Abstract
The deregulation of the immune response is a critical component in inflammatory disease. Recent in vitro data show that T-cell protein tyrosine phosphatase (TC-PTP) is a negative regulator of cytokine signaling. Furthermore, tc-ptp-/- mice display immune defects and die within 5 weeks of birth. We report here that tc-ptp-/- mice develop progressive systemic inflammatory disease as shown by chronic myocarditis, gastritis, nephritis, and sialadenitis as well as elevated serum interferon-γ. The widespread mononuclear cellular infiltrates correlate with exaggerated interferon-γ, tumor necrosis factor-α, interleukin-12, and nitric oxide production in vivo. Macrophages grown from tc-ptp-/- mice are inherently hypersensitive to lipopolysaccharide, which can also be detected in vivo as an increased susceptibility to endotoxic shock. These results identify T-cell protein tyrosine phosphatase as a key modulator of inflammatory signals and macrophage function. (Blood. 2004;103:3457-3464)
Introduction
Protein phosphorylation, the most common posttranslational modification in mammalian cells, is used as a rapid means of modulating enzyme function. The control of cellular phosphorylation levels depends on the reciprocal activity of kinases and phosphatases, and defects in either component can have widespread effects on cell function. T-cell protein tyrosine phosphatase (TC-PTP) is a nuclear phosphatase that is strongly expressed in the hematopoietic system. We have previously shown that it is essential for coordinate immune function and development.1 More recently, it has been identified as a negative regulator of signal transduction associated with cytokine signaling through Janus kinases 1 (JAK-1) and -32 as well as several members of the signal transducers and activators of transcription (STAT) family.3-5
The tc-ptp-/- mice show normal genotypic frequency at birth and appear to be physically normal until 10 to 14 days of age, after which their growth is retarded.1,6 Although the total number of peripheral T and B cells remains relatively unchanged, the tc-ptp-/- mice are severely immunosuppressed. They are unable to mount T-cell-dependent B-cell responses,1 and their splenocyte response to mitogens is totally suppressed. Moreover, thymic double-positive T cells decline sharply with age, and stromal defects in the bone marrow result in depletion of B-cell precursors by 3 weeks of age.1 The tc-ptp-/- mice progressively develop symptoms of wasting disease,7-9 including runting, hunched posture, diarrhea, and weight loss1 and die at 3 to 5 weeks of age.
Severe immune suppression accompanied by wasting disease is a hallmark of graft-versus-host disease (GVHD), which is characterized by a loss of T-cell proliferative responses and the systemic priming of macrophages.10-13 Unstimulated macrophages from normal animals respond to an interferon-γ (IFN-γ) plus lipopolysaccharide (LPS) activation by producing tumor necrosis factor-α (TNF-α)14,15 and nitric oxide (NO).16,17 Both are mediators of tissue damage7-9,18,19 that play an important role in septic shock. Excess NO has also been shown to cause immune suppression.20-22 The immune suppression and apparent onset of inflammatory symptoms together with the reported increase in number of splenic macrophages1 in tc-ptp-/- mice and the in vitro data for a role in cytokine signaling2,3 led us to hypothesize that in vivo deletion of TC-PTP may result in overproduction of cytokines and development of inflammatory disease, which would account, at least in part, for the early mortality seen in tc-ptp-/- mice.
In this report we show that the expression of IFN-γ, TNF-α, and inducible nitric oxide synthase (iNOS) is up-regulated in vivo in tc-ptp-/- mice. A significant increase can be detected as early as 3 days after birth, prior to the onset of any overt disease. The overproduction of cytokines contributes to the priming of inflammatory cells in vivo, as shown by an increase in sensitivity to LPS both in vitro and in vivo. Furthermore, tc-ptp-/- macrophages are inherently hypersensitive to LPS. We also demonstrate the presence of a widespread mononuclear infiltrate in several nonlymphoid organs. Taken together, our observations indicate that TC-PTP is a key negative regulator of cytokine signaling and inflammatory disease.
Materials and methods
Mice
The tc-ptp-/- mice were obtained from heterozygous matings and genotyped as previously described.1 The tc-ptp+/+ and tc-ptp+/- littermates were used as controls. All mice were kept in specific pathogen-free housing in the animal care facility. Protocols were approved by the McGill animal care ethics committee.
Reagents, antibodies, and media
Cell culture reagents were purchased from Invitrogen (Burlington, ON, Canada) unless otherwise specified. LPS was obtained from Calbiochem (San Diego, CA) and prepared as previously described.10 Recombinant murine IFN-γ (mIFN-γ) was purchased from Invitrogen and ConcavalinA (ConA) from Pharmacia (Baie-d'Urfé, QC, Canada). TC-PTP was detected with the monoclonal mouse antibody clone 4F4. The polyclonal rabbit anti-iNOS antibody was purchased from Upstate Biotechnology (Lake Placid, NY), and polyclonal rabbit antiserum for calnexin was kindly provided by Dr J. J. Bergeron (McGill University, Montreal, QC, Canada). Secondary antibodies used were horseradish peroxidase-conjugated anti-mouse (Jackson Immunoresearch, Bar Harbor, ME) and antirabbit immunoglobulin (Upstate Biotechnology) as appropriate. Fluorescein isothiocyanate (FITC)-conjugated anti-granulocyte differentiation (anti-Gr-1) antigen, phycoerythrin (PE)-conjugated anti-CD14, and allophycocyanin (APC)-conjugated anti-macrophage antigen-1 (anti-Mac1) antibodies for flow cytometry were purchased from BD Biosciences Pharmingen (Mississauga, ON, Canada).
Quantification of mRNA
Total RNA was extracted from tissues or cultured cells using TRIzol (Invitrogen) according to the manufacturer's specifications. The mRNA was quantified using the QuantiTect SYBR Green reverse transcriptase-polymerase chain reaction (RT-PCR) kit (Qiagen, Mississauga, ON, Canada) and the Light Cycler Real-Time PCR machine (Roche Diagnostics, Laval, QC, Canada). The samples were first incubated at 50°C for 20 minutes to synthesize first-strand cDNA, after which they were heated to 95°C for 15 minutes in order to activate the Taq polymerase. Conditions for amplification are described in “Primers.” After each cycle, total fluorescence was determined as a measure of double-stranded DNA in the sample, and the data were used as the basis for quantification. The identity of the PCR products was confirmed by melting curve analysis. Data were analyzed using the The LightCycler Data Analysis (LCDA) software (Roche Diagnostics). Glyceraldehyde phosphate dehydrogenase (GAPDH) was used as a housekeeping message, and the message of interest-to-GAPDH ratio was taken as the relative expression level. To compare between organs and between age groups, the tc-ptp+/+ expression level was always set as one, and the tc-ptp-/- level was expressed as the fold increase or decrease compared with age-matched tc-ptp+/+ mice.
Primers
The following oligonucleotides were synthesized at the Sheldon Biotechnology Centre (McGill University, Montreal, QC, Canada) and used as follows: GAPDH forward (FWD) 5′-AATGGTGAAGGTCGGTGTGAAC-3′ and GAPDH reverse (REV) 5′-TGGAAGATGGTGATGGGCTTC-3′ (15 s 95°C, 10 s 57°C, 20 s 72°C; 30 cycles); TNFα FWD 5′-CCTGTAGCCCACGTCGTAGC-3′ and TNFα REV 5′-TTGACCTCAGCGCTGAGTTG-3′ (15s95°C,8s57°C, 25 s 72°C; 35 cycles); iNOS FWD 5′-GCCGCATGAGCTTGGTGTTTG-3′ and iNOS REV 5′-TGATAACGTTTCTGGCTCTTGAG-3′ (15 s 95°C, 9 s 62°C, 25 s 72°C; 40 cycles); IFN-γ FWD 5′-TGGAGGAACTGGCAAAAGGATG-3′ and IFN-γ REV 5′-CGCTTCCTGAGGCTGGATTC-3′ (15 s 95°C, 10 s 61°C, 25 s 72°C; 30 cycles); interleukin 12 (IL-12; p40) FWD 5′-AGATGACATCACCTGGACCTCAG-3′ and IL-12 (p40) REV 5′-ACGTGAACCGTCCGGAGTAA-3′ (15 s 95°C, 8 s 62°C, 30 s 72°C; 35 cycles).
Histology
Complete autopsies were performed on all animals after they were killed. All of the organs from each animal were placed in an individual bottle of 10% buffered formalin for fixation. The bottles were labeled in code, so that the reviewing pathologist had no knowledge of the animal's genotype or age. After fixation, the tissue from each bottle was placed in a single cassette and processed. After overnight dehydration and impregnation of the tissue by paraffin, all of the tissue from each animal was embedded in a paraffin block and serially sectioned. The 5-μm-thick sections were stained with hematoxylin and eosin (H and E) and examined by light microscopy. A minimum of 4 H and E sections was reviewed from each animal; in several animals, serial sections (> 15) were cut, stained, and examined.
Measurement of serum IFN-γ
Nineteen- to 21-day-old mice were injected intraperitoneally with 150 μL sterile phosphate-buffered saline (PBS) containing 0 μg, 4 μg, or 8 μg LPS. Blood samples were collected 5 hours after injection, allowed to clot, and separated. Serum IFN-γ was measured with OptEIA mouse IFN-γ enzyme-linked immunosorbent assay (ELISA; BD Biosciences Pharmingen). In brief, 96-well plates were coated with a capturing monoclonal anti-mIFN-γ antibody overnight. The amount of bound cytokine from serum samples was measured using a second anti-mIFN-γ antibody with a different specificity and horseradish peroxidase/tetramethylbenzidine (TMB)-based detection system (BD Biosciences Pharmingen). Absorbance was measured at 450 nm on an ELISA plate reader (SLT Labinstruments, Salzburg, Austria) and compared with a recombinant mIFN-γ standard curve.
Spleen cultures
Splenocytes were harvested from 14-, 19-, and 21-day-old mice, passed through stainless steel mesh, and cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 10 U/mL penicillin, and 100 μg/mL streptomycin at 37°C with 5% CO2. For iNOS mRNA, cells were stimulated with 100 U/mL IFN-γ and 10 ng/mL LPS for 4 hours.
Splenic T cells were purified by positive selection for CD4 and CD8 using the EasySep magnetic nanoparticles (StemCell Technologies, Vancouver, BC, Canada). The PE-conjugated antibodies were obtained from BD Biosciences Pharmingen. For IFN-γ mRNA, total spleen cells or CD4/CD8-selected T cells were cultured in medium supplemented with 2.5 μg/mL ConA for 16 hours.
Spleen-derived macrophages were obtained essentially as previously described.23 In brief, spleen cells were cultured for 7 days in 12-well plates at 1.6 × 106 cells/well and in medium supplemented with 30% NCTC clone 929 (L-929)-conditioned medium and 10% FBS. The supplemented medium was refreshed on day 4. The L-929 medium was replaced with unsupplemented medium for 24 hours before stimulation with IFN-γ and/or LPS.
Nitrite assay
Measurement of NO production was performed essentially as described previously.13 In brief, spleen-derived macrophages were cultured in RPMI 1640 supplemented with 10% FBS, 10 U/mL penicillin, and 100 μg/mL streptomycin and additional activators, where indicated. To measure the concentration of nitrite in the supernatant after 48 hours, 100 μL Griess reagent was added to 100 μL supernatant, and the optical density was read at 550nm on an ELISA plate reader (SLT Labinstruments). The absorbance values were then compared with a standard curve of known concentrations of sodium nitrite.
Results
Up-regulation of inflammatory mediators in tc-ptp-/- mice
Recent reports showed that signaling components downstream of the IFN-γ receptor were hyperphosphorylated in tc-ptp-/- cells in vitro.2,3 Since IFN-γ is an important regulator of inflammatory responses, we examined the effect that the lack of TC-PTP would have on the expression of IFN-γ as well as 2 IFN-γ responsive genes, TNF-α and iNOS, in vivo.
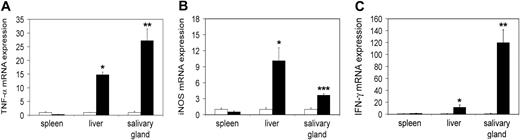
First we wished to study tc-ptp-/- mice at late stage of disease. We extracted RNA from different tissues of 20-day-old mice and analyzed it by real-time quantitative RT-PCR to determine the transcript levels in vivo. There was a significant increase in the level of TNF-α mRNA in the liver (approximately 15-fold) as well as in the salivary gland (27-fold; Figure 1A), mirrored by a respective 4- to 10-fold up-regulation of iNOS mRNA (Figure 1B). We observed similar results for IFN-γ in the liver and a particularly large increase in the salivary gland (120-fold; Figure 1C).
Increased transcription of inflammatory mRNA on day 20 in tc-ptp-/- animals. The mRNA levels for (A) TNF-α, (B) iNOS, and (C) IFN-γ in tc-ptp-/- mice (▪) and their wild-type littermates (□) at 20 days after birth. Bars represent the mean ± SE from 4 to 5 mice with tc-ptp-/- values shown as fold increase from those of age-matched tc-ptp+/+ mice. Statistical significance was determined by a 2-tailed unpaired Student t test. *P < .05; **P < .005; ***P < .001 tc-ptp-/- compared with tc-ptp+/+.
Increased transcription of inflammatory mRNA on day 20 in tc-ptp-/- animals. The mRNA levels for (A) TNF-α, (B) iNOS, and (C) IFN-γ in tc-ptp-/- mice (▪) and their wild-type littermates (□) at 20 days after birth. Bars represent the mean ± SE from 4 to 5 mice with tc-ptp-/- values shown as fold increase from those of age-matched tc-ptp+/+ mice. Statistical significance was determined by a 2-tailed unpaired Student t test. *P < .05; **P < .005; ***P < .001 tc-ptp-/- compared with tc-ptp+/+.
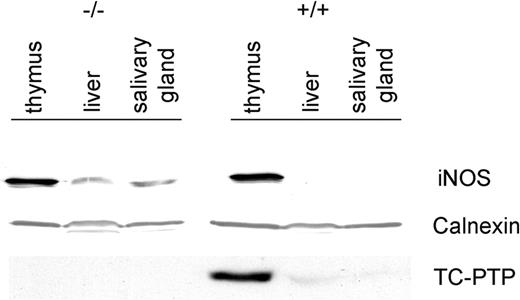
The increased expression of iNOS was not restricted to mRNA but was also reflected at the protein level (Figure 2). A 125-kDa band was clearly evident in tc-ptp-/- liver and salivary gland, while there was no detectable iNOS present in the corresponding tc-ptp+/+ tissues. The enzyme was present in the thymus of both tc-ptp+/+ and tc-ptp-/- animals.11 Membranes were stripped and reprobed for TC-PTP to confirm genotype and for calnexin, a resident protein within the endoplasmic reticulum, to ensure equal loading of protein in each lane.
Up-regulation of iNOS protein in tc-ptp-/- mice. Western blots showing TC-PTP and iNOS expression in the thymus, liver, and salivary gland of 3-week-old mice. Calnexin was used as a loading control. Data shown are representative of 3 separate experiments.
Up-regulation of iNOS protein in tc-ptp-/- mice. Western blots showing TC-PTP and iNOS expression in the thymus, liver, and salivary gland of 3-week-old mice. Calnexin was used as a loading control. Data shown are representative of 3 separate experiments.
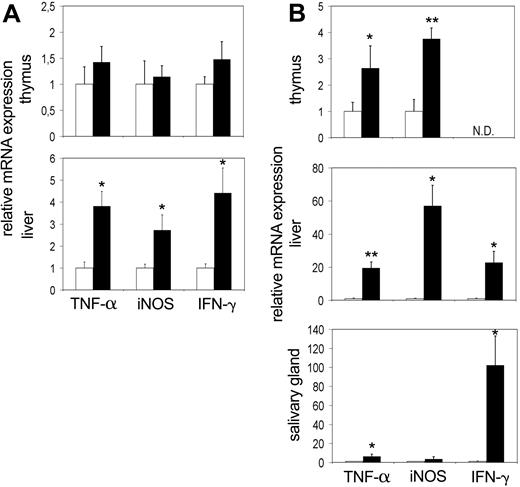
At birth, tc-ptp-/- mice appear normal and do not develop symptoms until 10 to 14 days of age.1 It is possible that the increased activation of inflammatory mediators is secondary to another defect brought on by the lack of TC-PTP and not a direct effect of its deletion. To further explore this possibility, we determined the expression levels of IFN-γ, TNF-α, and iNOS in thymus, liver, and salivary gland at the perinatal stage (3 days after birth) and at 12 to 14 days after birth, which coincides with the time that overt symptoms first appear. An up-regulation in expression of TNF-α and iNOS was already apparent in the liver at 3 days after birth (Figure 3A bottom). There was a corresponding increase in IFN-γ expression as well. We did not detect any appreciable difference within the thymus (Figure 3A top), and the expression levels in the spleen were not significantly increased (data not shown). The data from the salivary gland showed considerable variation from mouse to mouse (data not shown).
Up-regulation of TNF-α and iNOS mRNA precedes the symptoms of inflammatory disease and can be detected as early as day 3. The mRNA levels for TNF-α, iNOS, and IFN-γ in the thymus, liver, and salivary gland at (A) 3 days and (B) 2 weeks after birth. Bars represent the mean values ± SE from 4 to 5 mice, with tc-ptp-/- values (▪) shown as fold increase from those of age-matched tc-ptp+/+ mice (□). Statistical significance was determined by a 2-tailed unpaired Student t test. *P < .05; **P < .005 tc-ptp-/- compared with tc-ptp+/+. ND indicates not determined.
Up-regulation of TNF-α and iNOS mRNA precedes the symptoms of inflammatory disease and can be detected as early as day 3. The mRNA levels for TNF-α, iNOS, and IFN-γ in the thymus, liver, and salivary gland at (A) 3 days and (B) 2 weeks after birth. Bars represent the mean values ± SE from 4 to 5 mice, with tc-ptp-/- values (▪) shown as fold increase from those of age-matched tc-ptp+/+ mice (□). Statistical significance was determined by a 2-tailed unpaired Student t test. *P < .05; **P < .005 tc-ptp-/- compared with tc-ptp+/+. ND indicates not determined.
At 2 weeks of age, a significant increase in the TNF-α and iNOS transcripts was detected in the tc-ptp-/- thymus (Figure 3B top) accompanied by an even greater level of up-regulation in the liver (Figure 3B middle). Furthermore, TNF-α was up-regulated in the salivary gland of 14-day-old tc-ptp-/- pups (Figure 3B bottom). The increase in IFN-γ mRNA in the liver was already over 20-fold and the signal in the salivary gland had reached similar levels to those seen on day 20 (over 100-fold increase). The inflammatory reaction appears to be inherent, since it can be detected as early as 3 days after birth and quickly becomes amplified with time.
Increased IFN-γ production is preceded by an up-regulation of IL-12
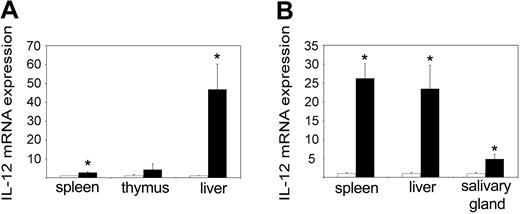
IL-12 is an IFN-γ-inducing cytokine that is normally produced by macrophages in response to microbial components.24 When we measured the levels of the inducible subunit p40 in 3-day-old pups, we could see a massive up-regulation in the tc-ptp-/- liver as well as to a lesser extent in the spleen (Figure 4A). At 2 weeks, there was a comparable increase in the tc-ptp-/- spleen, liver, and salivary gland (Figure 4B). Interestingly, the level of p40 mRNA returned to normal levels in all organs by day 21 (data not shown), suggesting that some negative feedback mechanism may be still in place despite the overall dysregulation of inflammatory cytokines.
Increased IL-12 p40 expression in tc-ptp-/- mice. The mRNA levels for IL-12 p40 subunit in (A) spleen, thymus, and liver at 3 days and (B) spleen, liver, and salivary gland at 2 weeks after birth. Bars represent the mean values ± SE from 4 mice, with tc-ptp-/- values (▪) shown as fold increase from those of age-matched tc-ptp+/+ mice (□). Statistical significance was determined by a 2-tailed unpaired Student t test. *P < .05 tc-ptp-/- compared with tc-ptp+/+.
Increased IL-12 p40 expression in tc-ptp-/- mice. The mRNA levels for IL-12 p40 subunit in (A) spleen, thymus, and liver at 3 days and (B) spleen, liver, and salivary gland at 2 weeks after birth. Bars represent the mean values ± SE from 4 mice, with tc-ptp-/- values (▪) shown as fold increase from those of age-matched tc-ptp+/+ mice (□). Statistical significance was determined by a 2-tailed unpaired Student t test. *P < .05 tc-ptp-/- compared with tc-ptp+/+.
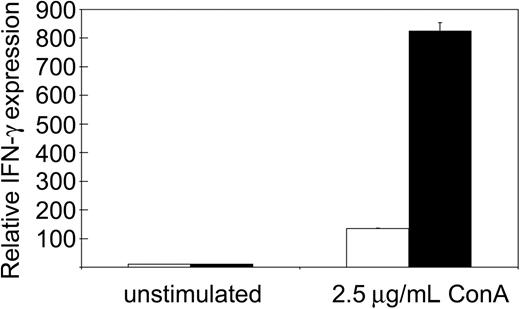
We reported previously that tc-ptp-/- splenic T cells display a severe proliferation defect in response to mitogenic stimulation.1,25 The strong IFN-γ production observed in tc-ptp-/- mice suggested that the T-cell defect may be restricted to proliferation and would not affect cytokine production. We purified T cells from the spleen using antibodies against CD4 and CD8 and stimulated them overnight with the T-cell mitogen ConA. There was no difference in IFN-γ production between unstimulated tc-ptp+/+ and tc-ptp-/- cells, but ConA-activated tc-ptp-/- T cells produced over 5 times more IFN-γ than their tc-ptp+/+ counterparts on a per cell basis (Figure 5). Similar results were obtained using whole spleen cultures. This indicates that proliferation and cytokine production are 2 independent events and that TC-PTP has opposite effects on the 2.
Increased IFN-γ production upon mitogenic stimulation by tc-ptp-/- splenic T cells. The mRNA levels for IFN-γ from splenic T cells from 19-day-old mice. The cells were either left unstimulated or stimulated overnight with 2.5 μg/mL ConA. Bars represent the mean values ± SE from triplicate cultures, with tc-ptp-/- values (▪) shown as fold increase from those of age-matched tc-ptp+/+ cells (□). Three separate experiments were performed and all gave similar results. The results shown are from one representative experiment.
Increased IFN-γ production upon mitogenic stimulation by tc-ptp-/- splenic T cells. The mRNA levels for IFN-γ from splenic T cells from 19-day-old mice. The cells were either left unstimulated or stimulated overnight with 2.5 μg/mL ConA. Bars represent the mean values ± SE from triplicate cultures, with tc-ptp-/- values (▪) shown as fold increase from those of age-matched tc-ptp+/+ cells (□). Three separate experiments were performed and all gave similar results. The results shown are from one representative experiment.
Development of systemic inflammation in tc-ptp-/- mice
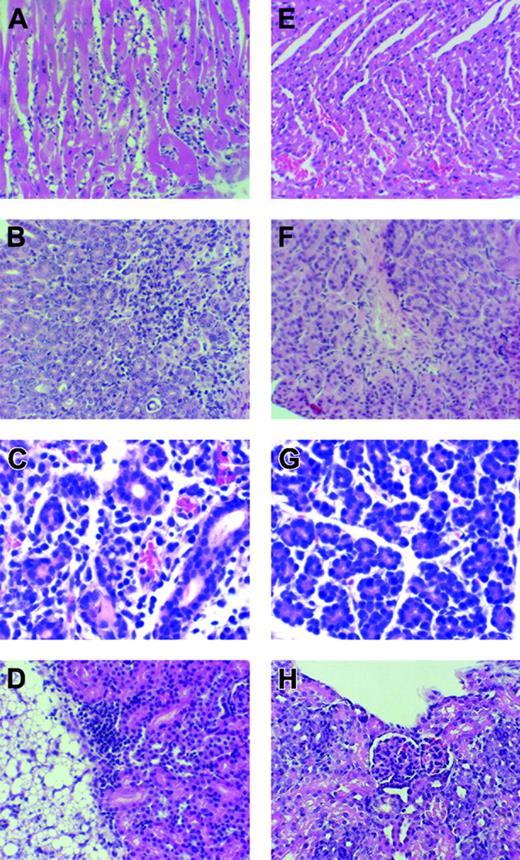
The presence of inflammatory cytokines in the salivary glands of tc-ptp-/- mice led us to examine the presence of an inflammatory infiltrate in nonlymphoid organs. A total of 9 tc-ptp-/- mice as well as 20 tc-ptp+/+ and tc-ptp+/- littermates were killed at 1, 2, or 3 weeks after birth, and tissue from each mouse was evaluated for pathology. At one week, subtle, focal infiltration of the salivary gland as well as small lymphocytic foci in the stomach could be detected in some tc-ptp-/- mice. In addition to the infiltration of the stomach and salivary gland, chronic lymphocytic myocarditis was observed in 2-week-old mice. All three 21-day-old tc-ptp-/- mice featured combined chronic myocarditis (Figure 6A), chronic gastritis (Figure 6B), and chronic sialadenitis (Figure 6C); in 2 of these mice, lymphocytic infiltrates were also present in the kidney (Figure 6D); in one mouse, chronic panniculitis was found in a random section of skin. The heart sections showed evidence of patchy myocyte degeneration and necrosis, consistent with chronic lymphocytic myocarditis, and it is not inconceivable that some tc-ptp-/- mice would die from a cardiac arrhythmia. Chronic gastritis was accompanied with glandular injury in at least 2 cases. One animal showed foci of lymphocytic infiltration of the renal cortex. Another had lymphocytic infiltrates in the perirenal adipose tissue that extended into the subjacent renal cortex. The liver sections revealed a mild degree of extramedullary hematopoiesis in the younger animals (day 6-7). Sections from one liver demonstrated patchy hepatocellular necrosis. Overall, the number and severity of inflammatory lesions increased with age, consistent with a progressive disease. The results are summarized in Table 1.
Systemic inflammation in tc-ptp-/- mice. (A) Light micrograph of myocardium of tc-ptp-/- mouse 21 days old (D21) featuring interstitial edema, prominent interstitial mononuclear cellular infiltrate, and patchy myocyte degeneration and necrosis. (B) Light micrograph of gastric mucosa of tc-ptp-/- mouse age D21 featuring dense mononuclear cellular infiltrate with patchy destruction of gastric epithelium. (C) Light micrograph of parotid gland of tc-ptp-/- mouse age D21 demonstrating mononuclear cellular infiltrate. (D) Light micrograph of kidney and adjacent adipose tissue of tc-ptp-/- mouse age D21 demonstrating mononuclear cellular infiltrate in the superficial renal cortex and adjacent perinephric adipose tissue. (E-H) Light micrographs of myocardium (E), gastric mucosa (F), parotid gland (G), and kidney (H) of tc-ptp+/- mice age D21 showing normal histology. All panels stained with H and E; original magnification × 200 for all panels.
Systemic inflammation in tc-ptp-/- mice. (A) Light micrograph of myocardium of tc-ptp-/- mouse 21 days old (D21) featuring interstitial edema, prominent interstitial mononuclear cellular infiltrate, and patchy myocyte degeneration and necrosis. (B) Light micrograph of gastric mucosa of tc-ptp-/- mouse age D21 featuring dense mononuclear cellular infiltrate with patchy destruction of gastric epithelium. (C) Light micrograph of parotid gland of tc-ptp-/- mouse age D21 demonstrating mononuclear cellular infiltrate. (D) Light micrograph of kidney and adjacent adipose tissue of tc-ptp-/- mouse age D21 demonstrating mononuclear cellular infiltrate in the superficial renal cortex and adjacent perinephric adipose tissue. (E-H) Light micrographs of myocardium (E), gastric mucosa (F), parotid gland (G), and kidney (H) of tc-ptp+/- mice age D21 showing normal histology. All panels stained with H and E; original magnification × 200 for all panels.
Age-dependent increase of inflammatory lesions in tc-ptp−/−mice
. | No. of mice with mononuclear infiltrate . | . | |
|---|---|---|---|
| Age and organ . | No infiltrate . | Mild to severe infiltrate . | |
| Days 6-7 | |||
| Salivary gland | ⅓ | ⅔ | |
| Stomach | ½ | ½ | |
| Days 13-14 | |||
| Salivary gland | ⅓ | ⅔ | |
| Stomach | ½ | ½ | |
| Heart | 0/2 | 2/2 | |
| Days 20-21 | |||
| Salivary gland | 0/3 | 3/3 | |
| Stomach | 0/3 | 3/3 | |
| Liver | ⅔ | ⅓ | |
| Heart | 0/3 | 3/3 | |
| Kidney | ⅓ | ⅔ | |
| Skin | ⅔ | ⅓ | |
. | No. of mice with mononuclear infiltrate . | . | |
|---|---|---|---|
| Age and organ . | No infiltrate . | Mild to severe infiltrate . | |
| Days 6-7 | |||
| Salivary gland | ⅓ | ⅔ | |
| Stomach | ½ | ½ | |
| Days 13-14 | |||
| Salivary gland | ⅓ | ⅔ | |
| Stomach | ½ | ½ | |
| Heart | 0/2 | 2/2 | |
| Days 20-21 | |||
| Salivary gland | 0/3 | 3/3 | |
| Stomach | 0/3 | 3/3 | |
| Liver | ⅔ | ⅓ | |
| Heart | 0/3 | 3/3 | |
| Kidney | ⅓ | ⅔ | |
| Skin | ⅔ | ⅓ | |
Summary of pathology results in tc-ptp−/− mice at 1, 2, and 3 weeks after birth. The tc-ptp+/+ and tc-ptp+/− littermate controls showed no infiltrate.
Neither tc-ptp+/+ nor tc-ptp+/- littermate controls demonstrated any histologic evidence of chronic lymphocytic myocarditis (Figure 6E) or gastritis (Figure 6F), and no lymphocytic infiltration was observed in the salivary gland (Figure 6G), renal parenchyma (Figure 6H), perirenal adipose tissue, or skin. The liver sections revealed a mild degree of extramedullary hematopoiesis in the younger animals (day 6-7), however, we detected no significant pathologic alterations. Sections of the spleen, thymus, lymph nodes, small intestine, esophagus, and lungs revealed no pathologic alterations.
Increased serum IFN-γ and LPS sensitivity in tc-ptp-/- mice
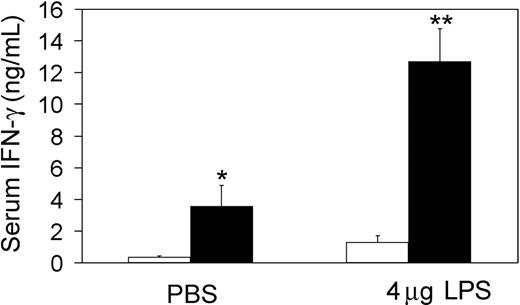
Our in vivo and in vitro results showing elevated IFN-γ production led us to examine the in vivo response to LPS and IFN-γ production in tc-ptp-/- animals. Twenty-day-old mice were injected intraperitoneally with 4 μg LPS and kept under constant surveillance until their blood was collected 5 hours after injection. Within an hour of injection, the majority of tc-ptp-/- mice were immobile. By 4 hours, they had developed symptoms of septic shock, including diarrhea, piloerection, and a hunched posture and their eyes were closed. None of the normal littermates or PBS-injected control mice showed similar signs or symptoms, even up to 6 hours after a higher dose of LPS (8 μg). Significant levels of serum IFN-γ were detected in PBS-injected tc-ptp-/- mice (Figure 7). These levels were further increased by up to 4-fold following LPS injection and reached levels that were 10-fold higher than those detected in age-matched LPS-injected tc-ptp+/+ mice (Figure 7). Administration of LPS did not significantly increase mean serum IFN-γ levels in tc-ptp+/+ (Figure 7) or tc-ptp+/- control mice even after administration of 8 μg LPS (data not shown).
Increased concentration of serum IFN-γ in tc-ptp-/- mice. Serum IFN-γ in tc-ptp-/- (▪) and tc-ptp+/+ mice (□) at 3 weeks after birth. Mice were injected with PBS or 4 μg LPS in 150 μL PBS and bled 5 hours after injection. Results are shown as ng of IFN-γ/mL of mouse serum. Bars represent the mean values ± SE from 4 to 8 mice. Statistical significance was determined by a 2-tailed unpaired Student t test. *P < .05 tc-ptp-/- +LPS compared with tc-ptp-/- +PBS. **P < .01 tc-ptp-/- +LPS compared with tc-ptp+/+ +LPS.
Increased concentration of serum IFN-γ in tc-ptp-/- mice. Serum IFN-γ in tc-ptp-/- (▪) and tc-ptp+/+ mice (□) at 3 weeks after birth. Mice were injected with PBS or 4 μg LPS in 150 μL PBS and bled 5 hours after injection. Results are shown as ng of IFN-γ/mL of mouse serum. Bars represent the mean values ± SE from 4 to 8 mice. Statistical significance was determined by a 2-tailed unpaired Student t test. *P < .05 tc-ptp-/- +LPS compared with tc-ptp-/- +PBS. **P < .01 tc-ptp-/- +LPS compared with tc-ptp+/+ +LPS.
LPS sensitivity of tc-ptp-/- macrophages in vitro
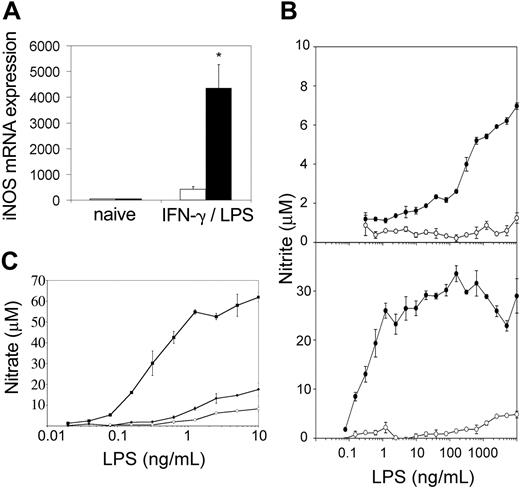
Evidence of excess IFN-γ in vivo led us to examine the activation status of tc-ptp-/- splenic macrophages. Cells from the macrophage/monocyte lineage are the only cell type that respond to IFN-γ and LPS by producing NO.26 Furthermore, IFN-γ alone is able to induce some NO production in normal macrophages, but the cells do not respond to low concentrations of LPS unless previously primed by IFN-γ.16 Both tc-ptp+/+ and tc-ptp-/- splenocytes responded to a combination of IFN-γ and LPS by expressing iNOS mRNA (Figure 8A), although in tc-ptp-/- cells up-regulation was close to 10-fold greater, suggesting that they have a stronger maximum response, which could be due to prior exposure to and priming by IFN-γ.
Splenic macrophages from tc-ptp-/- mice display an inflammatory phenotype as shown by increased iNOS and NO in response to LPS. (A) The iNOS mRNA levels in spleen cells from 2-week-old tc-ptp-/- (▪) mice and tc-ptp+/+ (□) after a stimulation with 100 U/mL IFN-γ and 10 ng/mL LPS for 4 hours. Expression level in naive cells was set as one. Bars represent the mean values ± SE from 5 mice. Statistical significance was determined by a 2-tailed unpaired Student t test. *P < .05 tc-ptp-/- compared with tc-ptp+/+. (B) NO production as measured by nitrite concentrations in the supernatants from spleen cells from 14-day-old (top) and 27-day-old (bottom) tc-ptp-/- (•) and tc-ptp+/+ (○) mice cultured with different concentrations of LPS. Five separate experiments were performed and all gave similar results. The results shown are from one representative experiment. (C) NO production as measured by nitrite concentrations in the supernatants from spleen-derived macrophages grown from 19-day-old tc-ptp-/- (▪), tc-ptp+/- (•), and tc-ptp+/+ (○) mice and stimulated with different concentrations of LPS. Three separate experiments were performed and all gave similar results. The results shown are from one representative experiment. The error bars represent the SE from triplicate cultures.
Splenic macrophages from tc-ptp-/- mice display an inflammatory phenotype as shown by increased iNOS and NO in response to LPS. (A) The iNOS mRNA levels in spleen cells from 2-week-old tc-ptp-/- (▪) mice and tc-ptp+/+ (□) after a stimulation with 100 U/mL IFN-γ and 10 ng/mL LPS for 4 hours. Expression level in naive cells was set as one. Bars represent the mean values ± SE from 5 mice. Statistical significance was determined by a 2-tailed unpaired Student t test. *P < .05 tc-ptp-/- compared with tc-ptp+/+. (B) NO production as measured by nitrite concentrations in the supernatants from spleen cells from 14-day-old (top) and 27-day-old (bottom) tc-ptp-/- (•) and tc-ptp+/+ (○) mice cultured with different concentrations of LPS. Five separate experiments were performed and all gave similar results. The results shown are from one representative experiment. (C) NO production as measured by nitrite concentrations in the supernatants from spleen-derived macrophages grown from 19-day-old tc-ptp-/- (▪), tc-ptp+/- (•), and tc-ptp+/+ (○) mice and stimulated with different concentrations of LPS. Three separate experiments were performed and all gave similar results. The results shown are from one representative experiment. The error bars represent the SE from triplicate cultures.
When the cells were stimulated with various concentrations of LPS without exogenous IFN-γ and NO production was measured in supernatants after 48 hours, only tc-ptp-/- cells were able to respond to ng/mL concentrations of LPS (Figure 8B top). Cells from 27-day-old tc-ptp-/- animals responded to a 1000-fold lower dose of LPS than those from day 14 and reached a plateau between 1 and 10 ng/mL LPS (Figure 8B bottom). The maximum level of NO production was at least 3-fold higher than for day-14 spleen cells. This demonstrates that tc-ptp-/- splenocytes have been primed by IFN-γ in vivo, and the priming effect increased with age, increasing both the sensitivity to LPS and the strength of the maximum response. To determine whether tc-ptp-/- cells were maximally stimulated by LPS alone, they were cultured with 10 ng/mL LPS and various concentrations of IFN-γ (10-500 U/mL). The maximum NO production under these conditions equaled the one reached in the absence of IFN-γ (21.7 ± 1.1 μM for tc-ptp-/- cells). The low NO produced by tc-ptp+/+ cells (1.35 ± 0.2 μM with10 ng/mL LPS + 100 U/mL IFN-γ) reflects the fact that normal unprimed splenocytes respond poorly to inflammatory stimuli, in contrast to the tc-ptp-/- cells.
To dissociate between the priming effect and an inherent sensitivity to LPS, spleen-derived macrophages grown from 7-day cultures were stimulated with various concentrations of LPS without exogenous IFN-γ and NO production was measured. Similar to the results obtained with whole spleen, only tc-ptp-/- macrophages were able to respond to pg/mL concentrations of LPS (Figure 8C). This demonstrates that tc-ptp-/- splenic macrophages are inherently sensitive to low doses of LPS, as nearly all the cells were de novo-derived in vitro and any priming effect due to prior exposure to IFN-γ would have disappeared.11 No endogenous IFN-γ production was detected under these conditions (< 30 pg/mL for both tc-ptp-/- and tc-ptp+/+ cultures). Although the maximum NO production in response to a combined IFN-γ and LPS stimulus under these conditions was slightly higher in tc-ptp-/- than tc-ptp+/+ cells (97.0 ± 7.7 μM vs 76.5 ± 4.9 μM, respectively), it most likely reflects their increased responsiveness to IFN-γ.2 We observed no difference in the number of live cells (as determined by MTT [3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay), cell morphology (size or granularity by flow cytometry), Gr-1 (Ly-6G) and Mac1 (CD11b) staining, or level of CD14 expression (data not shown), demonstrating that there was no apparent disparity in macrophage activation or development.
Discussion
The results presented here demonstrate that within days after birth tc-ptp-/- mice develop a systemic inflammatory disease. They are born at normal mendelian ratios, indicating that the loss of TC-PTP is not lethal during embryonic development. There are no defects in embryonic organ development as shown by normal tissue architecture on day 6. However, we show progressive mononuclear infiltrates in nonlymphoid organs, accompanied by active cytokine production in the liver and salivary gland. The increase in cytokine expression together with the mononuclear infiltrates causes tissue damage and ultimately the death of the animal. Moreover, tc-ptp-/- mice show a significantly increased in vivo sensitivity to exogenous LPS and develop symptoms of endotoxic shock. These findings indicate that TC-PTP is a necessary component in the regulation of inflammatory responses and that its absence results in systemic inflammatory disease.
Susceptibility to endotoxic shock has been reported in inflammatory models,10,27-29 and it has been shown to be dependent on IFN-γ and TNF-α production. Both cytokines are up-regulated in tc-ptp-/- mice without exogenous stimulation, and IFN-γ is strongly induced in response to LPS injection. LPS is constantly produced by resident enteric bacteria, but in healthy animals the liver detoxifies the small amount of LPS that crosses the gut epithelium and enters the portal circulation.30 Injury to the liver or intestine may result in further LPS spilling over into the systemic circulation and stimulating primed macrophages to produce TNF-α and other cytokines in peripheral organs.10,12 The earliest cytokine up-regulation in tc-ptp-/- mice is seen in the liver on day 3, which is likely to correspond to the activation of Kupffer cells that can also be primed to respond to LPS. The proliferation defect reported in tc-ptp-/- cells31 could lead to an increase in gut permeability due to an inadequate turnover of intestinal epithelium. We also detected liver injury in older animals at the time that the mice spontaneously develop symptoms of septic shock, such as diarrhea, runting, and hunched posture. Endogenous LPS translocating from the gastrointestinal tract may therefore play an important role in the pathogenesis of the tc-ptp-/- phenotype, similar to what has been previously shown in GVHD.10,12,32,33
It was recently shown that the characteristic injury to epithelial tissues that occurs during the systemic inflammatory response that accompanies acute GVHD is not antigen restricted.34 Similarly, wide-spread infiltrates seen in tc-ptp-/- mice also argue against an antigen-dependent mechanism and the pathology is more likely to be dependent on inflammatory cytokines as well as activation of components of the innate immune system. Splenic macrophages from tc-ptp-/- animals respond to very low concentrations of LPS alone, while the same cells from normal animals require additional prior stimulation by IFN-γ in order to produce NO in response to LPS.16,17 The increased sensitivity to LPS in vivo suggests that the tc-ptp-/- macrophages are capable of becoming activated in response to very low levels of stimulus due to a combination of in vivo exposure to IFN-γ and inherent hypersensitivity and migrate into epithelial tissues where they actively produce inflammatory mediators.
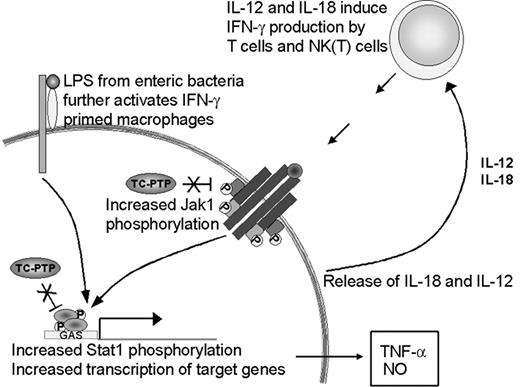
The priming effect observed in the absence of TC-PTP could be attributed to 2 independent factors. First, we have shown increased IFN-γ production in vivo, hence, increased levels of the priming stimulus. Second, recent findings implicate TC-PTP in the control of JAK and STAT dephosphorylation downstream of cytokine receptors.2-5 Bone marrow-derived macrophages from tc-ptp-/- mice have increased JAK1 phosphorylation levels after stimulation with IFN-γ in vitro.2 TC-PTP has also been identified as the phosphatase for STAT1 in fibroblast nuclei.3 Since these kinases and transcription factors require phosphorylation to be active,35,36 a lack of dephosphorylation in the tc-ptp-/- mice could result in constitutive signaling and lead to an exaggerated response, such as was seen with TNF-α and iNOS production. Both factors are likely to be important in the development of the tc-ptp-/- phenotype (Figure 9).
Up-regulation of the IFN-γ pathway in the absence of TC-PTP. The loss of TC-PTP results in increased activation of the IFN-γ pathway through hyperphosphorylation of JAK11 and STAT1.3 STAT1 activity induces TNF-α and iNOS expression. LPS increases IL-18 production, which together with IL-12 serves as a positive feedback to IFN-γ-producing cells. In the absence of TC-PTP this cycle cannot be turned off and results in the accumulation of IFN-γ, TNF-α, NO, and activated inflammatory cells.
Up-regulation of the IFN-γ pathway in the absence of TC-PTP. The loss of TC-PTP results in increased activation of the IFN-γ pathway through hyperphosphorylation of JAK11 and STAT1.3 STAT1 activity induces TNF-α and iNOS expression. LPS increases IL-18 production, which together with IL-12 serves as a positive feedback to IFN-γ-producing cells. In the absence of TC-PTP this cycle cannot be turned off and results in the accumulation of IFN-γ, TNF-α, NO, and activated inflammatory cells.
The source of IFN-γ production by the tc-ptp-/- animals in vivo is yet unclear. Macrophages respond to LPS by producing IL-12 and IL-18, 2 cytokines that synergistically activate IFN-γ production by T cells and natural killer T (NKT) cells.24,37 We show elevated IL-12 p40 expression in young tc-ptp-/- animals and their splenic T cells produce high amounts of IFN-γ in vitro in response to mitogenic stimuli despite lacking a proliferative response; however, NKT cells may be the cell population that most readily produces IFN-γ in vivo in response to LPS-induced IL-18 and IL-12.38 Cytokine-induced IFN-γ production in spleen cultures has also been attributed to NK1.1+ cells rather than T cells.39 Despite the increased IFN-γ production shown here, treatment with anti-IFN-γ does not significantly increase the life expectancy of tc-ptp-/- mice (data not shown), in contrast to what has been reported for the socs-1-/- mice.40 This may reflect the different roles played by suppressor of cytokine signaling-1 (SOCS-1) and TC-PTP; the latter deactivates an active pathway, whereas the former inhibits the activation of the JAK/STAT pathway through binding the activation loop on JAKs.41 Furthermore, while SOCS-1 is inducible42 by cytokines, TC-PTP levels are regulated in a cell cycle-dependent manner.43 We are currently breeding our tc-ptp-/- mice onto an ifng-/- background to further study the mechanisms of inflammatory activation in the absence of TC-PTP.
The data presented here agree with previous studies from our laboratory1 showing that the heterozygous tc-ptp+/- animals have no distinguishable phenotype. This is important because PTP-1B, a phosphatase closely related to TC-PTP, is now seen as a major target in the treatment of diabetes and obesity.44,45 Despite the obviously very different roles of TC-PTP and PTP-1B, to date, no small molecule inhibitors have been reported that significantly discriminate between the 2. Therefore, the absence of a tc-ptp+/- phenotype suggests that PTP-1B inhibitors could be safely used for the treatment of diabetes and obesity without inducing inflammatory side effects due to the partial loss of TC-PTP activity.
In conclusion, our results strongly indicate that TC-PTP is an important negative regulator of inflammatory reactions. The systemic inflammation seen in tc-ptp-/- mice leads us to hypothesize that the absence of the enzymatic activity or decreased expression of TC-PTP may be operative in many chronic inflammatory conditions, including GVHD. This implies that the up-regulation of TC-PTP could potentially be used to control inflammatory responses. Conversely, TC-PTP down-regulation could stimulate immune function in conditions where an augmented response may be beneficial, for example, the elimination of pathogenic organisms or in the treatment of neoplastic disease.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-09-3153.
K.M.H. received a Max Stern recruitment fellowship from the Faculty of Graduate Studies and Research as well as a Canadian Institutes of Health Research (CIHR) Cancer Consortium training award from McGill Cancer Centre. M.L.T. is a research scientist of the CIHR. This work was supported by the CIHR grant no. 3526 (W.S.L.) and the National Cancer Institute of Canada (NCIC) grant no. 010427 (M.L.T.). The purchase of the Light Cycler was funded by the Jean-Louis Lévesque Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Rosemary Siegrist-Johnstone and Ailsa Lee Loy for their excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal