Abstract
Arsenic trioxide induces c-jun N-terminal kinase (JNK) activation and apoptosis in acute promyelocytic leukemia (APL), where it has major clinical activity, but whether JNK is necessary to induce apoptosis is unknown. To clarify this necessity, we established 2 arsenic trioxide (As2O3)-resistant subclones of the APL cell line, NB4. Both resistant lines showed little activation of JNK1 following treatment with As2O3, even at doses sufficient to elicit robust activation in NB4 cells. One mechanism of resistance in these cells is up-regulated glutathione (GSH) content, and GSH depletion by l-buthionine-[S,R]-sulfoximine (BSO) restores JNK activation and As2O3 sensitivity. This correlation between JNK activation and apoptosis led us to test whether inhibition of JNK would protect cells from As2O3-induced apoptosis. SEK1-/- mouse embryo fibroblasts (MEFs) showed diminished JNK activation following As2O3 treatment and were protected from As2O3-induced but not doxorubicin-induced apoptosis. Furthermore, treatment of arsenic trioxide-sensitive APL cells with the JNK inhibitor, dicumarol, significantly increased growth and survival in response to As2O3 but did not protect cells from doxorubicin. Together, these data support an essential role for JNK signaling in the induction of growth inhibition and apoptosis by As2O3 and suggest that activating JNK may provide a therapeutic advantage in the treatment of cancers that do not respond to arsenic alone. (Blood. 2004;103:3496-3502)
Introduction
Acute promyelocytic leukemia (APL) is a myeloid leukemia arising as the result of a reciprocal chromosomal translocation and the subsequent expression of a novel fusion protein, PML/RARα.1-3 Composed of the N-terminal part of the promyelocytic leukemia (pmL) gene product, and most of the functional domains of the retinoic acid receptor alpha (RARα), PML/RARα induces a block to differentiation in affected promyeloctyes. Treatment of patients with APL or APL cells in vitro with pharmacologic doses of retinoic acid (RA), the natural ligand for RARs, induces the activation and subsequent degradation of PML/RARα, thereby relieving the block to RA signaling and allowing granulocytic differentiation to proceed.4,5
Although 70% to 90% of patients treated with RA in combination with chemotherapy achieve complete remission, many relapse and develop RA resistance.6 Our laboratory and others have described point mutations in the RARα moiety of the fusion protein, both in RA-resistant patient cells and in resistant APL cells derived in culture, providing one mechanism of resistance.7-10 A second, highly effective therapeutic option for the treatment of APL exists, however. Arsenic trioxide (As2O3), a centuries-old component of traditional Chinese medicine whose role in APL treatment was defined in China roughly a decade ago, induces remission in most patients, including those who have developed resistance to RA.11-13 Its effects are mediated in part by inducing degradation of PML/RARα and consequent differentiation of APL cells.14 In addition, unlike RA, arsenic trioxide induces growth inhibition (at lower concentrations) and apoptosis by mechanisms that remain to be fully elucidated.
The induction of apoptosis by arsenic has been linked, among other things, to the accumulation of free radicals and subsequent induction of oxidative stress.15 In keeping with this observation, an inverse correlation between levels of the intracellular antioxidant, reduced glutathione (GSH), and arsenic trioxide sensitivity has been reported.16,17 Arsenic has also been shown to cause activation of the stress-activated protein kinase, c-jun N-terminal kinase (JNK).18 Activation of the neuron-specific JNK3 isoform by arsenite was shown to contribute to neuronal apoptosis.19 Similarly, activation of JNK has been shown to correlate with the induction of apoptosis by arsenic in a mouse epidermal cell line.20 However, this activation of JNK in normal mouse cells was observed following short-term treatment (3 hours) with very high doses of arsenic (50-200 μM) that are beyond those tolerable in vivo with As2O3 therapy. In addition, the induction of apoptosis by JNK activation appears to require a sustained increase in activity.21 Therefore, to examine the effects of arsenic trioxide on JNK signaling that may contribute to in vivo responses, we carried out experiments using longer treatments with lower doses of As2O3. We found substantial activation of JNK1 in the human APL cell line, NB4. As2O3-resistant NB4 subclones established in our lab17 were used to further link activation of JNK to arsenic trioxide-induced apoptosis. To determine whether signaling through this pathway is necessary to elicit an apoptotic response, we used genetic and pharmacologic approaches to inhibit JNK activity. First, we examined the response to arsenic trioxide of a mouse embryo fibroblast (MEF) cell line lacking SEK1, an upstream activator of JNK, and therefore, impaired in JNK signaling. Finally, as an alternative means of inhibiting JNK, we used the chemical inhibitor, dicumarol, in NB4 cells to examine whether signal transduction through this pathway contributes to the exquisite sensitivity of APL cells to arsenic.
Materials and methods
Cell culture
Two separate arsenic trioxide-resistant APL cell lines, NB4-M-AsR2 and NB4-M-AsR3, hereafter referred to as AsR2 and AsR3, respectively, were generated by constantly culturing NB4 cells in the presence of As2O3 at concentrations that were gradually increased over time.17 When a population of cells were obtained that could be maintained in the presence of 1 μM As2O3, single clones were selected by plating in methylcellulose. A possible mechanism of resistance in these cell lines is their elevated intracellular GSH content, which is approximately 2-fold higher than that in NB4 cells.17 NB4, AsR2, and AsR3 cells were maintained in RPMI 1640 media supplemented with 10% fetal calf serum (FCS). AsR2 and AsR3 cells were also constantly grown in the presence of 2 μM As2O3 (Sigma, Oakville, ON, Canada). In experiments examining the response of AsR2 and AsR3 to As2O3, the cells were first washed thoroughly to remove arsenic trioxide from the media, then cultured for 24 hours in media alone prior to initiating the experiment. SEK1+/+ and SEK1-/- MEF cell lines were kindly provided by Dr Jim Woodgett, University of Toronto.22 MEFs were grown in Dulbecco modified Eagle media with 10% FCS. All cells were grown in a humidified chamber at 37°C with a 5% CO2 environment.
Growth assays
NB4 cells were seeded at 5 × 104 cells/mL in 6-well plates. Cells were treated with 0.5 μM As2O3 (Sigma), or 0.01 μg/mL doxorubicin alone or in combination with 1 μM dicumarol (Sigma) for 4 days. SEK1+/+ and SEK1-/- cells were seeded at 1 × 103 cells per well in 24-well plates and treated for 5 days with 1 or 2.5 μM As2O3, 0.5 or 1 μg/mL doxorubicin (Sigma). Viable cells were counted by trypan blue (Invitrogen, Burlington, ON, Canada) exclusion with a hemacytometer. NB4 cells were maintained at a density lower than 1 × 106 cells/mL through dilution as required. Cells were retreated with arsenic trioxide or doxorubicin on every third day and daily with dicumarol.
GSH depletion
GSH was depleted from APL cells by treatment with 100 μM l-buthionine- [S,R]-sulfoximine (BSO) (Sigma), a dose sufficient to decrease GSH to undetectable levels within 12 hours, yet not have toxic effects in APL cells when used as a single agent (data not shown). Depletion of GSH restores sensitivity to As2O3 in arsenic trioxide-resistant APL cells and, therefore, provides a method by which to investigate mechanisms of arsenic-induced apoptosis.17
Immune complex kinase assay
Twice-washed cells were seeded at 4 × 106 cells/10 mL serum-free media in T25 flasks and serum-starved for 24 hours. They were then treated at various times and concentrations of As2O3 alone or in combination with 100 μM BSO. Following treatment, cells were washed once with cold phosphate-buffered saline (PBS) and lysed on ice in the presence of phosphatase and protease inhibitors. JNK1 was immunoprecipitated from 200 μg extract with an anti-JNK1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and Protein A-Sepharose beads by nutation at 4°C for 1 hour. Following multiple, successive washes with kinase buffer containing 3 M urea, then kinase buffer alone, immune complexes were incubated with GST-c-jun and [γ-32P]ATP for 30 minutes at 30°C. GST-c-jun was then purified from the reaction mix with GSH-Sepharose beads, followed by resolution on a 12% acrylamide gel and autoradiography. Immunoprecipitated JNK1 could not be measured directly by Western blot because of the presence of immunoglobulin G (IgG) heavy chain from the immunoprecipitating antibody, which cross-reacts with the secondary antibody, obscuring the JNK1 band. To ensure equal pull-down of JNK1, samples were handled identically, and Western blotting was performed prior to the immunoprecipitation to ensure that As2O3 treatment did not affect absolute JNK1 expression levels.
Western blot analysis
Cell extracts were prepared by first washing 5 × 106 cells with cold PBS, then resuspending cell pellets in 0.4 mL lysis buffer (5 mM NaH2PO4, 1 mM DTT (dithiothreitol), 10% glycerol, 1 mM PMSF (phenylmethylsulfonyl fluoride), 10 μg/mL each aprotinin and leupeptin, pH 7.4) at 4°C. Extracts were then centrifuged at 14 000 g in a microfuge at 4°C, and supernatants were transferred to fresh tubes. Protein concentration was determined with the Bio-Rad protein assay (Bio-Rad, Mississauga, ON, Canada). To detect JNK1 or SEK1, 50 μg protein was added to an equal volume of 2 × sample buffer and run on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel. Proteins were transferred to nitrocellulose membranes (Bio-Rad), stained with 0.1% Ponceau S (in 5% acetic acid) to ensure equal protein loading, and blocked with 5% milk in PBS containing 0.5% Triton X-100 for 1 hour at room temperature. The membrane was then hybridized overnight at 4°C with antibody against JNK1 (Santa Cruz) (1:2000 dilution) or SEK1 (1:2000 dilution). Following 3 washes with PBS and 0.5% Triton X-100 for 15 minutes each, blots were incubated with a goat antirabbit antibody (PharMingen, Mississauga, ON, Canada) for 1 hour at room temperature. Bands were visualized by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, Baie d'Urfe, QC, Canada).
Detection of apoptosis
Apoptosis induced in SEK1+/+ and SEK1-/- cells treated as described in “Growth assays” was detected as previously described.23 Cells were washed in 4°C PBS/5% fetal bovine serum (FBS)/0.01 M NaN3, pelleted, and resuspended in 0.5 mL hypotonic fluorochrome solution containing 50 μg/mL propidium iodide (Sigma), 0.1% sodium citrate, and 0.1% Triton X-100. Cells were analyzed by flow cytometry. Cells undergoing DNA fragmentation and apoptosis were shown to be those in which propidium iodide (PI) fluorescence was weaker than the typical G0-G1 cell cycle peak.
Results
JNK1 activity is strongly induced by As2O3 in normal, but not arsenic trioxide-resistant, APL cells
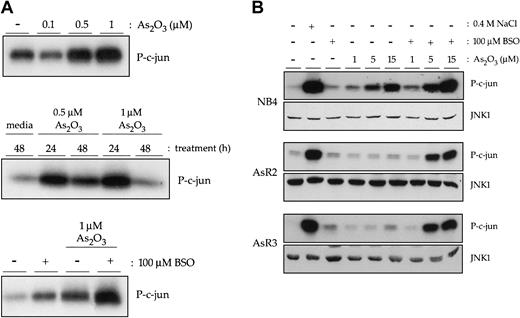
We examined the effects of arsenic trioxide on JNK1 activation in NB4 cells, as well as 2 NB4-derived arsenic-resistant subclones, AsR2 and AsR3, through the highly sensitive immune complex kinase assay, using GST-c-jun as an exogenous substrate. Figure 1A shows that, although 0.1 μM As2O3 has no effect, a 24-hour treatment with as little as 0.5 μM As2O3 induces significant JNK1 activation in NB4 cells (upper panel). A kinase assay performed with extracts from cells treated over a longer duration revealed that by 48 hours, this activation had declined, although not yet to baseline levels. Previous experiments have shown that peak As2O3-induced JNK activity in NB4 is reached by 16 hours (data not shown). These results are in keeping with earlier reports by our lab and others describing the induction of reactive oxygen species (ROS) and oxidative stress, a well-characterized inducer of JNK activity, by As2O3. Consistent with published results that GSH depletion increases the toxicity of As2O3 to a variety of cancer cells,16,17 Figure 1A (bottom panel) shows a further increase in JNK activity when cells are pretreated with BSO for 24 hours prior to As2O3 treatment.
Arsenic trioxide induces JNK1 activation in NB4 cells. (A) Immune complex kinase assays to measure JNK1 activity were performed with extracts from NB4 cells treated with 0.1 μM, 0.5 μM, or 1 μM As2O3 for 24 hours (top panel). Those doses that induced JNK activity were used in treatments carried out over longer time points (middle panel). Finally, JNK activity induced by a 16-hour treatment with 1 μM As2O3 following a 24-hour pretreatment with 100 μM BSO was measured in NB4 cells. (B) NB4, AsR2, and AsR3 cells were treated for 16 hours with a range of doses of As2O3 alone or in combination with BSO. Western blots of whole cell extracts were also performed to detect total JNK1 levels.
Arsenic trioxide induces JNK1 activation in NB4 cells. (A) Immune complex kinase assays to measure JNK1 activity were performed with extracts from NB4 cells treated with 0.1 μM, 0.5 μM, or 1 μM As2O3 for 24 hours (top panel). Those doses that induced JNK activity were used in treatments carried out over longer time points (middle panel). Finally, JNK activity induced by a 16-hour treatment with 1 μM As2O3 following a 24-hour pretreatment with 100 μM BSO was measured in NB4 cells. (B) NB4, AsR2, and AsR3 cells were treated for 16 hours with a range of doses of As2O3 alone or in combination with BSO. Western blots of whole cell extracts were also performed to detect total JNK1 levels.
If JNK activation contributes to the induction of apoptosis by arsenic, as we hypothesize, we would expect little or no activation of JNK in response to arsenic trioxide treatment of our resistant cells. Indeed, as shown in Figure 1B, immune complex kinase assays revealed only a small effect of As2O3 on JNK activity in one of 2 resistant lines, AsR3, and only at the highest concentration used. This finding is despite our observation that both AsR2 and AsR3 have normal or increased levels of JNK1 protein (Figure 1B). Furthermore, JNK signaling pathways were intact, as illustrated through their normal response to osmotic shock, induced by 0.4 M NaCl. This finding suggests that AsR2 and AsR3 resistance may be due to an event upstream of JNK signaling.
GSH depletion enhances As2O3-induced JNK1 activation in NB4 and restores its activation in arsenic trioxide-resistant cell lines
We have previously shown AsR2 and AsR3 cells to have IC50 (concentration that inhibits 50%) values for As2O3 roughly 10 times higher than their parental, NB4 cell line. This resistance could be overcome by depletion of GSH with BSO treatment, which allowed the restoration of ROS formation by As2O3 and subsequent induction of apoptosis.17 Given that GSH depletion could restore the sensitivity of AsR2 and AsR3 cells to arsenic through the increased generation of oxidative stress, we examined the effect of BSO treatment on arsenic trioxide-induced JNK activation in these resistant cells. Consistent with a role for JNK in arsenic-induced apoptosis, BSO restored activation of this signal transduction pathway in both arsenic-resistant cell lines without inducing a change in JNK protein levels (Figure 1B). The correlation between JNK activation and induction of apoptosis in AsR2 and AsR3 cells prompted us to further examine whether signaling through this pathway is required for the apoptotic response to arsenic trioxide.
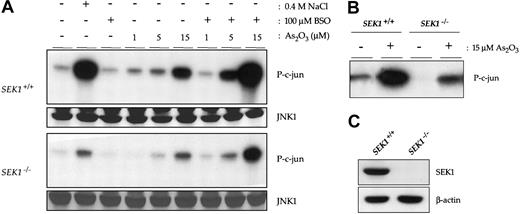
SEK1 knock-out cells show reduced JNK1 activation and are resistant to the apoptotic effects of arsenic trioxide
To investigate whether JNK activation is necessary for As2O3-induced apoptosis, we obtained cells with a genetic defect in JNK signaling. These MEF cells were derived from mice genetically engineered to lack expression of SEK1, an upstream activator of JNK whose activity is also induced by arsenic (data not shown).22 Therefore, although SEK1-/- cells continue to express normal JNK protein levels (Figure 2), the activation of this pathway in response to multiple inducers is dampened. To correlate the resistance of SEK1-/- cells with a lack of JNK activation by As2O3, we performed in vitro immune complex kinase assays, as described earlier. As with NB4 cells, SEK1+/+ cells showed a clear dose-dependent increase in JNK1 activity following a 16-hour treatment with As2O3 that was further enhanced on GSH depletion. As expected, JNK activation by As2O3 was considerably reduced in SEK1-/- cells, relative to their wild-type counterparts (Figure 2). Western blotting revealed that this result was not a consequence of decreased expression of total JNK1. No treatment induced a change in the enzyme's expression level, and SEK1+/+ and SEK-/- cells had comparable baseline levels of JNK1 protein. To exclude the possibility that differences between SEK1+/+ and SEK1-/- cells could be attributed to technical variation, the result of a control experiment in which extracts from untreated and 15 μM As2O3-treated cells of each type were run on a single gel is also shown. Of note, JNK activation by arsenic trioxide was not completely abolished in the knock-out MEFs, which express no detectable SEK1 protein (Figure 2), suggesting that SEK1-independent mechanisms play a role in the residual As2O3-induced JNK activity.
SEK1 partially mediates arsenic trioxide-induced JNK activation in MEF cells. (A) SEK1+/+ (upper panels) and SEK1-/- cells (lower panels) were treated for 16 hours with a range of doses of As2O3 alone or in combination with 100 μM BSO. Immune complex kinase assays were performed to determine the effect of arsenic trioxide on JNK1 activity. A control blot directly comparing the activation of JNK by As2O3 in SEK1+/+ and SEK1-/- cells is also shown (B). Western blots of whole cell extracts from SEK1+/+ and SEK1-/- MEFs to detect total JNK1 expression levels are shown, as is a Western blot detailing the expression level of SEK1 (C).
SEK1 partially mediates arsenic trioxide-induced JNK activation in MEF cells. (A) SEK1+/+ (upper panels) and SEK1-/- cells (lower panels) were treated for 16 hours with a range of doses of As2O3 alone or in combination with 100 μM BSO. Immune complex kinase assays were performed to determine the effect of arsenic trioxide on JNK1 activity. A control blot directly comparing the activation of JNK by As2O3 in SEK1+/+ and SEK1-/- cells is also shown (B). Western blots of whole cell extracts from SEK1+/+ and SEK1-/- MEFs to detect total JNK1 expression levels are shown, as is a Western blot detailing the expression level of SEK1 (C).
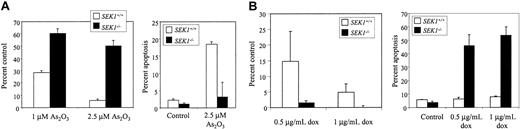
We then determined whether the reduced JNK response of SEK1-/- cells was associated with resistance to As2O3-induced apoptosis. A growth assay revealed that after a 5-day treatment with 2.5 μM As2O3, viable cell number of the SEK1-/- MEFs was more than 50% of controls, compared with only 5% of the SEK1+/+ wild-type cells (Figure 3). A PI assay confirmed the induction of apoptosis in the wild-type MEFs, with only a small increase observed in the SEK1-/- cells. Because PI staining only detects cells undergoing apoptosis at a single point in time, this assay likely underrepresents the actual percentage of cells that underwent apoptosis during the 5-day treatment, which is better reflected in the growth assay. Furthermore, because the percentage of cells undergoing necrosis, as determined by trypan blue staining, was negligible, any difference in viable cell counts between 2 groups that cannot be accounted for by the induction of apoptosis likely reflects growth inhibition.
Arsenic trioxide-induced growth inhibition is SEK1-dependent in MEF cells.SEK1+/+ and SEK1-/- cells were treated for 5 days with 1 or 2.5 μM As2O3 (A) or with 0.5 or 1.0 μg/mL doxorubicin (B). Growth inhibition after this time was assessed by counting live, trypan blue-negative cells, and was expressed as percentage of viable cells for each treatment relative to its respective untreated control. Trypan blue-positive cells never exceeded 3%. Apoptosis was detected by PI staining and quantitated by flow cytometric measurement of PI-positive cells. Each bar represents an average of 3 independent samples, and standard deviation error bars are shown.
Arsenic trioxide-induced growth inhibition is SEK1-dependent in MEF cells.SEK1+/+ and SEK1-/- cells were treated for 5 days with 1 or 2.5 μM As2O3 (A) or with 0.5 or 1.0 μg/mL doxorubicin (B). Growth inhibition after this time was assessed by counting live, trypan blue-negative cells, and was expressed as percentage of viable cells for each treatment relative to its respective untreated control. Trypan blue-positive cells never exceeded 3%. Apoptosis was detected by PI staining and quantitated by flow cytometric measurement of PI-positive cells. Each bar represents an average of 3 independent samples, and standard deviation error bars are shown.
To determine whether SEK1-/- cells are globally resistant to all apoptotic inducers, and not specifically to arsenic trioxide or some subset of apoptotic stimuli, we examined the effect of the chemotherapeutic agent, doxorubicin, on these cells. In contrast to our observations with As2O3, we found that SEK1-/- cells were more sensitive to doxorubicin than their wild-type counterparts (Figure 3). Although a 5-day treatment with 0.5 μg/mL doxorubicin induced 85% cell death in SEK1+/+ cells, the same treatment induced 99% cell death in SEK1-/- cells. Again, we found a strong correlation between decreased cell viability and increased induction of apoptosis. Furthermore, doxorubicin was found to induce significant JNK activity in a dose-dependent manner in the SEK1+/+ cells but not to any detectable level in either the SEK1 knock-out MEFs or NB4 cells (data not shown). Thus, JNK activation is clearly not a requirement for sensitivity to doxorubicin-induced apoptosis, and certainly, the resistance of SEK1-/- cells to arsenic trioxide is not merely a result of their global resistance to apoptotic stimuli.
Chemical inhibition of JNK inhibits As2O3-induced apoptosis of NB4 cells
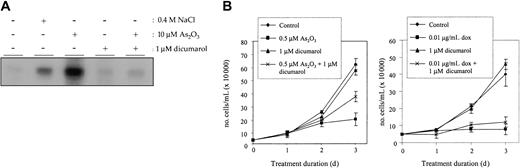
To expand our findings in SEK1-/- MEF cells to APL cells, we examined the effects of pharmacologic inhibition of JNK on arsenic trioxide signaling in NB4. The pan-JNK inhibitor, dicumarol, has previously been shown to markedly inhibit JNK activation by various agents, including anisomycin and sorbitol.24 In Figure 4A, we show that a 16-hour treatment of NB4 cells with 1 μM dicumarol completely inhibited 10 μM As2O3-induced JNK activation. Results are presented for 10 μM arsenic trioxide, as this concentration robustly induces apoptosis and activation of JNK in APL cells.
Inhibiting JNK activation abrogates arsenic trioxide-induced apoptosis in NB4 cells. (A) NB4 cells were treated with 10 μM As2O3 alone or in combination with 1 μM dicumarol for 16 hours. An immune complex kinase assay was performed to ensure inhibition of JNK1 at this dose of dicumarol. (B) NB4 cells were either left untreated ( ) or were treated for 3 days with 0.5 μM As2O3 (left panel) or 0.01 μg/mL doxorubicin (right panel) alone (▪) or in combination with 1 μM dicumarol (*), which was replenished daily. Growth inhibition was assessed by counting trypan blue-excluding cells, which always made up more than 97% of the total cells examined. Each data point shown represents an average of 3 independent replicates, and standard deviation error bars are shown.
) or were treated for 3 days with 0.5 μM As2O3 (left panel) or 0.01 μg/mL doxorubicin (right panel) alone (▪) or in combination with 1 μM dicumarol (*), which was replenished daily. Growth inhibition was assessed by counting trypan blue-excluding cells, which always made up more than 97% of the total cells examined. Each data point shown represents an average of 3 independent replicates, and standard deviation error bars are shown.
Inhibiting JNK activation abrogates arsenic trioxide-induced apoptosis in NB4 cells. (A) NB4 cells were treated with 10 μM As2O3 alone or in combination with 1 μM dicumarol for 16 hours. An immune complex kinase assay was performed to ensure inhibition of JNK1 at this dose of dicumarol. (B) NB4 cells were either left untreated ( ) or were treated for 3 days with 0.5 μM As2O3 (left panel) or 0.01 μg/mL doxorubicin (right panel) alone (▪) or in combination with 1 μM dicumarol (*), which was replenished daily. Growth inhibition was assessed by counting trypan blue-excluding cells, which always made up more than 97% of the total cells examined. Each data point shown represents an average of 3 independent replicates, and standard deviation error bars are shown.
) or were treated for 3 days with 0.5 μM As2O3 (left panel) or 0.01 μg/mL doxorubicin (right panel) alone (▪) or in combination with 1 μM dicumarol (*), which was replenished daily. Growth inhibition was assessed by counting trypan blue-excluding cells, which always made up more than 97% of the total cells examined. Each data point shown represents an average of 3 independent replicates, and standard deviation error bars are shown.
We treated NB4 cells with the combination of As2O3 and dicumarol, at 0.5 μM and 1 μM, respectively, to determine whether inhibition of JNK activity repressed arsenic trioxide-induced apoptosis. After 3 days of treatment with As2O3 alone, 20% of control cells remained viable (Figure 4B). Although dicumarol alone had little effect on cell growth, the combination of dicumarol and arsenic trioxide restored cell survival to approximately twice that observed for cells treated with As2O3 alone. This effect was statistically significant and is consistent with the reduction of arsenic trioxide-induced apoptosis seen in SEK1-/- MEF cells. This decrease in number of viable NB4 cells is not explained by the number of apoptotic cells present at the lower 0.5 μM arsenic trioxide concentration. When tested using several different assays for apoptosis, including Annexin V and PI staining as well as caspase-3 activation, we saw only a small increase in the number of apoptotic cells (5%-10%; data not shown). These data suggest that arsenic acts to inhibit cell proliferation at these lower doses and that JNK may mediate this action as well.
To determine if JNK inhibition by way of dicumarol is specific to arsenic signaling pathways, we performed a similar experiment with doxorubicin, which we showed induces apoptosis through a mechanism distinct from arsenic trioxide and independent of SEK1 expression (Figure 3). NB4 cells were treated with 0.01 μg/mL doxorubicin alone or in combination with 1 μM dicumarol for 3 days, and viable cells were again counted by trypan blue exclusion. In contrast to our finding that dicumarol decreased As2O3-induced growth inhibition, we found it to have no significant effect on doxorubicin-treated cells. This result strengthens our hypothesis that arsenic trioxide induces growth inhibition and apoptosis at least in part through JNK activation, a mechanism distinct from that induced by some other chemotherapeutic agents.
Discussion
We sought to clarify the role played by JNK activation in arsenic trioxide-induced apoptosis of APL cells. In general, there is evidence that the functional consequence of JNK activation is cell type and signal specific, having alternately been ascribed proapoptotic and antiapoptotic effects.25 Gene disruption studies have shown that embryonic fibroblasts from mice deficient in both JNK1 and JNK2 display profoundly suppressed apoptotic responses to multiple stressors, including UV radiation, anisomycin, and DNA alkylation.26 Although arsenic trioxide treatment has clearly been implicated in the activation of JNK and multiple other signaling pathways, the contribution of these pathways in mediating the effects of As2O3 has not been resolved. Therefore, we used genetic and pharmacologic approaches to clarify the role of JNK activation in the induction of apoptosis by arsenic.
Our initial experiments performed with the human APL cell line, NB4, revealed a strong activation of JNK1 by As2O3, which was further enhanced by cotreatment with BSO, a condition that strongly synergizes in the induction of apoptosis. Although JNK2 phosphorylation was also induced to some degree, the effect of arsenic trioxide on this isoform was less pronounced (data not shown). Similar results could be elicited in several cell types, including the solid tumor-derived cell line, HeLa (data not shown), revealing an overall positive correlation between JNK activation and the induction of apoptosis by arsenic trioxide. These data confirm data previously published by Huang et al,20 showing JNK activation at shorter time points with much greater doses of arsenic. We extend these to doses that can be achieved in patients and show that arsenic trioxide increases JNK activity at least 48 hours after treatment (Figure 1B). This sustained increase in JNK activity has been postulated to be an important indicator in the ability of JNK to mediate growth inhibition rather than cell survival.21
Although the highly sensitive NB4 cell line showed a dose-dependent increase in JNK1 activation, as determined through immune complex kinase assays, neither of 2 arsenic trioxide-resistant NB4 subclones showed significant activation of JNK on treatment with As2O3 alone. This result was consistent with a recent report describing the acquisition of apoptotic resistance during arsenic-induced malignant transformation, which correlated with perturbations of JNK signaling.27 Here, the researchers found that rat liver cells displaying progressively transformed phenotypes showed decreased JNK activation in response to arsenic, as compared with passage-matched control cells, even at the very high dose of 300 μM As3+ used throughout their report. In APL cells, we found a similar correlation between JNK activation and arsenic trioxide-induced apoptosis.
GSH depletion of arsenic trioxide-resistant APL cells by BSO restored JNK1 activation to a level approaching that of the wild-type parental cells, as well as sensitized these cells to As2O3-induced apoptosis. The synergy observed between arsenic trioxide and BSO in both wild-type and resistant APL cells likely results from an increase in oxidative stress generated under conditions of GSH depletion. Given that GSH serves as the major intracellular antioxidant and largest source of nonprotein thiol, one can easily envision how its depletion affects a cell's ability to escape damage induced by oxidative assaults. Arsenic alone has been well documented to cause accumulation of free radicals and ROS, and cotreatment with BSO enhances this accumulation.15,17 We have also previously found that increased GSH was one mechanism by which AsR2 and AsR3 cells resist the effects of arsenic trioxide and avoid ROS accumulation on treatment.17 Therefore, depletion of GSH from these cells may eliminate their enhanced ability to cope with oxidative stress and restore their sensitivity to As2O3. Because oxidative stress is a known inducer of JNK, the cumulative effects of arsenic trioxide and BSO on ROS accumulation likely mediate their enhanced activation of this enzyme.
Thus, we and others have found a correlation between JNK activation and arsenic-induced apoptosis. To demonstrate whether activation of JNK is necessary for As2O3-induced apoptosis, we examined the effects of arsenic trioxide on the growth of cells with a genetic defect in mitogen-activated protein kinase (MAPK) signaling, leading to greatly decreased JNK activity. SEK1-/- MEFs lack expression of SEK1, an immediate upstream activator of JNK in the stress-activated signaling pathway. The greater sensitivity of SEK1+/+ MEF cells to As2O3 supports our hypothesis that arsenic trioxide's apoptotic signal is transduced, at least in part, through JNK and implies that SEK1 expression is a requirement for this transduction. This hypothesis is consistent with the finding that apoptosis signal-regulating kinase 1 (ASK1), activated in response to various cytotoxic stresses, including ROS, is a direct upstream activator of SEK1 that is proposed to play a role in As2O3-induced apoptosis.28 JNK1 activity, although dampened, is not abrogated in SEK1-/- cells but rather can be induced somewhat by As2O3 alone and further induced by arsenic trioxide and GSH depletion. Therefore, other mechanisms for activation of this kinase by arsenic exist. Possible mechanisms are inhibition of a dual specificity JNK phosphatase, as reported by Cavigelli et al,18 or activation of SEK2, which also lies immediately upstream of JNK. This latter hypothesis is supported by the role described by Porter et al29 for the small GTP-binding proteins, Rac and Rho, in the activation of JNK by arsenic. These proteins lie upstream of and activate a number of effector kinases implicated in JNK signaling by arsenic, including MEKK2 (MEK [MAP/ERK kinase] kinase 2), MEKK3, and MEKK4,29 which in turn activate SEK1 and SEK2.21 Furthermore, SEK1 and SEK2 have recently been shown to synergistically phosphorylate JNK and to specifically target the tyrosine and threonine residues, respectively, of the Thr-Pro-Tyr motif in its activation domain.30
Results of the genetic knock-out MEF cells were confirmed by pharmacologic inhibition of JNK in APL cells. Although the chemical inhibitor, dicumarol, used in this study may have effects outside of JNK, its abrogation of As2O3-induced growth arrest and apoptosis correlated with inhibition of JNK activation but alone had no effect on cell growth. These results, together with those obtained in cells with genetic lesions of the JNK pathway, suggest that the effect of dicumarol is mediated through inhibition of JNK and not other intracellular enzymes. Further, our data suggest that the need for JNK activation may be relatively specific for As2O3-induced growth inhibition, whether the decrease in cell proliferation seen at lower concentrations or apoptosis. As Figure 3 shows, apoptosis induced by doxorubicin was not dependent on JNK activation, despite the fact that this common chemotherapeutic agent activates JNK in some cell systems.31,32 Indeed, SEK1-/- cells were more sensitive to doxorubicin-induced apoptosis than were SEK1+/+ cells, although JNK was activated in the SEK1+/+ but not the SEK1-/- MEFs (data not shown), in keeping with the hypothesis that JNK activation is not essential to the mechanism of action of all inducers of ROS and apoptosis.
The precise mechanism by which JNK activation induces apoptosis remains ill defined, but likely involves both transcriptional and nontranscriptional events. One mechanism by which JNK activation may transduce apoptotic signals is through the posttranslational modification of some Bcl-2 family members. Proteins known to be phosphorylated by JNK include antiapoptotic Bcl-2 and Bcl-XL and proapoptotic Bax, Bak, and Bid. Bcl-2 is phosphorylated at Ser70, Ser87, and Thr69 by JNK during normal cell cycling, and this phosphorylation is further enhanced on treatment with the microtubule-damaging agent, paclitaxel. Cells expressing nonphosphorylatable mutant Bcl-2 show resistance to apoptotic inducers, suggesting that Bcl-2 phosphorylation is an inactivating event rendering the cells more sensitive to apoptosis.33 However, results from our laboratory suggest that expression of Bcl-2 may not be an important factor in determining response to arsenic trioxide. The relatively As2O3-insensitive, estrogen receptor-negative breast cancer cell line, MDA-MB-231, was not sensitized to As2O3 when Bcl-2 expression was inhibited with an antisense oligonucleotide (data not shown). In addition to inactivating antiapoptotic proteins, JNK may also activate proapoptotic proteins. Although activated JNK alone has been shown to induce cytochrome c release and apoptosis of wild-type MEFs, Bak-/- Bax-/- MEFs were resistant to activated JNK.34 UV radiation also induced Bax activation in wild-type fibroblasts but not JNK-null MEFs. Furthermore, overexpression of Bax or Bak in JNK-null cells recapitulated the effect of activated JNK on the induction of apoptosis, suggesting that these apoptotic effectors may in part mediate the apoptotic effects of JNK.34 JNK-deficient cells resistant to UV-induced apoptosis also do not induce cleavage and subsequent activation of the proapoptotic protein, Bid, as do their wild-type counterparts.26 Not all of these proteins will likely be found necessary to mediate JNK-induced apoptosis in all cell systems. Clearly, however, there exist many potential targets for the regulation of apoptosis by JNK, and further work will be required to clearly delineate which of these mechanisms contribute to the induction of apoptosis by arsenic trioxide in APL cells.
Despite the need for further work to clarify the precise role of JNK in the induction of As2O3-induced apoptosis, our data suggest that it may provide a novel target in the development of anticancer treatment strategies. It will be of interest to determine whether these in vitro results may apply to patients treated with arsenic trioxide for relapsed APL or other potential malignant indications. Although we cannot exclude the possibility that As2O3-induced apoptosis involves other important signaling pathways, especially in vivo, we demonstrate that the induction of apoptosis by arsenic trioxide is JNK dependent and that combined As2O3 and BSO treatment potentiates both JNK activation and apoptosis. Importantly, restoring JNK activation in an arsenic trioxide-resistant cell line through combined treatment with BSO also restored sensitivity to As2O3-induced apoptosis. This suggests that the manipulation of JNK activity may provide a mechanism for sensitizing other, less responsive cancer cells to the effects of arsenic. This hypothesis is supported by a recent report by Muscarella et al,35 showing that an arsenic-resistant B-lymphocyte cell line could be made sensitive to As2O3 by activation of JNK through exposure to a brief, nonlethal period of hyperthermia. Our data also suggest that arsenic trioxide may still be effective for the treatment of cancers resistant to other chemotherapeutic drugs, such as doxorubicin, which we show induces apoptosis through a mechanism distinct from that induced by arsenic.
Prepublished online as Blood First Edition Paper, December 30, 2003; DOI 10.1182/blood-2003-05-1412.
Supported by the Canadian Institutes for Health Research (CIHR) and the Samuel Waxman Foundation. Wilson H. Miller Jr is an Investigator of the CIHR.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Myrian Colombo and Nancy St-Pierre for their excellent technical help and April Colosimo for her excellent technical help and critical reading of this manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal