We recently demonstrated that Notch signaling is involved in proliferation and survival of B cell-derived tumor cells of classic Hodgkin disease and described a novel mechanism for the oncogenic capacity of Notch. In this study we investigated whether Notch signaling is involved in the tight interactions between neoplastic plasma cells and their bone marrow microenvironment, which are essential for tumor cell growth in multiple myeloma (MM). Here we demonstrate that Notch receptors and their ligand Jagged1 are highly expressed in cultured and primary MM cells, whereas nonneoplastic counterparts show low to undetectable levels of Notch. Functional data indicate that ligand-induced Notch signaling is a growth factor for MM cells and suggest that these interactions contribute to myelomagenesis in vivo. (Blood. 2004;103:3511-3515)
Introduction
Notch signaling plays a key role in the development and differentiation of various hematopoietic lineages.1-3 In the hematopoietic system, Notch receptors are expressed in early hematopoietic stem cells, whereas Notch ligands are found in bone marrow stroma, which provides the microenvironment necessary for stem cell survival and differentiation.1,3-5 Further evidence indicates that Notch signaling is involved in the pathogenesis of human B-cell neoplasms; a recent study suggested that Notch2 is highly expressed in B-cell chronic lymphocytic leukemia (B-CLL).6 Our own data indicated that Notch receptors are highly expressed in tumor cells of B cell-derived classic Hodgkin disease.7 We demonstrated a novel mechanism for the oncogenic capacity of Notch by showing that the interaction between Notch receptors on tumor cells and their cognate ligand promotes tumor cell growth and survival.7 However, a pathogenetic role for Notch in multiple myeloma (MM), where tight interactions between neoplastic plasma cells and their microenvironment are essential for tumor cell growth, is unknown.8-11
To evaluate novel molecular mechanisms that support MM cell growth and may be involved in the blockage of plasma cell differentiation, we analyzed Notch gene expression in cultured and primary MM cells. Here we show that Notch1 and Notch2 receptors and their ligand Jagged1 are highly expressed in MM cells. We demonstrate that Notch signaling is activated in MM cells and that further stimulation by its ligand Jagged1 results in a strong increase of tumor cell growth. Therefore, we suggest that activated Notch signaling plays a pivotal role in the pathogenesis of MM.
Materials and methods
Cell culture
Human cell lines analyzed in this study were as follows: the MM cell lines RPMI-8226, OPM-2, LP-1, NCI-H929, U266; the Hodgkin cell line KM-H2; the Burkitt lymphoma cell line BJAB (DSMZ, Braunschweig, Germany). Cell lines were maintained in RPMI 1640 (Biochrom, Berlin, Germany) and 10% heat-inactivated fetal calf serum (FCS; Gibco, Karlsruhe, Germany). HtTA-jag10 cells expressing human Jagged1 under tetracycline control as described previously12 (HeLa-derived cell line) were maintained in Dulbecco modified Eagle medium (DMEM; Biochrom) supplemented with 10% FCS, hygromycin (225 U/mL; Calbiochem, Bad Soden, Germany), G418 (125 μg/mL; Gibco), and tetracycline (2 μg/mL; Sigma, Deisenhofen, Germany). For cocultivation assays HtTA-jag10 cells were plated in 6-cm dishes. Jagged1 expression was induced by washing HtTA-jag10 cells 3 times with DMEM without tetracycline 24 hours after plating. After 48 hours, cells were cocultered with MM and lymphoma cell lines (1 × 106 cells/dish) and with primary MM cells (2 × 105 cells/dish) for 24 hours. MM and lymphoma cells that were aggregated onto the HtTA-jag10 monolayer were harvested by firm tapping, prior to RNA extraction.
Isolation and preparation of CD19+ B cells and CD19low CD38 high plasma cells
CD19+ B cells were isolated from peripheral blood mononuclear cells (PBMCs) of citrated venous blood from healthy donors using centrifugation on Ficoll gradients as previously described.7 CD19+ B cells were isolated by magnetic-activated cell sorting (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany). For differentiation of cells with the characteristics of plasma cells, CD19+ B cells were cocultured with irradiated (50 Gy) EL4 B5 thymoma cells that constitutively express CD40L.13 B cells were cultured in RPMI, 10% FCS that contained 5% phorbol myristate acetate-phytohemagglutinin (PMA/PHA)-stimulated T-cell/monocyte culture supernatant. The B-to-thymoma cell ratio was 1:10. After 3 days B cells were isolated and cultured in RPMI supplemented with recombinant interleukin 2 (IL-2), IL-3, and IL-10 for another 5 days. Activation resulted in about 35% plasmablastic CD38++/CD19+/CD20- cells.13
Bone marrow samples were obtained from adult patients undergoing hip surgery. Cells were washed out of the head of femurs by vigorous pipetting with phosphate-buffered saline (PBS). Bone marrow mononuclear cells were isolated by a standard Ficoll-Hypaque PLUS gradient method (Amersham Biosciences, Freiburg, Germany) and subsequently stained for CD19 (phycoerythrin [PE]; BD Biosciences, Heidelberg, Germany) and CD38 (allophycocyanin [APC], BD Biosciences). Plasma cells were isolated by fluorescence-activated cell sorting (FACS DIVA, BD) according to their expression of CD38high and CD19low+. Cytospins of isolated CD38+++/CD19+ plasma cells of nonneoplastic bone marrow were analyzed by immunohistochemistry.
Enrichment of primary MM cells
Isolation of MM cells was performed as described previously.14 In brief, mononuclear cells from bone marrow aspirates of patients with MM were separated by Ficoll-Hypaque density gradient centrifugation (Amersham Biosciences), labeled with anti-CD138 magnetic beads, and separated using MACS columns. Primary MM cells were cultured in RPMI1640 supplemented with 20% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mM sodium pyruvate, 2 mM glutamine and, in the absence of bone marrow stromal cells (BMSCs), 2 ng/mL IL-6. Cytospins of enriched cells were subjected to Pappenheim stain and controlled by morphology. In every sample at least 90% purity could be achieved. Samples were taken from routine diagnostic specimens after informed consent of patients was obtained and permission was granted by the local ethics committee (University Medicine Berlin).
Immunohistochemistry
Sections, 4-μm thick, of formalin-fixed and paraffin-embedded specimens from 16 patients with the diagnosis of MM (8 medullar, 8 extramedullar manifestations) were retrieved from the files of the Reference Center for Lymph Node Pathology and Hematopathology at the Institute of Pathology, Charité, Campus Benjamin Franklin, Berlin, Germany. The neoplastic plasma cell infiltrates were first identified by conventional histology using hematoxylin-eosin or Giemsa stains. This was followed by appropriate immunostains for plasma cell characteristic molecules, that is, cytoplasmic immunoglobulin heavy and light chains (polyclonal), interferon regulatory factor (IRF4) (clone MUM; both from DakoCytomation, Glostrup, Denmark), and CD138 (clone B-B4; Serotec, Oxford, United Kingdom). In the case of bone marrow biopsy specimens the extent of remaining granulopoietic and erythropoietic cells was made visible by detection of myeloperoxidase (MPO7, polyclonal) or glycophorin C (clone Ret40), respectively (both antibodies were from DakoCytomation). For detection of Notch1, Notch2, and Jagged1 the monoclonal mouse anti-Notch1 (clone N1A; BD Biosciences), monoclonal rat anti-Notch2 (clone bhN6D; Developmental Studies Hybridoma Bank, Iowa City, IA), and polyclonal rabbit anti-Jagged1 (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies were used. Bound antibodies were visualized using the immunoalkaline phosphatase (alkaline phosphatase antialkaline phosphatase [APAAP]) method with reagents from DakoCytomation. For detection of immunoglobulin light or heavy chains and for the performance of double-labeling experiments, the streptavidin/biotin/peroxidase technique was also applied (also using reagents from DakoCytomation).
Immunoblotting
Cell extracts were prepared and quantitated as described.15 Proteins (12-30 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Protein load was normalized by Ponceau red staining. Membranes were incubated with monoclonal mouse anti-Notch1 antibodies (no. 552466; BD Biosciences), monoclonal rat anti-Notch1 and anti-Notch2 antibodies (bTAN20 and bhN6D; Developmental Studies Hybridoma Bank), and polyclonal rabbit anti-Jagged1 antibodies (sc-8303; Santa Cruz Biotechnology). Secondary antibodies were goat antimouse (Promega, Mannheim, Germany), goat antirat (Dianova, Hamburg, Germany), and goat antirabbit (Santa Cruz Biotechnology) horseradish peroxidase (HRP)-conjugated antibodies. They were detected by enhanced chemiluminescence (Amersham Pharmacia, Freiburg, Germany).
RT-PCR analysis
Reverse transcription-polymerase chain reaction (RT-PCR) analysis was performed using human Hes-1 (forward 5′-AGC ACA GAA AGT CAT CAA AGC C-3′ and reverse 5′-TTC ATG CAC TCG CTG AAG CC-3′; 270 bp) and human β2-microglobulin (210 bp)-specific primers. To serve as an internal control, β2-microglobulin was amplified from all cDNA templates generated. Total RNA was extracted from 1 × 106 cells and 2 × 105 primary MM cells using RNeasy Mini Kit (Quiagen, Hilden, Germany) following the manufacturer's instructions. For cDNA synthesis, the Superscript II RNase H Reverse Transcriptase Kit (Invitrogen, Karlsruhe, Germany) was used following the manufacturer's instructions. For PCR, 2 μL cDNA was amplified using AmpliTaq DNA polymerase (Roche, Darmstadt, Germany). PCR conditions (32 cycles) were the following: denaturation at 94°C for 5 minutes, annealing at 63°C for 30 seconds and extension at 72°C for 90 seconds. A final step at 72°C for 10 minutes was included before samples were cooled to 4°C.
Proliferation assays
HtTA-jag10 cells were plated in microtiter wells (2 × 103 cells/100 μL DMEM). Plates were washed with DMEM without tetracycline for induction of Jagged1 after 24 hours. After 48 hours, HtTA-jag10 cells were irradiated with 120 Gy and cocultered with MM and lymphoma cells (2-4 × 104 cells/well). Triplicate samples were cultured for 24 hours at 37°C in the presence or absence of tetracycline. 3[H]-Thymidine (1 μCi/100 μL [0.037 MBq/100 μL] DMEM; 0.037 MBq) was added to each well for 20 hours before determination of radioisotope incorporation into DNA. For inhibition of Notch signaling the γ-secretase inhibitor DAPT (kindly provided by M. Wolfe, Harvard Medical School, Boston, MA) was used. Jagged1-stimulated MM and lymphoma cells were cultured for 48 hours with 50 μM DAPT dissolved in dimethyl sulfoxide (DMSO) or with DMSO alone as control. 3[H]-Thymidine (1 μCi/100 μL [0.037MBq/100 μL] DMEM) was added to each well for 20 hours before determination of radioisotope incorporation into DNA.
Results
Notch1, Notch2, and the Notch ligand Jagged1 are highly expressed in primary MM cells
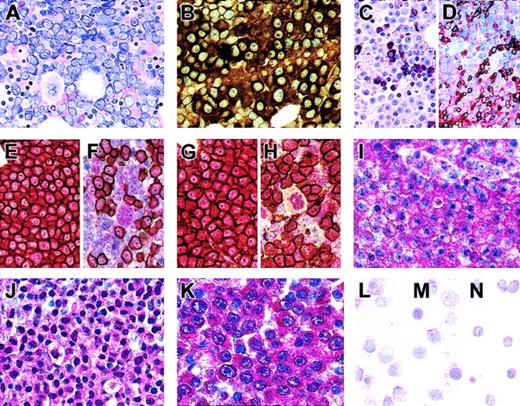
We analyzed 16 cases of MM (8 medullar and 8 extramedullar manifestations) for expression of Notch1 and Notch2 by immunohistochemistry. By conventional histology, neoplastic plasma cell infiltrates could be identified by their enlarged nuclei, frequently containing prominent nucleoli (Figure 1A). By immunostains they showed a monotypic expression of cytoplasmic immunoglobulin light (Figure 1B) or heavy chains, of CD138, and of IRF4 (data not shown). Medullary and extramedullary MM manifestations were positively labeled with anti-Notch1 and anti-Notch2 antibodies. The expression pattern of both molecules was cytoplasmic (Figure 1I-J), whereas an additional nuclear staining was detectable in some cases (Figure 1J for Notch2). The expression intensity was higher than in nonneoplastic adjacent cells. This was substantiated by performing double-labeling experiments in a representative case by combining immunostains for CD138 with that for Notch1 detection. Thus it could be shown that Notch1 was clearly highly expressed in neoplastic CD138 myeloma cells (Figure 1E), whereas only some CD138- nonneoplastic bone marrow cells, mainly megakaryocytes, showed a weaker Notch1 expression (Figure 1F).
High Notch1, Notch2, and Jagged1 expression in primary MM cells. (A-H) Bone marrow biopsy specimen of one case of MM. (A) Giemsa staining demonstrating bone marrow infiltration by atypical plasma cells (MM cells) with large nuclei and prominent nucleoli. (B) Immunostained MM cells show monotypic expression of immunoglobulin light-chain κ. (C-D) Immunostains for myeloperoxidase (C) and glycophorin C (D) mark granulocytic and erythroid precursors showing that MM cells are surrounded by nonmalignant bone marrow cells. (E-F) Double labeling for CD138 (brown reaction product; streptavidin/biotin/peroxidase technique) and Notch1 (red reaction product; immunoalkaline phosphatase method) reveals that CD138+ MM cells strongly coexpress Notch1. (G-H) Double labeling for CD138 (brown reaction product) and the Notch ligand Jagged1 (red reaction product) demonstrates coexpression of CD138 and Jagged1 on MM cells. (F,H) Some CD138- nonmalignant bone marrow cells, mainly megakaryocytes, show weak immunoreactivity against Notch1 or Jagged1. (I-K) Extramedullar (liver) biopsy specimen of MM immunostained for Notch1 (I), Notch2 (J), and Jagged1 (K) and counterstained with hematoxylin. MM cells are intensely labeled by anti-Notch1, anti-Notch2, and anti-Jagged1 antibodies. (L-N). Isolated plasma cells (CD38+++/CD19+) from the nonneoplastic bone marrow of healthy donors (cytospins) show low to undetectable immunoreactivity against Notch1 (L), Notch2 (M), and Jagged1 (N). Original magnification × 200.
High Notch1, Notch2, and Jagged1 expression in primary MM cells. (A-H) Bone marrow biopsy specimen of one case of MM. (A) Giemsa staining demonstrating bone marrow infiltration by atypical plasma cells (MM cells) with large nuclei and prominent nucleoli. (B) Immunostained MM cells show monotypic expression of immunoglobulin light-chain κ. (C-D) Immunostains for myeloperoxidase (C) and glycophorin C (D) mark granulocytic and erythroid precursors showing that MM cells are surrounded by nonmalignant bone marrow cells. (E-F) Double labeling for CD138 (brown reaction product; streptavidin/biotin/peroxidase technique) and Notch1 (red reaction product; immunoalkaline phosphatase method) reveals that CD138+ MM cells strongly coexpress Notch1. (G-H) Double labeling for CD138 (brown reaction product) and the Notch ligand Jagged1 (red reaction product) demonstrates coexpression of CD138 and Jagged1 on MM cells. (F,H) Some CD138- nonmalignant bone marrow cells, mainly megakaryocytes, show weak immunoreactivity against Notch1 or Jagged1. (I-K) Extramedullar (liver) biopsy specimen of MM immunostained for Notch1 (I), Notch2 (J), and Jagged1 (K) and counterstained with hematoxylin. MM cells are intensely labeled by anti-Notch1, anti-Notch2, and anti-Jagged1 antibodies. (L-N). Isolated plasma cells (CD38+++/CD19+) from the nonneoplastic bone marrow of healthy donors (cytospins) show low to undetectable immunoreactivity against Notch1 (L), Notch2 (M), and Jagged1 (N). Original magnification × 200.
To compare the high expression of Notch receptors in MM cells to plasma cells of healthy donors, we isolated CD38+++/CD19+ plasma cells of nonneoplastic bone marrow (Figure 1L-M). Immunocytochemical staining of cytospins of isolated normal plasma cells showed that Notch1 and Notch2 were expressed in some plasma cells of healthy donors but to a lower extent compared with MM cells (Figure 1E-J). Most plasma cells were devoid of the proteins. In addition, we found low to undetectable levels of Notch1 and Notch2 in plasma cells of reactive lymphoid tissue (data not shown).
Using immunohistochemistry we further found high expression levels of the Notch ligand Jagged1 in primary MM cells (Figure 1G,H,K) compared to normal plasma cells (Figure 1N). Double labeling for Jagged1 and CD138 in representative specimens (Figure 1G-H) revealed intensive labeling for both molecules, proving that primary MM cells highly expressed Jagged1.
Jagged1 induces Notch signaling in primary and cultured MM cells
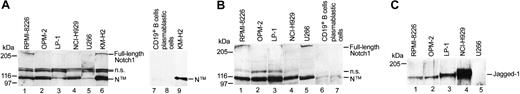
To verify high expression of Notch1, Notch2, and Jagged1 in cultured MM cells, we performed Western blot analysis of 5 MM cell lines (Figure 2). According to our data in primary MM cells, we found that both Notch receptors were highly expressed in all MM cell lines except for U266 cells (Figure 2A-B lanes 1-5). U266 cells expressed high amounts of Notch2 but only low amounts of Notch1 and were used as a negative control in the present and in our previous study.7 The Hodgkin cell line KM-H2 served as a positive control (Figure 2A lanes 6 and 9), known to express high amounts of Notch1 and Notch2 as previously described.7 In contrast, freshly isolated mature CD19+ B cells and CD19+ B cells, which we differentiated to CD38+ plasmablastic cells in vitro, were almost completely devoid of Notch expression (Figure 2A-B lanes 7-8 and lanes 6-7, respectively). Our data suggest that most cultured MM cells differ from their nonneoplastic counterparts with respect to strong Notch1 and Notch2 expression. In addition, Western blot analysis demonstrated that cultured MM cells except for U266 cells expressed the Notch ligand Jagged1 (Figure 2C).
High Notch1, Notch2, and Jagged1 expression in cultured MM cells. Western blot analysis of cell lysate proteins from cultured MM cells (RPMI-8226, OPM-2, LP-1, NCI-H929, U266; panels A-C, lanes 1-5), mature CD19+ B cells (panel A, lane 7; panel B, lane 6), CD38+/CD19+ plasmablastic cells (panel A, lane 8; panel B, lane 7), and the Hodgkin cell line KM-H2 (panel A, lanes 6 and 9), showing expression of Notch1 (A) and Notch2 (B) as full-length proteins (300 kDa) and their major cleavage product (N™, 110 kDa). In panel A, lanes 1-6, and panels B and C, 30 μg proteins were loaded. In panel A, lanes 7-9, 12 μg proteins were loaded. In panel A, lanes 1-6, proteins were incubated with mouse monoclonal anti-Notch1 antibodies (BD Biosciences); and for lanes 7-9, proteins were incubated with rat monoclonal anti-Notch1 antibodies (bTAN20; Developmental Studies Hybridoma Bank). (C) Expression of the Notch ligand Jagged1 (about 130 kDa) in cultured MM cells (left margin). Size markers are in kilodaltons, and n.s. indicates not significant.
High Notch1, Notch2, and Jagged1 expression in cultured MM cells. Western blot analysis of cell lysate proteins from cultured MM cells (RPMI-8226, OPM-2, LP-1, NCI-H929, U266; panels A-C, lanes 1-5), mature CD19+ B cells (panel A, lane 7; panel B, lane 6), CD38+/CD19+ plasmablastic cells (panel A, lane 8; panel B, lane 7), and the Hodgkin cell line KM-H2 (panel A, lanes 6 and 9), showing expression of Notch1 (A) and Notch2 (B) as full-length proteins (300 kDa) and their major cleavage product (N™, 110 kDa). In panel A, lanes 1-6, and panels B and C, 30 μg proteins were loaded. In panel A, lanes 7-9, 12 μg proteins were loaded. In panel A, lanes 1-6, proteins were incubated with mouse monoclonal anti-Notch1 antibodies (BD Biosciences); and for lanes 7-9, proteins were incubated with rat monoclonal anti-Notch1 antibodies (bTAN20; Developmental Studies Hybridoma Bank). (C) Expression of the Notch ligand Jagged1 (about 130 kDa) in cultured MM cells (left margin). Size markers are in kilodaltons, and n.s. indicates not significant.
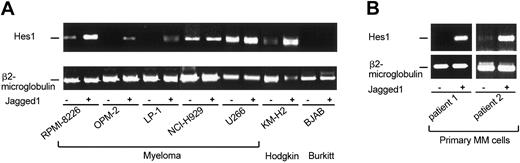
Next, we examined whether in cultured and primary MM cells Notch signaling is activated and whether stimulation by exogenous Jagged1 can induce Notch downstream target gene expression. To that end, we performed RT-PCR analysis of mRNA expression of Hes-1, a member of the hairy enhancer of split family of transcriptional repressors that are direct transcriptional targets of activated Notch.2,3 In the MM cell lines RPMI-8266, NCI-H929, and U266, Notch signaling was already activated because Hes-1 transcripts were detected in unstimulated MM cells (Figure 3A). After stimulation by Jagged1 through ligand-expressing HtTA cells,12 Hes-1 expression markedly increased in the MM cell lines RPMI-8266, OPM-2, and LP-1. In NCI-H929 and U266 cells the Notch target gene expression could not further be induced (Figure 3A). The Hodgkin cell line KM-H2 was used as a positive control (Figure 3A) because we had recently demonstrated that in tumor cells of Hodgkin lymphoma Notch signaling can be significantly induced by Jagged1-expressing HtTA-jag10 cells.7 The Burkitt lymphoma cell line BJAB served as a negative control because BJAB cells did not express Notch (data not shown).
Jagged1-induced Notch target gene activation in MM cells. (A) Cocultivation of HtTA-jag10 cells expressing the Notch-ligand Jagged1 under tetracycline control11 (induction of Jagged1 expression by tetracycline withdrawal) and MM, Hodgkin, and Burkitt lymphoma cell lines expressing various amounts of endogenous Notch1 and Notch2 receptors. RT-PCR analysis showed increased levels of endogenous Hes-1 transcripts in cultured MM cells (RPMI-8226, OPM-2, LP-1) and in the Hodgkin lymphoma cell line KM-H2 after activation by Jagged1. KM-H2 cells served as a positive control. (B) Strong induction of Notch signaling by Jagged1 in primary MM cells.
Jagged1-induced Notch target gene activation in MM cells. (A) Cocultivation of HtTA-jag10 cells expressing the Notch-ligand Jagged1 under tetracycline control11 (induction of Jagged1 expression by tetracycline withdrawal) and MM, Hodgkin, and Burkitt lymphoma cell lines expressing various amounts of endogenous Notch1 and Notch2 receptors. RT-PCR analysis showed increased levels of endogenous Hes-1 transcripts in cultured MM cells (RPMI-8226, OPM-2, LP-1) and in the Hodgkin lymphoma cell line KM-H2 after activation by Jagged1. KM-H2 cells served as a positive control. (B) Strong induction of Notch signaling by Jagged1 in primary MM cells.
Results of RT-PCR analysis of U266 cells were in contrast to our previous study, where we used Northern blot analysis for detection of Hes-1-specific transcripts and did not detect any transcripts in U266 cells prior and after stimulation by Jagged1. This discrepancy could possibly be explained by the higher sensitivity of RT-PCR analysis compared to Northern blot analysis for detection of Hes-1-specific transcripts.
To improve the relevance of our data we further isolated primary MM cells as described previously.14 We showed that stimulation of primary MM cells of different patients by Jagged1 markedly increased Hes-1 expression, indicating that Notch signaling was functional in primary MM cells (Figure 3B). In the second patient Notch signaling was already slightly activated (Hes-1 transcripts) prior to stimulation by Jagged1 and could further be strongly induced (Figure 3B). Taken together, these data suggest that Notch signaling is activated and can further be induced by exogenous Jagged1 in cultured and primary MM cells.
Activated Notch signaling accelerates proliferation of cultured MM cells that can be blocked by the γ-secretase inhibitor DAPT
To evaluate the biologic significance of Jagged1-induced Notch activation in MM cells and whether activation promotes tumor cell growth, we performed proliferation assays and measured 3[H]thymidine uptake using cocultivation assays. Stimulation by Jagged1 strongly increased MM cell growth up to 2-fold in 20 hours and correlated with Hes-1 target gene activation (RPMI-8266, OPM-2, LP-1; Figure 4A). These data indicated an exponential increase in already well-proliferating tumor cells. In contrast, we could not detect an increase in proliferation rates in NCI-H929 cells and U266 cells (data not shown7 ), where Hes-1 was not significantly up-regulated (Figure 4A). The Hodgkin cell line KM-H2 and the Burkitt lymphoma cell line BJAB were used as positive and negative controls, respectively. We previously demonstrated that in this cocultivation assay promotion of Hodgkin lymphoma cell growth did indeed occur through the engagement of Notch on the target cells and not through Jagged1 that could also activate Notch on the feeder cells and change expression of other proteins that modify lymphoma cell proliferation.7 In our previous study, we used purified, clustered soluble Jagged1 and confirmed our data with Jagged1-expressing feeder cells.7 We therefore conclude that Jagged1-induced Notch signaling can strongly accelerate proliferation of cultured MM cells and thereby might contribute to the MM cell transformation process in vivo.
Activated Notch signaling promotes proliferation of MM cells and can specifically be blocked by the γ-secretase inhibitor DAPT. (A) Proliferative responses of Notch-expressing MM, Hodgkin, and Burkitt lymphoma cells after activation by Jagged1. HtTA-jag10 cells were plated in microtiter wells. Plates were washed with DMEM without tetracycline for induction of Jagged1 after 24 hours. Controls were washed with tetracycline-containing medium. After 48 hours, HtTA-jag10 cells were irradiated (120 Gy) and cocultured with MM, Hodgkin, and Burkitt lymphoma cells. Triplicate samples were cultured for 24 hours at 37°C in the presence or absence of tetracycline. 3[H]-thymidine was added to each well for 20 hours before determination of radioisotope incorporation into DNA. The results are shown as the mean of 3[H]-thymidine incorporation (± SD) of 3 independent proliferation assays. (A) Counts of activated cells (presence of Jagged1; ▪) are given relative to counts of nonactivated cells (absence of Jagged1; □), which were set arbitrarily at 100% for each cell line. (B) Jagged1-stimulated cells treated either with the γ-secretase inhibitor DAPT (▪) or with DMSO (□) alone as control. Counts of DAPT-treated cells are given relative to counts of control treated cells, which were set arbitrarily at 100% for each cell line. *P < .05; n.s. indicates not significant using the Student t test.
Activated Notch signaling promotes proliferation of MM cells and can specifically be blocked by the γ-secretase inhibitor DAPT. (A) Proliferative responses of Notch-expressing MM, Hodgkin, and Burkitt lymphoma cells after activation by Jagged1. HtTA-jag10 cells were plated in microtiter wells. Plates were washed with DMEM without tetracycline for induction of Jagged1 after 24 hours. Controls were washed with tetracycline-containing medium. After 48 hours, HtTA-jag10 cells were irradiated (120 Gy) and cocultured with MM, Hodgkin, and Burkitt lymphoma cells. Triplicate samples were cultured for 24 hours at 37°C in the presence or absence of tetracycline. 3[H]-thymidine was added to each well for 20 hours before determination of radioisotope incorporation into DNA. The results are shown as the mean of 3[H]-thymidine incorporation (± SD) of 3 independent proliferation assays. (A) Counts of activated cells (presence of Jagged1; ▪) are given relative to counts of nonactivated cells (absence of Jagged1; □), which were set arbitrarily at 100% for each cell line. (B) Jagged1-stimulated cells treated either with the γ-secretase inhibitor DAPT (▪) or with DMSO (□) alone as control. Counts of DAPT-treated cells are given relative to counts of control treated cells, which were set arbitrarily at 100% for each cell line. *P < .05; n.s. indicates not significant using the Student t test.
To further determine the role of Notch signaling in tumor cell proliferation in MM, we specifically blocked the Notch pathway by the γ-secretase inhibitor DAPT. γ-Secretase is a critical component of the Notch signaling transduction pathway. Inhibition of Notch by γ-secretase interferes with the ligand induced proteolytic formation of Notch and thereby blocks its activation. Treatment with DAPT resulted in 40% to 75% decrease in proliferation rates of MM and Hodgkin cell lines. The decrease in proliferation rates in BJAB cells (negative control) was not significant. Our data demonstrate that Jagged1-stimulated Notch activation and induction of proliferation of MM cells can be efficiently blocked by DAPT.
Discussion
In this study we investigated the role of the Notch signaling pathway in the pathogenesis of neoplastic plasma cells. We show here that Notch1 and Notch2 proteins are highly expressed in primary MM cells in medullar and extramedullar manifestations of MM. To definitively conclude on the high expression in MM cells compared to plasma cells from healthy donors, we isolated CD38+++/CD19+ plasma cells of nonneoplastic bone marrow of healthy donors. Some isolated normal plasma cells showed low expression of Notch1 and Notch2, but most plasma cells were devoid of the proteins.
Moreover, we asked whether heterotypic or homotypic cell-cell interactions between MM cells and their medullar environment may activate Notch signaling in vivo. Abundant evidence suggests that the Notch-ligand Jagged1 is expressed in bone marrow stromal cells, so that neoplastic plasma cells can be heterotypically stimulated.16 Furthermore, we found high expression levels of Jagged1 in primary MM cells. In addition, a recent array study showed mRNA overexpression of the Notch-ligand Jagged2 in MM samples compared to lymphoblastoid cell lines.17 Therefore, we suggest that heterotypic as well as homotypic cell-cell interactions can activate Notch signaling in vivo and thereby contribute to the pathogenesis of MM.
Notch receptors consist of 2 fragments, the extracellular (NEC) and the transmembrane fragment (N™), that are linked and appear as the ligand-accessible form on the cell surface.18 After ligand binding N™ is cleaved and the resulting intracellular fragment (NIC) translocates to the nucleus where it functions as a transcription factor.18 Both anti-Notch antibodies that we used for immunohistochemistry were directed against epitopes of the intracellular fragments.18 Interestingly, we observed in primary MM cells of some MM cases not only intensive cytoplasmic but also nuclear staining with Notch1 (data not shown) and Notch2-specific antibodies (Figure 1J). Because normal ligand-induced signaling does not generally result in detectable levels of nuclear protein,7 we asked whether Notch activity in MM cells results from expression of a constitutively active, truncated form of Notch or from continuous activation by ligand.
To that end, we performed Western blot analysis of unstimulated and ligand-stimulated (Jagged1) cultured MM cells (Figure 2 and data not shown). We observed significant expression of N™ with the expected size of about 110 kDa (Notch1 and Notch2) in cultured MM cells (Figure 2A-B; except for U266 cells). Thereby, no obvious differences in sizes (truncated forms) of Notch-specific bands were found either in unstimulated or Jagged1-stimulated cell lines (data not shown). Taken together, we suggest that Notch activity in MM cells results from continuous activation by ligands rather than from truncated Notch forms.
Furthermore, stimulation by Jagged1 resulted in activation of the Notch downstream target gene Hes-1 in cultured and primary MM cells and caused a strong increase in growth rates of cultured MM cells, which could be efficiently blocked by the γ-secretase inhibitor DAPT. Given the strong coexpression of Notch receptors and their ligand Jagged1 in MM cells and ligand expression in bone marrow stromal cells, it seems likely that possible homotypic and heterotypic interactions also lead to activated Notch signaling in vivo. Our data suggest that Jagged1-induced Notch signaling might thereby contribute to the pathobiology of MM.
From our study it cannot be excluded that Notch signaling in MM cells is a marker of a particular cell type or differentiation stage. It has been demonstrated that expression of components (eg, manic fringe, presenilin 2), which modify Notch signaling, was altered in normal plasma cells compared to IgM+ B cells.19 However, in our study neither activated plasmablastic CD38+ cells in vitro nor normal plasma cells in vivo revealed high-level Notch expression.
Constitutive activation of Notch alleles induces T-cell leukemias and lymphomas.20-22 In addition, our previous study demonstrates that Notch signaling promotes tumor cell growth and survival in B cell-derived Hodgkin disease.7 We therefore hypothesize from this study that Notch signaling is also involved in the pathogenesis of neoplastic plasma cells. Manipulation of the Notch pathway may be a novel therapeutic approach in these neoplasms.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-07-2254.
Supported in part by the Deutsche Forschungsgemeinschaft through JU 426/1-3.
F.J. and K.S.P. contributed equally to this work.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Katharina Kley and Sina Heydrich for excellent technical assistance. HtTA-jag10 cells were kindly provided by Dr Celine Gelinas from the University of Medicine and Dentistry of New Jersey (Newark, NJ). The γ-secretase inhibitor DAPT was kindly provided by M. Wolfe of Harvard Medical School (Boston, MA).

![Figure 4. Activated Notch signaling promotes proliferation of MM cells and can specifically be blocked by the γ-secretase inhibitor DAPT. (A) Proliferative responses of Notch-expressing MM, Hodgkin, and Burkitt lymphoma cells after activation by Jagged1. HtTA-jag10 cells were plated in microtiter wells. Plates were washed with DMEM without tetracycline for induction of Jagged1 after 24 hours. Controls were washed with tetracycline-containing medium. After 48 hours, HtTA-jag10 cells were irradiated (120 Gy) and cocultured with MM, Hodgkin, and Burkitt lymphoma cells. Triplicate samples were cultured for 24 hours at 37°C in the presence or absence of tetracycline. 3[H]-thymidine was added to each well for 20 hours before determination of radioisotope incorporation into DNA. The results are shown as the mean of 3[H]-thymidine incorporation (± SD) of 3 independent proliferation assays. (A) Counts of activated cells (presence of Jagged1; ▪) are given relative to counts of nonactivated cells (absence of Jagged1; □), which were set arbitrarily at 100% for each cell line. (B) Jagged1-stimulated cells treated either with the γ-secretase inhibitor DAPT (▪) or with DMSO (□) alone as control. Counts of DAPT-treated cells are given relative to counts of control treated cells, which were set arbitrarily at 100% for each cell line. *P < .05; n.s. indicates not significant using the Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/9/10.1182_blood-2003-07-2254/6/m_zh80090460500004.jpeg?Expires=1769106011&Signature=AmyX--RtiCm18P3ZlXWkqOpAtppS7DAl65CfEIM8ZJKX17TtK0Z6Mr3aCo3iXKJki49cmS2P9eEwXmmszE4m5v-sErlZ0atTWJIWCXFkfo5XbvDJj7byE0SjO4H6ZqliLwZc4SB9YxJQrbNyTtt1ILfjHBGJI9fMAF9mI6UppzWDFpJ8AS-BYXcgWAqKsGVicfi7QCqeO81dMqnVkPff0UI5SA02DvxwbYVT9ZqdfCcorEiJBmeVbCoCrXnKg0iNoLoQcMQHJxPw5Qg3EVz3vaBv38igEQtGCQdV6nHnujyRCGOR-GywK2-MHQSUxlqURkWjY4V089OJ2t9btDQ93w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal