Abstract
The stem cell leukemia (SCL) gene is essential for the development of hematopoietic stem cells in the embryo. Here, we used a conditional gene targeting approach to examine the function of SCL in adult hematopoietic stem cells (HSCs). Flow cytometry of bone marrow from SCL-deleted mice demonstrated a 4-fold increase in number of Linneg c-kit+ Sca-1+ cells. Despite this increase in the number of phenotypic HSCs, competitive repopulation assays demonstrated a severe multilineage defect in repopulation capacity by SCL-deleted bone marrow cells. SCL-heterozygous cells also showed a mild repopulation defect, thus suggesting haploinsufficiency of SCL. The transplantation defect of SCL-deleted cells was observed within 4 weeks of transplantation, indicating a defect in a multipotent progenitor or short-term repopulating HSCs. Although the defect persisted in secondary transplants, it remained relatively stable, suggesting that SCL was not required for self-renewal of the HSCs. Generation of SCL-deleted cells within SCL-wild-type mice rescued the early repopulating defect. Together, our results suggest that SCL is required for the normal function of short-term repopulating HSCs. (Blood. 2004;103:3342-3348)
Introduction
Adult hematopoiesis is a hierarchical system arising from a rare population (< 0.01% in bone marrow) of hematopoietic stem cells (HSCs). Properties of HSCs include self-renewal, extensive proliferative capacity, and the ability to generate all lineages of the hematopoietic system. HSCs can be divided into long-term HSCs (LT-HSCs), short-term HSCs (ST-HSCs), and multipotent progenitors (MPPs). LT-HSCs, which are relatively quiescent, provide multilineage engraftment over several months.1 LT-HSCs can self-renew for the lifetime of the animal and in the mouse this property can be tested by serial transplantation.2 LT-HSCs give rise to ST-HSCs and MPPs, which have limited self-renewal capacity but can rapidly provide multilineage repopulation of lethally irradiated recipients.3-6 Early engraftment by multilineage progenitors is an important but poorly understood process that requires homing to the spleen and bone marrow, rapid proliferation, and multilineage differentiation within the first few weeks after transplantation.
Expression of the cell surface antigens c-kit and Sca-1 in the absence of lineage markers can be used to enrich for HSCs.7 In vitro assays including the high proliferative potential assay and long-term culture initiating cell assay are based upon the requirement of primitive progenitors for multiple cytokines or stromal cell support.8,9 While these assays have been used as surrogate in vitro assays of HSCs, the competitive repopulation assay remains the gold standard.10,11 The competitive repopulation assay is a sensitive in vivo assay that can detect subtle differences in repopulating ability. Repopulation as early as 3 weeks after transplantation reflects the activity of multipotent progenitors.12,13 Although the competitive repopulation assay quantitates function of stem cell populations rather than individual HSCs, it has been validated against limiting dilution analysis as a method of comparing the functional capacity of HSCs from different donor populations.12,13
The ability to generate hematopoietic cells of all lineages requires finely controlled homeostatic mechanisms balancing self-renewal, proliferation, lineage commitment, differentiation, and cell death.14 The critical signals and transcription factors that regulate HSC fate are still not completely understood. Gene deletion studies have identified several transcription factors including stem cell leukemia (SCL), acute myeloid leukemia 1 (AML1), and GATA-2 that are critical for the development of primitive and definitive hematopoiesis in the embryo.15-19 Embryonic lethality has precluded the use of gene-targeted mice to study the role of these genes in the function of adult HSCs. To circumvent this, we and others generated a conditional SCL knockout mouse model to directly examine the role of SCL in adult hematopoiesis.20,21 Our initial studies demonstrated that SCL was critical for the growth of erythroid burst-forming units (BFU-Es) and megakaryocyte colony-forming cells (Mk-CFCs) but was dispensable for oligolineage granulocyte/macrophage progenitor cells.20,21 Others confirmed these results and also demonstrated that SCL was dispensable for HSC activity, raising the possibility that once HSCs are formed in development, SCL is no longer required for HSC function.21 In this study, we have used SCL conditional knockout mice to examine HSC function using competitive repopulation assays. We demonstrate that SCL is required for the normal function of multipotent ST-HSCs.
Materials and methods
Mice
Mice with a loxP-targeted SCL allele (SCLloxP) were generated as previously described20 and backcrossed onto a C57Bl/6 (Ptprcb [Ly5.2], Hbbs) inbred genetic background for 8 generations. Mice with the lacZ reporter gene knocked into the SCL locus were used as a source of an SCL null allele (SCL-).22 The interferon-inducible MxCre transgenic mice have been previously reported.23 All mice were bred at the Walter and Eliza Hall Institute of Medical Research Animal House Facility. Mice were fed acidified water and sterilized lab chow. The Melbourne Health Research Directorate Animal Ethics Committee approved all procedures.
Deletion of SCL
Deletion of the SCLloxP allele was achieved by injecting 300 μg polyinosinic-polycytidylic acid (poly(I:C); Sigma Chemical Company, St Louis, MO) dissolved in normal saline intraperitoneally on alternate days for 3 doses. Poly(I:C)-treated MxCre SCL-/loxP and MxCre SCL+/loxP mice generated SCL-deleted and SCL-heterozygous marrow, respectively. Deletion of the SCLloxP allele (SCLΔ) was determined by preparing genomic DNA from bone marrow (BM) cells, spleen, and thymus of animals. Genotypes of tissues were routinely determined by Southern blot analyses using an SCL probe that distinguished loxP, Δ, wild-type, and null SCL alleles.20 SCL genotyping of fluorescence-activated cell sorter (FACS)-isolated cells was performed by boiling for 5 minutes followed by digestion with proteinase K at 55°C for 1 hour prior to polymerase chain reaction (PCR). Primers sequences used for PCR amplification of loxP and Δ alleles were 5′-TCCCAAGCCCAAAGATTTCCCCAATG-3′, 5′-GCAAGCTGGATGGATCAACATGGACCT-3′, and 5′-ATGCTTGGATGCTTGGTTCAGAG-3′.
Transplantation assays
The competitive repopulation assay was performed as described previously.12 Bone marrow cells for transplantation were harvested after poly(I:C) by flushing femurs and tibias with phosphate-buffered saline/5% fetal bovine serum using a 21-gauge needle. Viability of single-cell suspensions was checked by trypan blue exclusion. Age- and sex-matched B6.SJL (Ptprca [Ly5.1]) or B6-Hbbd congenic mice were used as a source of competitor marrow cells. Mice used as a source of competitor cells were not exposed to poly(I:C). Age- and sex-matched B6.SJL (Ptprca [Ly5.1]) recipients were lethally irradiated with 2 doses of 5.5 Gy (550 rads) administered 3 hours apart from a 60Co gamma source at a dose rate of 0.45 Gy/minute (45 rads/min) 2 to 4 hours before transplantation. A minimum of 3 recipients was used for each donor cell population. In most experiments, the donor cell dose was 2 × 106 cells and competitor cell dose was 5 × 105 cells. Secondary recipients received at least 1 × 107 bone marrow cells pooled from 3 primary recipients. Mice that received transplants were fed antibiotic-treated water and maintained in hooded cages for the first 4 weeks.
To examine homing of SCL-deleted marrow, bone marrow was labeled with carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) as previously described.24 CFSE-labeled mononuclear cells (4 × 108) were injected into 3 lethally irradiated recipients, bone marrow and spleens were harvested after 4 hours, and single-cell suspensions were analyzed by flow cytometry. For calculation of the percentage of cells homing to the bone marrow, the number of cells in both femurs was assumed to be 15% of the total bone marrow.
Measurement of donor cell-derived hematopoiesis
Donor contribution to each lineage was determined using either 50 μL of heparinized blood obtained from the retro-orbital plexus or single-cell suspensions of bone marrow or thymus at the time that the mice were killed. After lysis of erythrocytes, donor and competitor hematopoiesis were distinguished by costaining samples with phycoerythrin (PE)-conjugated lineage markers, fluorescein isothiocyanate (FITC)-conjugated anti-CD45.2 (104), and biotinylated anti-CD45.1 (A20), the latter being visualized with a streptavidin-allophycocyanin (APC) conjugate. All purified rat monoclonal antibodies to mouse cell surface antigens were obtained from BD PharMingen (San Diego, CA). All analyses were performed on a BD LSR (Becton Dickinson, Mountain View, CA), and dead cells were excluded by propidium iodide staining. Repopulating units (RUs) of donor marrow were calculated from the measured percentage of donor-derived cells (%) using the formula RU = % C/(100 - %), where C indicates the number of competitor marrow cells/105.12 The proportions of donor-derived cells were compared by a Student t test.
Immature cell populations were identified using PE-conjugated anti-CD3e (145-2C11), anti-macrophage antigen-1α (anti-Mac-1α; M1/70), anti-B220 (RA3-6B2), antierythroid (TER119); FITC-conjugated anti-Sca-1 (E13-161.7); and APC-conjugated anti-c-kit (2B8). The appropriate conjugated rat antimouse isotypes were used as negative controls.
Hemoglobin electrophoresis was used to determine the donor erythroid contribution when competitor cells were derived from B6-Hbbd congenic mice.25 Gels were scanned to estimate donor contribution using a 300 Series computing densitometer (Molecular Dynamics, Sunnyvale, CA), and scans were analyzed by ImageQuant software (Amersham Biosciences, Piscataway, NY).
Results
Stem cell immunophenotype defects in SCL-deleted mice
Using SCL-conditional targeted mice, we have previously shown that SCL was required for normal megakaryopoiesis and erythropoiesis.20 Deletion of SCL also limited the differentiation potential of multipotent day 12 spleen colony-forming unit 12 (CFU-S12) to myeloid cells.20 To analyze the effect of deleting SCL in HSCs, we enumerated the number of HSCs in SCL-conditional targeted mice. Control (MxSCL+/loxP) and experimental (MxSCL-/loxP) mice were injected with poly(I:C) on alternate days for 3 doses to generate SCL-heterozygous (MxSCL+/Δ) and SCL-deleted (MxSCL-/Δ) bone marrow cells, respectively. Bone marrow from poly(I:C)-treated MxSCL-/loxP mice is referred to as “SCL-deleted” rather than “SCL-null,” recognizing that residual nondeleted SCL-expressing (MxSCL-/loxP) cells may be present. As previously described,20 Southern blot of hematopoietic tissues the day after the third poly(I:C) injection demonstrated approximately 90% deletion of the SCLloxP allele in bone marrow cells but less efficient deletion in spleen and thymus (Figure 1A). However, Southern blot analysis of SCL-deleted mice 1 to 3 months after poly(I:C) administration did not detect any nondeleted cells in the bone marrow or thymus (Figure 1A). Given that bone marrow cells and thymocytes are the progeny of HSCs, it was likely that at least 90% of HSCs (sensitivity of Southern blot) were SCL deleted. Deletion of the SCLloxP allele in most, if not all, HSCs was confirmed by repopulation assays described below (Figure 2), which further assessed the progeny of the HSC.
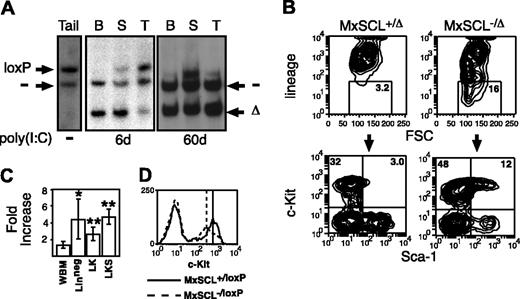
SCL-deleted marrow contains increased lineage-negative cells. (A) Southern blot of tissues from a MxSCL-/loxP mouse tail DNA prior to poly(I:C) or hematopoietic tissues 6 or 60 days after poly(I:C). B indicates bone marrow; S, spleen; and T, and thymus. The probe detects loxP-targeted (loxP), deleted loxP (Δ), and null (-) SCL alleles. (B) Representative contour plots of bone marrow cells from SCL-heterozygous (MxSCL+/loxP) and SCL-deleted (MxSCL-/loxP) mice 3 months after administration of poly(I: C). Lineage markers include Mac-1, B220, TER119, and CD3. The percentage of Linneg cells in the gate is shown. Gated cells were analyzed for the expression of c-kit and Sca-1. (C) Fold increase of unfractionated whole bone marrow (WBM), Linneg, Linneg c-kit+ Sca-1- (LK), and Linneg c-kit+Sca-1+ (LKS) cells in SCL-deleted mice (n = 9) compared with SCL-heterozygous mice (n = 10). Results are expressed as the mean and SEM. Comparison of cell numbers was performed by a 2-sided paired Student t test. *P < .05; **P < .005. (D) Representative histogram plot of c-kit expression on Linneg bone marrow cells from SCL-heterozygous and SCL-deleted mice. The peak fluorescence of Linneg c-kit+ cells is shown.
SCL-deleted marrow contains increased lineage-negative cells. (A) Southern blot of tissues from a MxSCL-/loxP mouse tail DNA prior to poly(I:C) or hematopoietic tissues 6 or 60 days after poly(I:C). B indicates bone marrow; S, spleen; and T, and thymus. The probe detects loxP-targeted (loxP), deleted loxP (Δ), and null (-) SCL alleles. (B) Representative contour plots of bone marrow cells from SCL-heterozygous (MxSCL+/loxP) and SCL-deleted (MxSCL-/loxP) mice 3 months after administration of poly(I: C). Lineage markers include Mac-1, B220, TER119, and CD3. The percentage of Linneg cells in the gate is shown. Gated cells were analyzed for the expression of c-kit and Sca-1. (C) Fold increase of unfractionated whole bone marrow (WBM), Linneg, Linneg c-kit+ Sca-1- (LK), and Linneg c-kit+Sca-1+ (LKS) cells in SCL-deleted mice (n = 9) compared with SCL-heterozygous mice (n = 10). Results are expressed as the mean and SEM. Comparison of cell numbers was performed by a 2-sided paired Student t test. *P < .05; **P < .005. (D) Representative histogram plot of c-kit expression on Linneg bone marrow cells from SCL-heterozygous and SCL-deleted mice. The peak fluorescence of Linneg c-kit+ cells is shown.
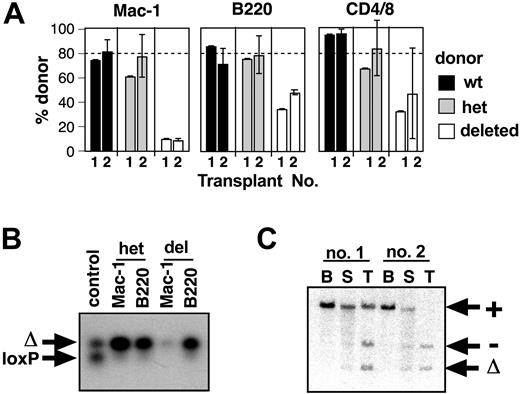
Multilineage repopulation defect of SCL-heterozygous and SCL-deleted bone marrow. (A) Percentage of donor-derived cells in a competitive repopulation assay. Donor bone marrow cells were obtained from SCL+/+ (wt, ▪), MxSCL+/loxP (het, ▦), or MxSCL-/loxP (deleted, □) mice 6 days after poly(I:C) administration. The proportion of donor-derived myeloid (Mac-1) and B (B220) cells in peripheral blood of recipient mice was assayed at 4 and 16 weeks after transplantation. Mice were killed at 16 weeks to assess donor contribution to T cells (CD4+/8+ thymocytes) and Linneg c-kit+ Sca-1+ bone marrow cells (LKS). Competitor bone marrow cells were obtained from congenic Ptprca [Ly5.1] mice. Each bar represents the mean and SD of 3 to 5 recipients. The dashed line indicates the expected donor contribution (80%). Comparison of SCL-wild-type and SCL-heterozygous donor cell contribution was performed by a one-sided Student t test. *P < .05; **P < .01. (B) Southern blot of hematopoietic tissues of a recipient 16 weeks after competitive transplantation with a 4:1 mix of SCL-deleted (MxSCL-/Δ) and SCL-wild-type competitor cells. The blot was probed with a genomic fragment that can distinguish competitor wild-type (+), loxP targeted (loxP), deleted loxP (Δ), and null (-) SCL alleles. (C) Representative Hb electrophoresis gels of peripheral blood at 16 weeks after transplantation. Competitor cells (20% of transplantation inoculum) were obtained from congenic B6-Hbdd mice. The βd (competitor origin) and βS (donor origin) are indicated. The average erythroid repopulation by donor bone marrow (determined as percentage of total Hb that was βS) for each population is underneath their respective samples accompanied by the standard deviation. Hb levels were quantified using ImageQuant software analysis. Comparison of SCL-wild-type and SCL-heterozygous donor cell contribution to erythroid cells was performed by a one-sided Student t test. **P < .01.
Multilineage repopulation defect of SCL-heterozygous and SCL-deleted bone marrow. (A) Percentage of donor-derived cells in a competitive repopulation assay. Donor bone marrow cells were obtained from SCL+/+ (wt, ▪), MxSCL+/loxP (het, ▦), or MxSCL-/loxP (deleted, □) mice 6 days after poly(I:C) administration. The proportion of donor-derived myeloid (Mac-1) and B (B220) cells in peripheral blood of recipient mice was assayed at 4 and 16 weeks after transplantation. Mice were killed at 16 weeks to assess donor contribution to T cells (CD4+/8+ thymocytes) and Linneg c-kit+ Sca-1+ bone marrow cells (LKS). Competitor bone marrow cells were obtained from congenic Ptprca [Ly5.1] mice. Each bar represents the mean and SD of 3 to 5 recipients. The dashed line indicates the expected donor contribution (80%). Comparison of SCL-wild-type and SCL-heterozygous donor cell contribution was performed by a one-sided Student t test. *P < .05; **P < .01. (B) Southern blot of hematopoietic tissues of a recipient 16 weeks after competitive transplantation with a 4:1 mix of SCL-deleted (MxSCL-/Δ) and SCL-wild-type competitor cells. The blot was probed with a genomic fragment that can distinguish competitor wild-type (+), loxP targeted (loxP), deleted loxP (Δ), and null (-) SCL alleles. (C) Representative Hb electrophoresis gels of peripheral blood at 16 weeks after transplantation. Competitor cells (20% of transplantation inoculum) were obtained from congenic B6-Hbdd mice. The βd (competitor origin) and βS (donor origin) are indicated. The average erythroid repopulation by donor bone marrow (determined as percentage of total Hb that was βS) for each population is underneath their respective samples accompanied by the standard deviation. Hb levels were quantified using ImageQuant software analysis. Comparison of SCL-wild-type and SCL-heterozygous donor cell contribution to erythroid cells was performed by a one-sided Student t test. **P < .01.
In view of the important role for SCL in megakaryopoiesis and erythropoiesis,20,21 we were surprised to observe that SCL-deleted (MxSCL-/Δ) mice were viable for at least 3 months after poly(I:C) administration without any significant expansion of SCL-positive (MxSCL-/loxP) hematopoiesis in the bone marrow. A small but significant population of nondeleted MxSCL-/loxP cells were present in the spleen (Figure 1A), perhaps providing sufficient erythropoiesis for survival. The prolonged survival of SCL-deleted mice allowed us to enumerate the numbers of HSCs in SCL-deleted bone marrow by flow cytometry (Figure 1B). Compared with control SCL-heterozygous bone marrow, SCL-deleted bone marrow contained significantly increased numbers of lineage-negative (Linneg) cells (P < .05). Furthermore, there was an expansion of the Linneg c-kit+ Sca-1+ (LKS) subpopulation, which is known to contain all HSCs (P < .005). The more mature Linneg c-kit+ Sca-1- (LK) subpopulation, which contains the lineage-restricted myeloid progenitors, was also significantly increased in SCL-deleted mice (P < .005). Overall, there was a 3- to 4-fold increase in the number of LKS and LK cells in SCL-deleted mice (Figure 1C). A proposed target gene of SCL is c-kit.26 Consistent with SCL as an enhancer of c-kit expression, we observed a small but reproducible reduction (mean, 2.3-fold) of c-kit expression in the LK population but not in the more immature LKS population (Figure 1D).
SCL is required for normal function of multilineage repopulating cells
To assess the function of SCL in HSCs, we used the competitive repopulation assay. The advantage of this assay is that the competitor cells remove any selective pressure for outgrowth of residual nondeleted SCL-/loxP cells. Donor cells were derived from poly(I:C)-treated SCL-wild-type, MxSCL+/loxP, or MxSCL-/loxP mice. Lethally irradiated mice received transplants of a 4:1 mix of donor and competitor cells. The proportion of donor-derived cells was measured by flow cytometry at 4 and 16 weeks after transplantation (Figure 2A). SCL-wild-type donor marrow cells contributed to myeloid, B-, and T-cell lineages in approximately the expected proportions (80%) at all time points measured. SCL-heterozygous cells (MxSCL+/Δ) had less repopulating activity, contributing 54% ± 9% of myeloid cells and 61% ± 7% of B cells at 4 weeks. This defect of repopulating activity was observed in 3 independent experiments and in all cases was significantly different to SCL-wild-type cells (Student t test; P < .05). Differences between SCL-wild-type and SCL-heterozygous cells at 16 weeks were less obvious due to greater variation between animals but still evident in the B-cell lineage. The defect in SCL-heterozygous function was further confirmed using bone marrow cells from SCLlacZ knock-in heterozygous mice (n = 3 experiments; data not shown). These results provided evidence of haploinsufficiency of SCL function in repopulating activity.
SCL-deleted HSCs had more severe defects in repopulation than SCL-heterozygous HSCs (Figure 2A). At 4 weeks, SCL-deleted donor cells (MxSCL-/Δ) contributed only 10% ± 4% of myeloid cells and 40% ± 9% of B cells. Reduced contribution by SCL-deleted HSCs to myeloid and B-cell lineages was also seen at 16 weeks. To confirm the defect was multilineage, mice were killed to measure the relative proportion of donor cells in the T-cell lineage (CD4+/8+ thymocytes). Consistent with a defect in a multipotent progenitor, the proportion of SCL-deleted T cells was reduced (29% ± 3% compared with wild-type controls 90% ± 5%). The relative proportion of SCL-deleted LKS cells was also reduced. We analyzed hematopoietic organs from mice that received transplants at 16 weeks to exclude outgrowth of nondeleted donor cells. As expected, we saw no evidence of nondeleted SCL-/loxP cells (Figure 2B). The deletion of the SCLloxP allele in HSCs was extremely efficient: in transplantations involving bone marrow samples from over 20 donor MxSCLloxP mice that provided transplants to 60 recipient mice, there was no evidence of hematopoietic reconstitution with SCL-nondeleted cells.
Competitive repopulation assays using the congenic Hbdd mouse strain as competitor were performed to assess the donor contribution to erythropoiesis. Mice that received SCL-wild-type transplants, heterozygous transplants, or deleted donor cells were analyzed by Hb electrophoresis at 16 weeks (Figure 2C). Similar to the reduced myeloid and B-cell lineages, there was a small but significant defect in erythropoietic activity of SCL-heterozygous donor cells (P < .05). Consistent with the inability of SCL-deleted BFU-Es to grow in vitro,20 SCL-deleted donor cells did not contribute to erythroid repopulation.
Repopulating abilities of SCL-deleted (MxSCL-/Δ) and SLC-heterozygous (MxSCL+/Δ) bone marrow for 3 independent transplantation assays were calculated as previously described.12,13 Overall, SCL-deleted bone marrow contained between 4- and 22-fold less repopulating activity than SCL-heterozygous bone marrow, depending upon which lineage was used for the calculation (Table 1).
Relative repopulating abilities of SCL-heterozygous and SCL-deleted bone marrow
Experiment (donor RUs/competitor RUs, × 105) . | SCL-heterozygous . | SCL-deleted . | Fold reduction . |
|---|---|---|---|
| 1 (20/5) | |||
| Myeloid lineage | 67 (10) | 11 (0.62) | 16.5 |
| B-cell lineage | 85 (28) | 34 (2.6) | 10.8 |
| T-cell lineage | 90 (45) | 29 (2.0) | 22.5 |
| 2 (20/5) | |||
| Myeloid lineage | 69 (11) | 22 (1.4) | 7.9 |
| B-cell lineage | 82 (22) | 53 (5.6) | 4.0 |
| T-cell lineage | ND | ND | ND |
| 3 (20/2) | |||
| Myeloid lineage | 87 (13) | 34 (1.0) | 13 |
| B-cell lineage | 86 (12) | 56 (2.5) | 4.8 |
| T-cell lineage | 87 (13) | 41 (1.4) | 9.3 |
Experiment (donor RUs/competitor RUs, × 105) . | SCL-heterozygous . | SCL-deleted . | Fold reduction . |
|---|---|---|---|
| 1 (20/5) | |||
| Myeloid lineage | 67 (10) | 11 (0.62) | 16.5 |
| B-cell lineage | 85 (28) | 34 (2.6) | 10.8 |
| T-cell lineage | 90 (45) | 29 (2.0) | 22.5 |
| 2 (20/5) | |||
| Myeloid lineage | 69 (11) | 22 (1.4) | 7.9 |
| B-cell lineage | 82 (22) | 53 (5.6) | 4.0 |
| T-cell lineage | ND | ND | ND |
| 3 (20/2) | |||
| Myeloid lineage | 87 (13) | 34 (1.0) | 13 |
| B-cell lineage | 86 (12) | 56 (2.5) | 4.8 |
| T-cell lineage | 87 (13) | 41 (1.4) | 9.3 |
Repopulating units (RUs) are defined as the repopulating ability of 1 × 105 fresh marrow cells from the SCL-wild-type competitor. The mean percentage of donor-derived cells for each lineage at 16 weeks are given, each calculated from a group of 3 to 5 recipients. The method of calculation for the RUs given in parentheses is described in “Materials and methods.” The fold reduction was calculated by dividing the RUs of SCL-heterozygous donor cells by the RUs of SCL-deleted donor cells.
ND indicates not done.
The appearance of the multilineage defect at 4 weeks favored a defect at the level of the MPP or ST-HSCs.6 Although we cannot exclude multiple defects in more mature lineage-restricted progenitors, this is unlikely because we have previously shown that day 7 granulocyte-macrophage colony-forming cells (GM-CFCs) are normal.20 An alternate explanation for the transplantation defect was a homing defect of SCL-deleted marrow cells. CFSE-labeled bone marrow cells were used to assess homing of SCL-deleted bone marrow cells.27 Four hours after injection of bone marrow cells, there was no difference in the number of SCL-wild-type or SCL-deleted cells homing to the spleen (18% ± 4% and 24% ± 3% of cells injected, respectively) or bone marrow (5.4% ± 0.4% and 7.4% ± 0.8%, respectively). Furthermore, we have previously shown that the number of SCL-deleted CFU-S12 is, at most, 2-fold reduced.20 Together with the CFSE-labeling results, this suggests that the early repopulating defect of SCL-deleted marrow cells was unlikely to be explained by a homing defect. Although these experiments cannot exclude impaired homing of a progenitor more immature than the CFU-S12, this is unlikely given the data in secondary recipients discussed below (Figure 3). In summary, in primary transplant recipients there was a defect in MPP or ST-HSC function, with reduced contribution of SCL-deleted cells to myeloid, B-cell, and T-cell lineages. This was unlikely to be due to a homing defect of SCL-deleted marrow cells.
Secondary transplantation of SCL-deleted HSCs. (A) Comparison of donor contribution to myeloid, B, and T cells in primary (1) and secondary (2) transplants. Donor cells for primary transplantation were obtained from SCL+/+ (wt, ▪), MxSCL+/loxP (het, ▦), or MxSCL-/loxP (deleted, □) mice 6 days after poly(I:C) administration. Secondary recipients received pooled bone marrow cells from primary recipients 4 months after transplantation. The mean and SD of 3 to 5 recipients are shown. The dashed line indicates the expected donor contribution (80%). (B) PCR genotype analysis of donor-derived myeloid or B cells in secondary recipients of donor bone marrow cells from poly(I:C)-treated MxSCL+/loxP (het) or MxSCL-/loxP (del) mice. FACS-isolated cells were used for genotyping. The PCR was designed to amplify only the loxP-targeted (loxP) and deleted loxP (Δ) SCL alleles. The control sample was a 50:50 mix of loxP and Δ alleles. (C) Southern blot of bone marrow (B), spleen (S), and thymus (T) from 2 secondary recipients 4 months after transplantation with marrow from primary recipients reconstituted with SCL-deleted donor cells and SCL-wild-type competitor cells. Genomic DNA was probed with a genomic fragment that can distinguish wild-type (+), loxP-targeted (loxP), deleted loxP (Δ), and null (-) SCL alleles. Note the skewing of SCL-deleted hematopoiesis, especially in recipient no. 2 where bone marrow cells are predominantly competitor (+) while the thymus is completely SCL-deleted (-/Δ).
Secondary transplantation of SCL-deleted HSCs. (A) Comparison of donor contribution to myeloid, B, and T cells in primary (1) and secondary (2) transplants. Donor cells for primary transplantation were obtained from SCL+/+ (wt, ▪), MxSCL+/loxP (het, ▦), or MxSCL-/loxP (deleted, □) mice 6 days after poly(I:C) administration. Secondary recipients received pooled bone marrow cells from primary recipients 4 months after transplantation. The mean and SD of 3 to 5 recipients are shown. The dashed line indicates the expected donor contribution (80%). (B) PCR genotype analysis of donor-derived myeloid or B cells in secondary recipients of donor bone marrow cells from poly(I:C)-treated MxSCL+/loxP (het) or MxSCL-/loxP (del) mice. FACS-isolated cells were used for genotyping. The PCR was designed to amplify only the loxP-targeted (loxP) and deleted loxP (Δ) SCL alleles. The control sample was a 50:50 mix of loxP and Δ alleles. (C) Southern blot of bone marrow (B), spleen (S), and thymus (T) from 2 secondary recipients 4 months after transplantation with marrow from primary recipients reconstituted with SCL-deleted donor cells and SCL-wild-type competitor cells. Genomic DNA was probed with a genomic fragment that can distinguish wild-type (+), loxP-targeted (loxP), deleted loxP (Δ), and null (-) SCL alleles. Note the skewing of SCL-deleted hematopoiesis, especially in recipient no. 2 where bone marrow cells are predominantly competitor (+) while the thymus is completely SCL-deleted (-/Δ).
SCL is not required for self-renewal of HSCs
To examine the function of SCL in self-renewal, we performed serial transplantation experiments. After 4 to 6 months, bone marrow was harvested from the primary transplant recipients discussed above (Figure 2) and transplanted into lethally irradiated secondary recipients. Secondary recipients were analyzed 4 months after transplantation (Figure 3A). The more mild defect of SCL-heterozygous cells observed in primary recipients was not seen in secondary recipients, probably because of the greater interindividual variation in secondary recipients. In contrast, the defect in SCL-deleted bone marrow cells was seen again in secondary recipients. The contribution of SCL-deleted cells to myeloid and lymphoid lineages was similar to that seen in the primary recipients. To confirm that the small number of donor-derived myeloid cells in secondary recipients were SCL-deleted, we performed PCR genotype analyses of FACS-isolated cells. This demonstrated that donor-derived myeloid and B cells within the bone marrow were SCL deleted rather than a product of HSCs escaping poly(I:C) deletion of the SCLloxP allele (Figure 3B). The persistence of SCL-deleted cells in secondary recipients argues against a defect of self-renewal of LT-HSCs, where a defect should produce progressive loss of donor cells with subsequent transplantations. Similarly, a homing defect of SCL-deleted HSCs would expect to result in a worsening of the defect in secondary transplantations. Equally, the observation that HSC function did not recover in secondary recipients indicates that the defect was cell intrinsic.
Although the mean proportion of SCL-deleted thymocytes in secondary recipients was similar to that observed in primary recipients, the proportions were highly variable, with some mice having more than 95% SCL-deleted T cells despite very low numbers (< 10%) of SCL-deleted myeloid cells. The defect of myeloid repopulation by SCL-deleted HSCs and the preference for SCL-deleted T cells were confirmed by Southern blot (Figure 3C).
Together, the results of serial transplantation suggested that the defect of SCL-deleted cells in long-term repopulation was persistent and cell intrinsic. The increased numbers of SCL-null T cells in secondary recipients raised the possibility that loss of SCL favored T-lymphoid differentiation of HSCs.
Rescue of the repopulating defect by SCL-wild-type hematopoiesis
Mikkola et al21 recently reported that SCL-deleted HSCs had normal function. In an attempt to reconcile this observation with our data showing a significant transplantation defect, we assayed SCL-deleted HSCs using their experimental design (Figure 4A). Bone marrow from MxSCL+/loxP or MxSCL-/loxP mice was transplanted with SCL-wild-type competitor marrow cells into primary recipients to generate mixed chimeras. Two months after transplantation, mixed chimeras were treated with poly(I:C) to delete the SCLloxP allele and bone marrow was then assayed by secondary transplantation. Consistent with the reported results,21 there was no defect at 4 weeks of SCL-deleted cells compared with SCL-heterozygous cells in myeloid and B-cell repopulation. Analysis 16 weeks after transplantation did reveal a loss of SCL-deleted myeloid cells that was not observed in the previous studies.21
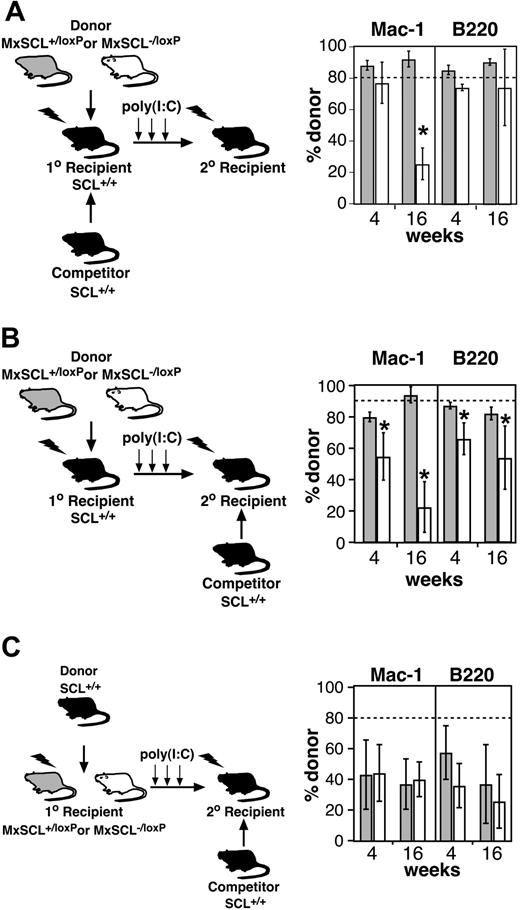
Rescue of early transplantation defect by wild-type hematopoiesis. (A) Deletion of SCL in mixed chimeras. Donor cells (MxSCL+/loxP or MxSCL-/loxP) mixed with wild-type competitor cells (SCL+/+) in a ratio of 80:20 were transplanted into lethally irradiated wild-type primary recipients. Reconstituted primary recipients were then treated with poly(I:C) to generate donor SCL-heterozygous (MxSCL+/Δ) or -deleted (MxSCL-/Δ) hematopoiesis in the presence of wild-type hematopoiesis and stroma. Bone marrow cells from these recipients were transplanted into secondary recipients 6 days after poly(I:C). The percentage of donor-derived myeloid (Mac-1) and B (B220) cells was measured in secondary recipients 4 and 16 weeks after transplantation. Each bar represents the mean and SD of 3 to 5 recipients. The dashed line indicates the expected donor contribution (80%). *P < .05. (B) Deletion of SCL in chimeras. Donor cells (MxSCL+/loxP or MxSCL-/loxP) were transplanted into lethally irradiated wild-type primary recipients. Reconstituted primary recipients were then treated with poly(I:C) to generate SCL-heterozygous (MxSCL+/Δ) or -deleted (MxSCL-/Δ) hematopoiesis in the presence of wild-type stroma. The SCL-heterozygous and SCL-deleted bone marrow cells were then mixed with SCL-wild-type cells in a ratio of 90:10 for assay by competitive repopulation. Competitor cells had also been serially transplanted so that their repopulating potential would be similar to the donor cells. The percentage of donor-derived myeloid (Mac-1) and B (B220) cells was measured in secondary recipients 4 and 16 weeks after transplantation. Each bar represents the mean and SD of 3 to 5 recipients. *P < .05. (C) Reciprocal transplantations to assess the ability stroma in SCL-deleted mice to support short-term repopulating cells. Donor cells (SCL-wild-type) were transplanted into lethally irradiated MxSCL+/loxP (n = 4) or MxSCL-/loxP (n = 4) mice. Reconstituted primary recipients were then treated with poly(I:C) to generate SCL-wild-type hematopoiesis within a SCL-heterozygous (MxSCL+/Δ) or -deleted (MxSCL-/Δ) stroma. The SCL-wild-type hematopoietic cells were then assayed by competitive repopulation in a ratio of 80:20 with SCL-wild-type competitor cells. Unlike the donor cells, the competitor cells had not been serially transplanted and thus provided greater than the expected repopulation (20%) in the secondary recipients. The percentage of donor-derived myeloid (Mac-1) and B (B220) cells was measured in secondary recipients 4 and 16 weeks after transplantation. Each bar represents the mean and SD of at least 3 recipients.
Rescue of early transplantation defect by wild-type hematopoiesis. (A) Deletion of SCL in mixed chimeras. Donor cells (MxSCL+/loxP or MxSCL-/loxP) mixed with wild-type competitor cells (SCL+/+) in a ratio of 80:20 were transplanted into lethally irradiated wild-type primary recipients. Reconstituted primary recipients were then treated with poly(I:C) to generate donor SCL-heterozygous (MxSCL+/Δ) or -deleted (MxSCL-/Δ) hematopoiesis in the presence of wild-type hematopoiesis and stroma. Bone marrow cells from these recipients were transplanted into secondary recipients 6 days after poly(I:C). The percentage of donor-derived myeloid (Mac-1) and B (B220) cells was measured in secondary recipients 4 and 16 weeks after transplantation. Each bar represents the mean and SD of 3 to 5 recipients. The dashed line indicates the expected donor contribution (80%). *P < .05. (B) Deletion of SCL in chimeras. Donor cells (MxSCL+/loxP or MxSCL-/loxP) were transplanted into lethally irradiated wild-type primary recipients. Reconstituted primary recipients were then treated with poly(I:C) to generate SCL-heterozygous (MxSCL+/Δ) or -deleted (MxSCL-/Δ) hematopoiesis in the presence of wild-type stroma. The SCL-heterozygous and SCL-deleted bone marrow cells were then mixed with SCL-wild-type cells in a ratio of 90:10 for assay by competitive repopulation. Competitor cells had also been serially transplanted so that their repopulating potential would be similar to the donor cells. The percentage of donor-derived myeloid (Mac-1) and B (B220) cells was measured in secondary recipients 4 and 16 weeks after transplantation. Each bar represents the mean and SD of 3 to 5 recipients. *P < .05. (C) Reciprocal transplantations to assess the ability stroma in SCL-deleted mice to support short-term repopulating cells. Donor cells (SCL-wild-type) were transplanted into lethally irradiated MxSCL+/loxP (n = 4) or MxSCL-/loxP (n = 4) mice. Reconstituted primary recipients were then treated with poly(I:C) to generate SCL-wild-type hematopoiesis within a SCL-heterozygous (MxSCL+/Δ) or -deleted (MxSCL-/Δ) stroma. The SCL-wild-type hematopoietic cells were then assayed by competitive repopulation in a ratio of 80:20 with SCL-wild-type competitor cells. Unlike the donor cells, the competitor cells had not been serially transplanted and thus provided greater than the expected repopulation (20%) in the secondary recipients. The percentage of donor-derived myeloid (Mac-1) and B (B220) cells was measured in secondary recipients 4 and 16 weeks after transplantation. Each bar represents the mean and SD of at least 3 recipients.
In the mixed chimeras (Figure 4A), SCL-deleted HSCs were generated in the presence of SCL-wild-type stroma and hematopoiesis (competitor cells). To further dissect the differences between our results and those reported previously, we generated chimeras by repopulating SCL-wild-type recipients with donor (MxSCL+/loxP or MxSCL-/loxP) cells in the absence of SCL-wild-type competitor cells (Figure 4B). Two months after transplantation, chimeras were treated with poly(I:C) to delete the SCLloxP allele and bone marrow was assayed by competitive repopulation assay. In this context, a multilineage repopulation defect similar to our original experiments was observed. Thus, it appeared that the early transplantation defect of SCL-deleted cells could be rescued by the presence of wild-type competitor cells rather than wild-type stroma.
Finally, to exclude a potential defect of stroma in SCL-deleted mice, we performed reciprocal transplantation experiments where SCL-wild-type hematopoietic cells were transplanted into lethally irradiated MxSCL+/loxP or MxSCL-/loxP mice (Figure 4C). Two months after transplantation, chimeras were treated with poly(I:C) to potentially delete the SCLloxP allele in the bone marrow stroma. Bone marrow cells from these poly(I:C)-treated chimeras were then assayed by competitive repopulation assay. Analysis of competitive transplantations at 4 and 16 weeks did not demonstrate any significant defect in the maintenance of MPPs or ST-HSCs by SCL-deleted mice compared with SCL-heterozygous mice. Normal function of stroma in SCL-deleted mice was not surprising because endothelial cells are the only cell type within the stroma that expresses SCL, and, in preliminary experiments, MxCre-mediated deletion in stromal cells was inefficient (data not shown). Thus, unlike the Steel mutant, the early transplantation defect of SCL-deleted bone marrow was unlikely to be explained by a defect of microenvironment.
Discussion
We used the competitive repopulation assay to demonstrate a severe multilineage repopulating defect of SCL-deleted bone marrow cells. The appearance of the defect at 4 weeks suggests that SCL is required for the function of a multilineage progenitor such as an MPP or ST-HSC, cells required for rapid multilineage engraftment.7,28 Although the mechanism of the repopulation defect remains to be elucidated, SCL is unlikely to be required for homing of HSCs because numbers of CFU-S12 are not reduced more than 2-fold,20 normal numbers of SCL-null bone marrow cells home to the spleen and bone marrow within 4 hours, and the transplantation defect does not worsen in secondary recipients (Figure 3A). The defect was not worse at 16 weeks nor in secondary recipients (Figure 3B), suggesting that SCL is not required for properties of LT-HSCs including self-renewal.
Our data raise the possibility that SCL is required for proliferation and/or differentiation of HSCs. The observation that nondeleted SCL cells do not expand in the bone marrow of SCL-deleted mice (Figure 1A) despite the repopulation defect may indicate 100% efficiency of deletion. Alternatively, it may indicate that SCL is more important for rapid proliferation and differentiation of HSCs following transplantation than for steady-state hematopoiesis. Stem cell factor (SCF) is required for growth and survival of HSCs and multipotent progenitors.26,29 The reduced expression of c-kit on SCL-deleted LK cells raises the possibility that SCL-deleted HSCs have impaired SCF signaling. Alternatively, the increased numbers of LKS and LK cells observed in SCL-deleted mice may indicate a block in differentiation of HSCs, particularly for myeloid cell differentiation. Finally, increased numbers of SCL-deleted T cells in some secondary recipients may indicate altered lineage commitment of SCL-deleted HSCs. Mikkola et al21 also noted a skewing of SCL-deleted hematopoiesis toward T cells. Relative levels of transcription factors are likely to regulate the cell fate of multipotent progenitors. For example, relative levels of Pu.1 and GATA-1 appear to regulate myeloid cell fate.30 It is possible that loss of the transcription factor, SCL, favors the formation of lymphoid progenitors by increasing the number of E2A homodimers. The opposite scenario (increased levels of SCL resulting in reduced E2A homodimers) is a proposed mechanism of inhibiting T-cell differentiation.31 Competitive limiting dilution analysis or clonal analyses of multipotent cell lines derived from SCL-deleted mice would be necessary to more clearly define the role of SCL in lineage commitment. Further detailed analysis of these conditionally targeted mice will further define the function of SCL in adult HSCs.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-09-3202.
Supported in part by grants from the National Health and Medical Research Council (Australia). A National Health and Medical Research Council CJ Martin Fellowship support D.J.C.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to acknowledge Jessica Salmon for excellent technical assistance, Ivan Bertoncello for assistance with the homing assay, Giovanni Siciliano for animal husbandry, and the Walter and Eliza Hall Institute (WEHI) Flow Cytometry Laboratory for FACS analyses.

![Figure 2. Multilineage repopulation defect of SCL-heterozygous and SCL-deleted bone marrow. (A) Percentage of donor-derived cells in a competitive repopulation assay. Donor bone marrow cells were obtained from SCL+/+ (wt, ▪), MxSCL+/loxP (het, ▦), or MxSCL-/loxP (deleted, □) mice 6 days after poly(I:C) administration. The proportion of donor-derived myeloid (Mac-1) and B (B220) cells in peripheral blood of recipient mice was assayed at 4 and 16 weeks after transplantation. Mice were killed at 16 weeks to assess donor contribution to T cells (CD4+/8+ thymocytes) and Linneg c-kit+ Sca-1+ bone marrow cells (LKS). Competitor bone marrow cells were obtained from congenic Ptprca [Ly5.1] mice. Each bar represents the mean and SD of 3 to 5 recipients. The dashed line indicates the expected donor contribution (80%). Comparison of SCL-wild-type and SCL-heterozygous donor cell contribution was performed by a one-sided Student t test. *P < .05; **P < .01. (B) Southern blot of hematopoietic tissues of a recipient 16 weeks after competitive transplantation with a 4:1 mix of SCL-deleted (MxSCL-/Δ) and SCL-wild-type competitor cells. The blot was probed with a genomic fragment that can distinguish competitor wild-type (+), loxP targeted (loxP), deleted loxP (Δ), and null (-) SCL alleles. (C) Representative Hb electrophoresis gels of peripheral blood at 16 weeks after transplantation. Competitor cells (20% of transplantation inoculum) were obtained from congenic B6-Hbdd mice. The βd (competitor origin) and βS (donor origin) are indicated. The average erythroid repopulation by donor bone marrow (determined as percentage of total Hb that was βS) for each population is underneath their respective samples accompanied by the standard deviation. Hb levels were quantified using ImageQuant software analysis. Comparison of SCL-wild-type and SCL-heterozygous donor cell contribution to erythroid cells was performed by a one-sided Student t test. **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/9/10.1182_blood-2003-09-3202/6/m_zh80090460630002.jpeg?Expires=1769802302&Signature=PjGQXJdn7Q~CTlKwSPiyvbGv4FzerLWjIXWs2sntEm9Qhn08vBb9wUD~MzHFrZSz-~6s3CXhQsM4tlzIYFxJ~nbZA8iGK5Jw0eNIXza5r7slPrHi2MA-V~OWYXgydpi8LaLBcGJZy8mBNEVevfW9QbESchwSWHbt7E2J9OByxbvGpxuwY4pFsd4W0RkOoUeRY9h-vBL02obRrJmYB065Dii81vUGp8ZggOZf80bBKbfrLNjsWeTL6fYUnBv-gbOdgoMaFOoORzMi3kG04UlaNtWh5E2ycQafs9BUEJ3-54fbEPti5T2Q7C9OJCj62qvxZtM1G~4fxeJ8AFKGBIZJfQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal