Abstract
The granulocyte colony-stimulating factor receptor (G-CSFR) transduces intracellular signals for myeloid cell proliferation, survival, and differentiation through the recruitment of nonreceptor protein tyrosine kinases Lyn and janus kinase 2 (Jak2). This results in the tyrosine phosphorylation of a small set of positive and negative adapters and effectors. Grb2-associated binder-2 (Gab2) is a newly described adapter molecule, preferentially expressed in hematopoietic cells and associated with phosphatidylinositol 3 (PI3) kinase. Studies suggest that Gab2 plays both positive and negative roles in cytokine receptor signaling. To investigate the role Gab2 plays in G-CSF receptor-mediated signaling, we have analyzed its activation state and correlated that with wild-type and mutant G-CSF receptors stably expressed in the murine factor-dependent Ba/F3 cell lines. G-CSF-induced tyrosine phosphorylation of Gab2 occurred in the wild-type and single Y-to-F mutants (Y704F, Y729F, and Y744F), but not in the ADA and W650R loss-of-function mutants. Cells expressing truncated proximal G-CSFR, the tyrosine-null (Y4F) G-CSFR, or Y764F mutant receptors had decreased phosphorylation of Gab2. Specific inhibitors of Src kinase (PD173 and PP1) but not Jak2 kinase (AG490) blocked Gab2 phosphorylation. Phosphorylation of Gab2 occurred in wild-type, but not Lyn-deficient, G-CSFR-transfected DT40 B cells. These data propose that Lyn, not Jak2, phosphorylates Gab2 and that maximal phosphorylation of Gab2 requires Y764, a Grb2-binding site. Serine phosphorylation of Akt, a marker of PI3-kinase activity, was detected in both wild-type and truncated proximal domain receptors, but not in the ADA and W650R mutants. Levels of phospho-Akt and phospho-extracellular signal-regulated kinase (phospho-ERK) were greater in proximal truncated than in wild-type G-CSFR cells, suggesting that Gab2 is dissociated from PI3 kinase or ERK activities. Overexpression of Gab2 enhanced the phosphorylation state of Akt, but not of ERK. This inhibited the proliferation of wild-type and truncated G-CSFR-transfected Ba/F3 cells and enhanced their myeloid differentiation. All together, these data indicate that G-CSF treatment leads to Lyn-mediated tyrosine phosphorylation of Gab2, which may serve as an important intermediate of enhanced Akt activity and myeloid differentiation, not growth/survival response. (Blood. 2004; 103:3305-3312)
Introduction
Granulocyte colony-stimulating factor (G-CSF) plays a crucial role in the process of hematopoiesis through its effects on proliferation, differentiation, and survival of neutrophilic progenitor cells. During myeloid maturation, neutrophilic progenitor cells undergo a series of tightly regulated biochemical and morphologic changes mediated through its cognate receptor. A member of the hematopoietin/cytokine receptor superfamily, the G-CSF receptor (G-CSFR) forms a homodimer complex with 2 G-CSF molecules.1 This sets off rapid changes in protein tyrosine phosphorylation mediated by janus kinase 2 (Jak2) and Src kinases.2-4 The cytoplasmic domain of the human G-CSFR contains 4 conserved tyrosine residues (Y704, Y729, Y744, and Y764) that can serve as potential docking sites for Src homology type 2 (SH2) or phosphotyrosine binding (PTB) domains of signaling proteins.5-9 Deletion studies have shown that the membrane-proximal cytoplasmic region of the G-CSFR, lacking all 4 tyrosines, is indispensable for transduction of growth signals, whereas the carboxy-terminal region is involved in the induction of neutrophilic maturation and function.10-12 However, the precise roles of nonreceptor protein tyrosine kinases and their substrates or binding partners in mediating proliferation and differentiation responses have not been fully elucidated.
A newly described family of signaling molecules is Grb2-associated binder (Gab).13-16 Distantly related to insulin receptor substrate (IRS) and more proximally related to daughter-of-sevenless (DOS) gene product in Drosophila, Gab contains multiple signaling modules: an amino-terminal pleckstrin homology (PH) domain, an amino-terminal and a carboxy-terminal potential 14-3-3 docking site, 2 proline-rich docking sites for SH3-containing proteins, and approximately 17 phosphotyrosine sites for SH2/PTB-containing proteins. Gab members (Gab1, Gab2, and Gab3) become tyrosine phosphorylated by a variety of stimuli, such as growth factors, cytokines, and ligands for T- and B-cell antigen receptors.16-20 The Gab family functions as scaffold proteins by interacting with multiple signaling molecules, such as SHP-2, p85 subunit of phosphatidylinositol 3 (PI3) kinase, and Grb2 via phosphotyrosine residues, proline-rich motif, and pleckstrin domain.21-24 Insights into Gab function from genetically ablated mice indicate a vital role for Gab1, mast cell defects for Gab2, and negligible effects for Gab3.25,26 Nonetheless, the role of Gab2 is confusing. A recent report suggests that Gab2 plays a critical role in Bcr-Abl-mediated transformation and estrogen-dependent breast cancer.27,28 Gab2 facilitates the complex formation of Bcr-Abl and Grb2 via phospho-Y177. This recruitment of Grb2 to tyrosine-phosphorylated Bcr-Abl serves as a major determinant for leukemogenicity, probably through the subsequent activation of the Ras-extracellular signal-regulated kinase (ERK) pathway.27 Gab2 also increases ERK activity via the tyrosine phosphatase SHP-2. Furthermore, Gab2-deficient bone marrow-derived myeloid cells are resistant to transformation by Bcr-Abl. On the other hand, overexpression of Gab2 led to growth arrest, cell spreading, and terminal differentiation in the K562 chronic myelogenous leukemia (CML)-derived cell line.29 Gab2, a substrate of ZAP-70, may serve as a switch toward inhibition of the T-cell receptor or FcϵRI signaling.18,19,30 By activating the ERK pathway via Shp2, Gab2 drives macrophage-CSF (M-CSF)-induced differentiation.31 Through β1 integrin signaling, Gab2 also promotes cell adhesion and migration.32 Therefore, Gab2 contributes to more than just blood cell proliferation.
Gab2 deficiency is associated with qualitative and quantitative defects in mast cells; some of these appear to be related to the Src kinase Lyn.25 Mast cells deficient in Lyn or Gab2 highlight the complexity of these 2 molecules. Lyn-deficient mast cells have intact degranulation and cytokine production. These responses are impaired in mast cells from Gab2-deficient mice, but FcϵR signaling activates other kinases besides Lyn. Lyn is involved in G-CSF proliferative signaling2,33,34 ; we sought to determine the Gab2 role in G-CSF receptor-mediated signaling. Therefore, we have analyzed the effects of Lyn and Jak2 on Gab2 phosphorylation state and correlated that with wild-type and mutant G-CSF receptors stably expressed in the murine factor-dependent Ba/F3. Through the use of tyrosine kinase-specific inhibitors and Src-deficient cell lines, we demonstrated that Lyn, not Jak2, principally phosphorylates Gab2. G-CSF-induced tyrosine phosphorylation of Gab2 did not correlate with proliferation. Tyrosine phosphorylation of Gab2 is also independent of PI3-kinase and ERK activation. When overexpressed, Gab2 inhibited the proliferation of wild-type and truncated G-CSFR-transfected Ba/F3 cells. Overexpression of Gab2 led to G-CSF-induced differentiation by morphologic, histochemical, and cell surface phenotype analysis. All together, these results suggest that Gab2 plays a role in Lyn-dependent pathways in differentiation, but is not directly related to growth and survival.
Materials and methods
Cell culture
Ba/F3 cells were grown in RPMI medium supplemented with 10% fetal calf serum (FCS) and 2 ng/mL murine recombinant interleukin 3 (rIL-3) (Peprotech, Rocky Hill, NJ). Source of human rG-CSF was Neupogen (Amgen, Thousand Oaks, CA). DT40 cells were grown in RPMI medium supplemented with 10% FCS and 1% chicken serum (Sigma, St Louis, MO) supplemented with β-mercaptoethanol.
Construction of expression plasmids
Different forms of the human G-CSFR cDNA with a hemagglutinin (HA) tag were constructed by polymerase chain reaction (PCR) and site-directed mutagenesis techniques and cloned into pcDNA3 vector. The original cDNA for G-CSFR-ADA was provided by Dr Belinda Avalos (Ohio State University, Columbus, OH) and for G-CSFR-W650R by Dr Ivo Touw (Erasmus University, Rotterdam, Netherlands). Automated sequencing was performed to verify fidelity. Expression plasmid containing human full-length Gab2 cDNA was constructed by releasing HindIII and XbaI fragments from pcDNA3/Gab2 and inserting this into the sites of HindIII and XbaI in pcDNA3.1/Zeo(+).
Transfection and surface expression analysis
For stable transfections, linearized plasmids were transfected by electroporation into the factor-dependent murine Ba/F3 cell line and chicken DT40 cells. Stable cell lines were established by selection with 500 μg/mL of G-418 sulfate (Geneticin) (GIBCO BRL, Gaithersburg, MD) or with 100 μg/mL Zeocin (Invitrogen, Carlsbad, CA) for 2 to 3 weeks. To determine G-CSFR expression levels, cells were then incubated at 4°C for 60 minutes with 5 μg/mL phycoerythrin (PE)-conjugated mouse anti-human G-CSFR (CD114) (PharMingen, San Diego, CA). Samples were analyzed by flow cytometry by means of a FACScan (Becton Dickinson, San Jose, CA). Cells showing similar fluorescence intensity with the CD114 antibody were selected by fluorescence-activated cell sorting (FACS) to obtain sets of transfected cells with comparable levels of receptor expression. Receptor expression was confirmed by Western blotting.
Measurement of proliferation in response to G-CSF
To measure the growth of the transfected cells in response to G-CSF, all of the transfected cells were seeded into fresh culture medium at a density of 1 × 105 cells per milliliter in the presence of 100 ng/mL recombinant human G-CSF (rhG-CSF). Cell numbers were determined daily for 3 days after exposure to rhG-CSF with the use of direct count on the basis of trypan blue exclusion. To corroborate the cell counting, monotetrazolium (MTT) assays were performed by means of the MTT Cell Proliferation Kit (American Collection Type Culture, Manassas, VA), following the manufacturer's instructions. For each cell line, the linear relationship between cell number and the signal produced was determined. Briefly, serial dilutions of cells from 1 × 106 to 1 × 103 cells per milliliter were prepared in culture medium containing 100 ng/mL G-CSF, plated in triplicate as 100 μL into wells of a 96-well microtiter plate. To these wells, 10 μL MTT reagent were added to each well and incubated for 3 hours. When precipitate became visible, 100 μL detergent reagent was added to all wells, gently swirled, and left covered in the dark for 3 hours. Absorbance was then measured, and the average values from the triplicate readings were calculated and standard curves were generated. After standard curves were established for each cell line, 100 μL 1 × 105 cells were placed into triplicate wells in the presence of 100 ng/mL G-CSF medium. Absorbance was measured at 24, 48, and 72 hours, and cells numbers were calculated.
Immunoprecipitation and Western blotting
The following antibodies were used: Lyn, Grb2, and ERK1 (Santa Cruz Biotechnology, Santa Cruz, CA); Gab2, Jak2, and 4G10 phosphotyrosine monoclonal antibody (mAb) (UBI, Lake Placid, NY); phospho-Akt and phospho-ERK1/2 (Cell Signaling, Beverly, MA); and HA (Roche, Indianapolis, IN). Cell lysis with 1% Nonidet P-40 (NP40) detergent was performed as described elsewhere.2 Proteins were immunoprecipitated with protein A/G agarose (Santa Cruz Biotechnology). For blotting, bands were visualized by enhanced chemiluminescence (Amersham, Piscataway, NJ).
PI3-kinase assay
Cells were serum starved for 4 hours and stimulated with or without 100 ng/mL G-CSF for 10 minutes at 37°C. Cell lysates were prepared in 1% NP40 lysis buffer, as described above. PI3-kinase activity was obtained by immunoprecipitation of cleared lysates with anti-p85 antibody, followed by solid-phase recovery with protein A/G agarose. Immunoprecipitated proteins were washed 3 times with phosphate-buffered saline (PBS)/1% NP40, twice with 10 mM Tris (tris(hydroxymethyl)aminomethane)/500 mM LiCl, and twice with 10 mM Tris, 100 mM NaCl, and 1 mM EDTA (ethylenediaminetetraacetic acid). Then, 200 μg/mL phosphatidylinositol dissolved in dimethyl sulfoxide (DMSO) (Avanti Polar Lipids, Birmingham, AL) was added with 20 μL kinase-reaction buffer. Reaction proceeded for 20 minutes before being stopped with the addition of 80 μL of 1 N HCl, and phosphatidylinositides were extracted by CHCl3/MeOH (1:1). The organic layer was spotted on silica gel plates, which were run in a chromatography tank. Phosphatidylinositol 4-phosphate (Sigma) was spotted as a control standard. Running solvent was CHCl3/MeOH/ddH20/NH4OH in a 60:47: 11.2:2 ratio. Plates were exposed to film, and autoradiographs were analyzed by densitometry.
Morphologic examination
Cells were collected every 24 hours and spun onto glass slides. Cell morphology was examined after May-Grünwald-Giemsa staining.
Flow cytometry analysis of Mac-1 and Gr-1 expression
The stable G-CSFR-transfected Ba/F3 cell line and G-CSFR-transfected cell line with the overexpression of Gab2 were washed twice with PBS and grown in the presence of 100 ng/mL G-CSF medium for 24, 48, and 72 hours. The cells were harvested and washed twice with PBS and blocked for 20 minutes in PBS containing 2% bovine serum albumin (BSA). Cells were then incubated with R-PE-conjugated rat antimouse CD11b (Mac-1) and rat antimouse Ly-6G (Gr-1) monoclonal antibodies (PharMingen, Franklin Lakes, NJ) or R-PE-conjugated rat immunoglobulin G2bκ (IgG2bκ) monoclonal immunoglobulin isotype control in 100 μL wash buffer (PBS plus 1% BSA) for 30 minutes in the dark. After incubation, cells were washed twice with the wash buffer and then resuspended in 0.5 mL wash buffer before analyzing with a flow cytometer. Fluorescent signal from 10 000 cells in each sample was analyzed. Isotype control antibody was used in each condition.
Results
Correlation of G-CSFR and mutant G-CSFR expression with G-CSF-dependent growth in Ba/F3 transfectants
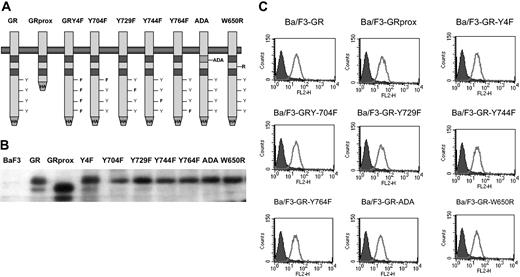
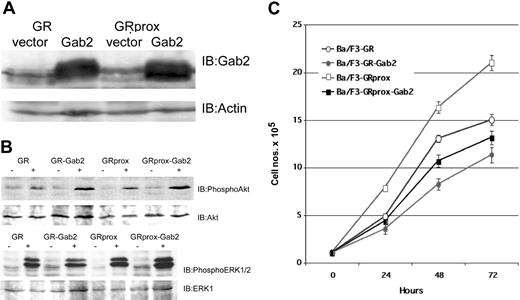
The factor-dependent Ba/F3 cell line was electroporated with cDNAs for a variety of epitope-tagged G-CSFR (Figure 1A). Following selection by G418 resistance, stable transfectants were grown. Expression of ectopic G-CSFR was confirmed by Western blotting (Figure 1B) and flow cytometry (Figure 1C). Cells with comparable levels of expressed receptors were then sorted by FACS. Western blotting and flow cytometry indicated comparable cell surface expression of the different G-CSFR forms. Stable transfectants were grown in medium containing recombinant murine IL-3, then washed several times, and resuspended in medium containing recombinant human G-CSF. Ba/F3 control cells and those expressing the ADA or W650R mutants failed to survive (Figure 2). The most robust growth occurred with the proximal truncated G-CSF receptor (GRprox) as determined by MTT assay and trypan blue exclusion cell counting, Since both flow cytometry and Western blotting show comparable levels of receptor expression and because of the principle of receptor excess, we doubt that growth or biochemical changes are due to minor variations in receptor number.
Structure and expression of wild-type and mutant G-CSFR on Gab2 and growth. GRprox indicates truncated proximal G-CSFR. (A) Schematic representation of wild-type and mutant G-CSFR proteins. The cDNAs for the various G-CSFR forms were engineered to express the hemagglutinin (HA) tag at the C terminus. (B) HA blotting of cell lysates from cell lines expressing the various G-CSFR forms. Cell lysates were prepared, electrophoresed, and transferred onto poly(vinylidene difluoride) (PVDF) filter. The filter was then blotted with anti-HA mAb. (C). Flow cytometric analysis of G-CSFR expression on the cell surface of transfectants. Receptor expression on the control, untransfected Ba/F3 cells are shown in the filled curves.
Structure and expression of wild-type and mutant G-CSFR on Gab2 and growth. GRprox indicates truncated proximal G-CSFR. (A) Schematic representation of wild-type and mutant G-CSFR proteins. The cDNAs for the various G-CSFR forms were engineered to express the hemagglutinin (HA) tag at the C terminus. (B) HA blotting of cell lysates from cell lines expressing the various G-CSFR forms. Cell lysates were prepared, electrophoresed, and transferred onto poly(vinylidene difluoride) (PVDF) filter. The filter was then blotted with anti-HA mAb. (C). Flow cytometric analysis of G-CSFR expression on the cell surface of transfectants. Receptor expression on the control, untransfected Ba/F3 cells are shown in the filled curves.
Growth characteristics of wild-type and mutant G-CSFR in factor-dependent Ba/F3 cells. Cells maintained in IL-3-containing medium were washed extensively and transferred to 100 ng/mL G-CSF-containing medium. Viable cells were determined at the indicated times by MTT cell proliferation assay, confirmed by trypan blue cell counting (data not shown). To facilitate inspection, data are presented in 2 graphs and are represented as the mean ± standard deviation.
Growth characteristics of wild-type and mutant G-CSFR in factor-dependent Ba/F3 cells. Cells maintained in IL-3-containing medium were washed extensively and transferred to 100 ng/mL G-CSF-containing medium. Viable cells were determined at the indicated times by MTT cell proliferation assay, confirmed by trypan blue cell counting (data not shown). To facilitate inspection, data are presented in 2 graphs and are represented as the mean ± standard deviation.
G-CSF-induced tyrosine phosphorylation of Gab2 and its dissociation from growth/survival
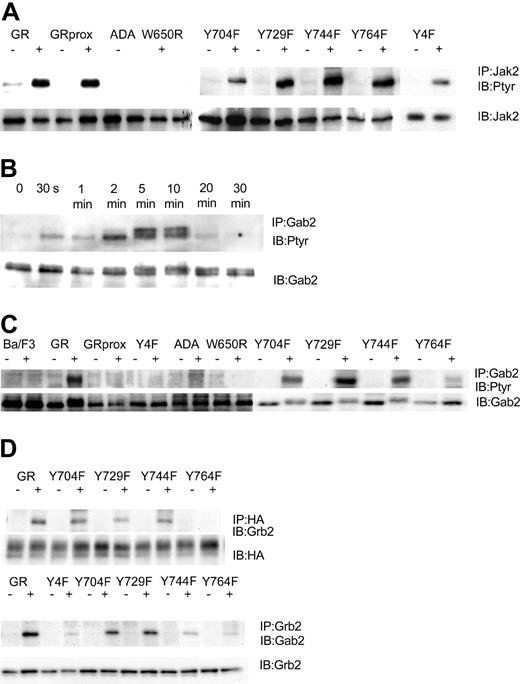
Having established cell lines that expressed comparable levels of wild-type and mutant G-CSFR forms, we next determined that they could respond to G-CSF with tyrosine phosphorylation of Jak2. We found that after stimulation with 100 ng/mL G-CSF for 10 minutes, G-CSF induced tyrosine phosphorylation of Jak2 in wild-type GR-, GRprox-, and the single or complete tyrosine-mutated G-CSFR-transfected Ba/F3 cells. No Jak2 tyrosine phosphorylation was observed in ADA and W650R transfected cells (Figure 3A), cell lines that will not respond to G-CSF with survival and proliferation. In Ba/F3 cell lines that express wild-type G-CSFR, G-CSF induced the tyrosine phosphorylation of Gab2, which peaked at 5 minutes and lasted less than 20 minutes (Figure 3B). When Ba/F3 cells expressing other forms of the G-CSFR were analyzed, tyrosine phosphorylation of Gab2 was decreased in the Y764F mutant and, interestingly, was absent in those cell lines that responded robustly (GRprox as well as Y4F) and those that did not respond (GR-ADA, and GRW650R) to G-CSF with growth/survival (Figure 3C). Thus, Gab2 tyrosine phosphorylation was partially dependent on tyrosine residue 764 but not required for growth/survival responses. Because Gab2 is basally associated with Grb2 via the Grb2 SH3, we next determined whether association of Gab2 with Grb2 is also phosphorylation dependent and localized to Y764. Our results confirmed that Grb2's association with G-CSFR required tyrosine phosphorylation of Y764 and that the G-CSF-induced increase in Gab2 association with Grb2 required that phosphorylation (Figure 3D). Therefore, Gab2 becomes tyrosine phosphorylated through the activation of protein tyrosine kinases in the cytosol, which may be optimized by its association with the G-CSFR via Grb2.
G-CSF-induced tyrosine phosphorylation patterns are G-CSFR dependent. IP indicates immunoprecipitation; IB, immunoblot; Ptyr, phosphotyrosine. (A) G-CSF-induced tyrosine phosphorylation of Jak2 after 10 minutes' stimulation. Cell lysates were subjected to immunoprecipitation by anti-Jak2 antibody, followed by transfer onto PVDF, and were then blotted with antiphosphotyrosine mAb 4G10. Membrane was stripped and reprobed for Jak2. (B) Time course for G-CSF-induced tyrosine phosphorylation of Gab2. Cell lysates were subjected to immunoprecipitation by anti-Gab2 antibody, followed by transfer onto PVDF, and were then blotted with antiphosphotyrosine mAb 4G10. Membrane was stripped and reprobed for Gab2. (C) G-CSF-induced tyrosine phosphorylation of Gab2 after 10 minutes' stimulation. (D) G-CSF-induced association of Grb2 with Y764 and association of Grb2 with Gab2.
G-CSF-induced tyrosine phosphorylation patterns are G-CSFR dependent. IP indicates immunoprecipitation; IB, immunoblot; Ptyr, phosphotyrosine. (A) G-CSF-induced tyrosine phosphorylation of Jak2 after 10 minutes' stimulation. Cell lysates were subjected to immunoprecipitation by anti-Jak2 antibody, followed by transfer onto PVDF, and were then blotted with antiphosphotyrosine mAb 4G10. Membrane was stripped and reprobed for Jak2. (B) Time course for G-CSF-induced tyrosine phosphorylation of Gab2. Cell lysates were subjected to immunoprecipitation by anti-Gab2 antibody, followed by transfer onto PVDF, and were then blotted with antiphosphotyrosine mAb 4G10. Membrane was stripped and reprobed for Gab2. (C) G-CSF-induced tyrosine phosphorylation of Gab2 after 10 minutes' stimulation. (D) G-CSF-induced association of Grb2 with Y764 and association of Grb2 with Gab2.
In contrast, Gab1, which is present but at lower levels in Ba/F3 cells as shown by Western blotting, was not tyrosine phosphorylated in response to G-CSF. These results of G-CSF-induced tyrosine phosphorylation of Gab2, but not Gab1, are similar to those for thrombopoietin-stimulated Ba/F3 cells expressing thrombopoietin receptor.35
Gab2 tyrosine phosphorylation is Lyn dependent
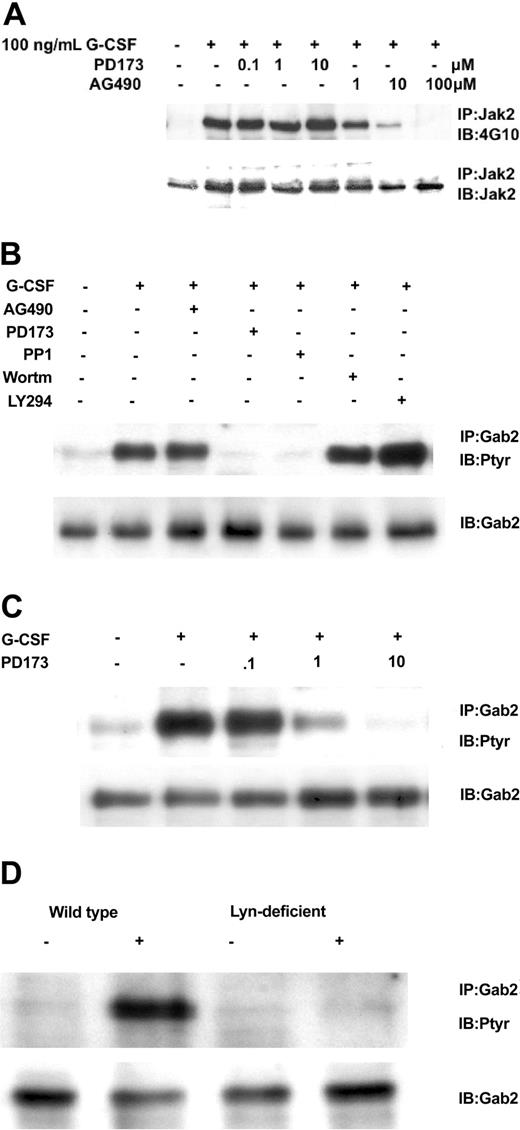
We next addressed which kinase might mediate G-CSF's phosphorylation of Gab2. Control experiments verified that the Src kinase inhibitor PD173 did not affect Jak2 tyrosine phosphorylation but the Jak2 inhibitor AG490 did (Figure 4A). At the same time, we determined the Gab2 tyrosine phosphorylation state in Ba/F3GR cells after the cells were treated first with Src inhibitors (PD173 or PP1), a Jak2 inhibitor AG490, and PI3-kinase inhibitors (Ly294002 and wortmannin) and then with G-CSF. Only the Src inhibitors resulted in the hypophosphorylation of Gab2 following G-CSF stimulation (Figure 4B). Gab2 appears hyperphosphorylated in cells treated first with Ly294002, probably owing to a PI3-kinase-dependent negative regulatory pathway first observed in heregulin signal transduction.36 A PD173 dose inhibition of Gab2 tyrosine phosphorylation shows a 50%-inhibiting concentration (IC50) of less than 1 μM (Figure 4C), similar to that for Lyn kinase (data not shown).37 To confirm a primary role for Lyn, we used the chicken DT40 B-cell line and its mutagenized Lyn-deficient strain. In the DT40 cell line, the only Src kinase expressed is Lyn, so no other Src members can substitute for it in the Lyn-deficient strain.38 We have previously shown that G-CSFR signaling pathways and responses can be reconstituted in these cell lines.2,33,34 As shown in Figure 4D, only wild-type DT40 GR cells showed tyrosine phosphorylation of Gab2 following G-CSF treatment. Even though Gab2 contains multiple tyrosine residues, providing potential phosphorylation sites for several tyrosine kinases, these data indicate that, in response to G-CSF, Lyn is the primary tyrosine kinase. In Ba/F3 cells as in human myeloid cell lines such as HL60, Mo7e, and TF1, Lyn is the predominant Src family kinase (data not shown).
Relationship of Gab2 and Lyn. Gab2 is a Lyn substrate. (A) Inhibition of Jak2 tyrosine phosphorylation by AG490, but not by the Src inhibitor PD173. Ba/F3GR cells were first incubated with varying concentrations of Jak2 inhibitor (AG490) or Src kinase inhibitor (PD173), then stimulated with 100 ng/mL G-CSF for 10 minutes. Jak2 was immunoprecipitated and then blotted sequentially with antiphosphotyrosine mAb 4G10 and anti-Jak2. (B) Ba/F3 G-CSFR cells were first incubated with Src kinase inhibitors (10 μM PD173 or 10 μM PP1), Jak2 inhibitor (100 μM AG490), or PI3-kinase inhibitor (10 μM wortmannin or 10 μM LY294003) for 60 minutes, then stimulated with 100 ng/mL G-CSF for 10 minutes. Gab2 tyrosine phosphorylation was then assayed. (C) Dose inhibition of Src inhibitor PD173 on G-CSF-induced tyrosine phosphorylation of Gab2. (D) Lyn-deficient DT40 GR cells without Gab2 tyrosine phosphorylation. DT40 GR and Lyn-deficient DT40 GR were studied for tyrosine phosphorylation of immunoprecipitated Gab2 following stimulation with 100 ng/mL G-CSF.
Relationship of Gab2 and Lyn. Gab2 is a Lyn substrate. (A) Inhibition of Jak2 tyrosine phosphorylation by AG490, but not by the Src inhibitor PD173. Ba/F3GR cells were first incubated with varying concentrations of Jak2 inhibitor (AG490) or Src kinase inhibitor (PD173), then stimulated with 100 ng/mL G-CSF for 10 minutes. Jak2 was immunoprecipitated and then blotted sequentially with antiphosphotyrosine mAb 4G10 and anti-Jak2. (B) Ba/F3 G-CSFR cells were first incubated with Src kinase inhibitors (10 μM PD173 or 10 μM PP1), Jak2 inhibitor (100 μM AG490), or PI3-kinase inhibitor (10 μM wortmannin or 10 μM LY294003) for 60 minutes, then stimulated with 100 ng/mL G-CSF for 10 minutes. Gab2 tyrosine phosphorylation was then assayed. (C) Dose inhibition of Src inhibitor PD173 on G-CSF-induced tyrosine phosphorylation of Gab2. (D) Lyn-deficient DT40 GR cells without Gab2 tyrosine phosphorylation. DT40 GR and Lyn-deficient DT40 GR were studied for tyrosine phosphorylation of immunoprecipitated Gab2 following stimulation with 100 ng/mL G-CSF.
Gab2 dissociation from PI3-kinase: Akt and ERK activities
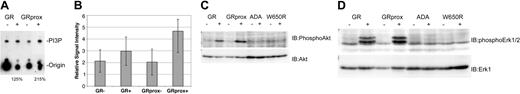
To identify the function of Gab2 in G-CSFR signaling, we examined its role in signaling by receptor mutants linked to specific physiologic effects such as survival and proliferation. First, we assayed for 2 biochemical effectors that have been linked to Gab2, PI3 kinase, and ERK activity,23,27,29,32,39-41 and correlated them with receptor mutants. G-CSF induced greater PI3-kinase activity in GRprox than wild-type GR cells (Figure 5A-B); this correlated with enhanced growth (Figure 2) but not with Gab2 tyrosine phosphorylation (Figure 3B). Phospho-Akt and phospho-ERK1/2 levels were increased in Ba/F3 cells expressing wild type or GRprox, and showed no change in ADA or W650R mutants (Figure 5C-D). All together, these data suggest that receptors that mediate G-CSF-dependent growth/survival involve PI3-kinase, Akt, and ERK, but that there is dissociation between Gab2 tyrosine phosphorylation and proliferation/survival responses.
Gab2 dissociation from PI3-kinase biochemical markers, Akt, and ERK. (A) In vitro assay for G-CSF-stimulated PI3-kinase activity in cells expressing either wild-type G-CSFR or GRprox. This picture demonstrates greater PI3-kinase activity in G-CSF-treated GRprox than GR cells (215% versus 125%) and represents 1 of 3 independent experiments. (B) Three independent experiments were performed, and the in vitro kinase activity was quantitated by densitometry by means of a Kodak (Rochester, NY) 1000 Image Station and software. The average and standard error in the relative signal intensity are shown. (C) G-CSF-stimulated Akt serine phosphorylation in wild-type, GRprox cells, and 2 loss-of-function mutants: ADA and W650R. (D) Western blot for phospho-ERK in the same cell lines. Cell lysates were first probed with anti-phospho-ERK1/2 antibody (Cell Signaling); then the blot was stripped and probed with anti-ERK1 antibody (Santa Cruz Biotechnology) to demonstrate comparable levels of ERK protein.
Gab2 dissociation from PI3-kinase biochemical markers, Akt, and ERK. (A) In vitro assay for G-CSF-stimulated PI3-kinase activity in cells expressing either wild-type G-CSFR or GRprox. This picture demonstrates greater PI3-kinase activity in G-CSF-treated GRprox than GR cells (215% versus 125%) and represents 1 of 3 independent experiments. (B) Three independent experiments were performed, and the in vitro kinase activity was quantitated by densitometry by means of a Kodak (Rochester, NY) 1000 Image Station and software. The average and standard error in the relative signal intensity are shown. (C) G-CSF-stimulated Akt serine phosphorylation in wild-type, GRprox cells, and 2 loss-of-function mutants: ADA and W650R. (D) Western blot for phospho-ERK in the same cell lines. Cell lysates were first probed with anti-phospho-ERK1/2 antibody (Cell Signaling); then the blot was stripped and probed with anti-ERK1 antibody (Santa Cruz Biotechnology) to demonstrate comparable levels of ERK protein.
Gab2 overexpression inhibits proliferation, but enhances differentiation
To address the hypothesis that Gab2 activation does not drive proliferation/survival, we studied the effects of overexpression of Gab2 in wild-type and truncated G-CSFR-transfected Ba/F3 cells (Figure 6A) and evaluated their biochemical and functional phenotypes. G-CSF treatment in Gab2-overexpressing cells resulted in either comparable or enhanced phospho-Akt or phospho-ERK levels (Figure 6B). Overexpression of Gab2 caused decreased growth (doubling times) when cells were incubated in either IL-3 (control medium) or G-CSF (Figure 6C). Cell cycle analysis also showed that overexpression of Gab2 is associated with a decrease in the number of S-phase cells (data not shown). These data suggest that neither Akt nor ERK can overcome negative signaling pathways caused by overexpression of Gab2. Alternatively, Gab2 may be redirecting Akt or ERK toward nonproliferative pathways.
Effect of Gab2 overexpression. Overexpression of Gab2 inhibits G-CSF-induced growth of Ba/F3 cells, but is not associated with inhibition of Akt or ERK. (A) Western blot of ectopically expressed Gab2 in either GR- or GRprox-expressing cells. (B) Western blots show that overexpression of Gab2 enhanced serine phosphorylation of Akt (upper panel), but not of ERK 1/2 (lower panel). (C) Growth characteristics of wild-type and truncated G-CSFR-transfected Ba/F3 cells with overexpression of Gab2 as determined by MTT cell proliferation assay.
Effect of Gab2 overexpression. Overexpression of Gab2 inhibits G-CSF-induced growth of Ba/F3 cells, but is not associated with inhibition of Akt or ERK. (A) Western blot of ectopically expressed Gab2 in either GR- or GRprox-expressing cells. (B) Western blots show that overexpression of Gab2 enhanced serine phosphorylation of Akt (upper panel), but not of ERK 1/2 (lower panel). (C) Growth characteristics of wild-type and truncated G-CSFR-transfected Ba/F3 cells with overexpression of Gab2 as determined by MTT cell proliferation assay.
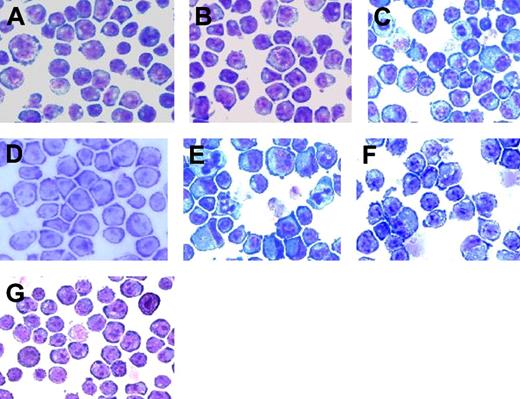
To determine whether Gab2 instead promoted G-CSF-induced differentiation, we analyzed the morphologic features of transfected Ba/F3 cells by May-Grünwald-Giemsa staining. Wild-type Ba/F3 cells resemble other immature hematopoietic cells and have a blastic appearance, with large nuclei containing finely dispersed chromatin, prominent nucleoli, and only small amounts of cytoplasm. Ba/F3 cells transfected with vector alone (Figure 7A) retain the primitive appearance of the wild-type cells even when treated with G-CSF (consistent with the absence of a G-CSF receptor in Ba/F3 cells). Transfection of Ba/F3 cells with the G-CSFR did not alter morphology when the cells were maintained in IL-3 (Figure 7B), but when stimulated by G-CSF, some G-CSFR-transfected Ba/F3 cells developed morphologic features suggestive of myeloid maturation with increased amounts and granulation of the cytoplasm and decreased nuclear size with chromatin condensation (Figure 7C). In particular, there was an increase in cells resembling myelocytes, showing eccentric nuclei and increased, granulated cytoplasm, and a small number of cells showed evidence of further nuclear maturation and chromatin condensation, acquiring the nuclear configuration of metamyelocytes or bands. A cytochemical stain for myeloperoxidase was also positive in these more mature-appearing cells (data not shown). In contrast, Ba/F3 cells transfected with the truncated GRprox receptor, showed little or no morphologic evidence of myeloid differentiation (Figure 7D). Overexpression of Gab2 with the full-length receptor accentuated the morphologic changes of myeloid differentiation in response to G-CSF. (Figure 7E). When Gab2 is overexpressed in GRproxtransfected Ba/F3 cells, intermediate effects are seen (Figure 7F). This evidence of partial differentiation in response to overexpression of Gab2, in the absence of a G-CSF receptor that promotes differentiation (ie, GRprox), re-emphasizes the importance of Gab2 as a mediator of myeloid differentiation signals and suggests that Gab2 overexpression can partially substitute for the differentiation signals from the C terminus of the G-CSFR that are lost with receptor truncation. Because Y764 contributes to Gab2 tyrosine phosphorylation, we looked for and found no morphologic evidence of differentiation in Ba/F3cells expressing the Y764F mutant receptor (Figure 7G). All together, our results and those reported for M-CSF31 suggest a role for Gab2 in myeloid differentiation.
Morphologic differentiation of transfectants in response to G-CSF. Cytokine-treated cells were photographed 48 hours after addition of IL-3 or G-CSF and after May-Grünwald-Giemsa staining. Original magnification × 200. (A) BaF3 cells transfected with vector alone retain the immature morphologic appearance of untransfected BaF3 cells. (B) Ba/F3 cells transfected with G-CSFR have a similar immature morphology when grown in IL-3. (C) When grown in G-CSF, Ba/F3GR cells acquire morphologic features of myeloid differentiation. (D) Ba/F3 cells transfected with the GRprox mutant, however, show little evidence of differentiation in response to G-CSF. (E) The appearance of myeloid differentiation is accentuated when Gab2 is overexpressed in cells expressing full-length G-CSFR. (F) Overexpression of Gab2 in GRprox cells partially restores the morphologic effects of the wild-type receptor. (G) Cells expressing a mutated Y764F receptor did not show differentiation.
Morphologic differentiation of transfectants in response to G-CSF. Cytokine-treated cells were photographed 48 hours after addition of IL-3 or G-CSF and after May-Grünwald-Giemsa staining. Original magnification × 200. (A) BaF3 cells transfected with vector alone retain the immature morphologic appearance of untransfected BaF3 cells. (B) Ba/F3 cells transfected with G-CSFR have a similar immature morphology when grown in IL-3. (C) When grown in G-CSF, Ba/F3GR cells acquire morphologic features of myeloid differentiation. (D) Ba/F3 cells transfected with the GRprox mutant, however, show little evidence of differentiation in response to G-CSF. (E) The appearance of myeloid differentiation is accentuated when Gab2 is overexpressed in cells expressing full-length G-CSFR. (F) Overexpression of Gab2 in GRprox cells partially restores the morphologic effects of the wild-type receptor. (G) Cells expressing a mutated Y764F receptor did not show differentiation.
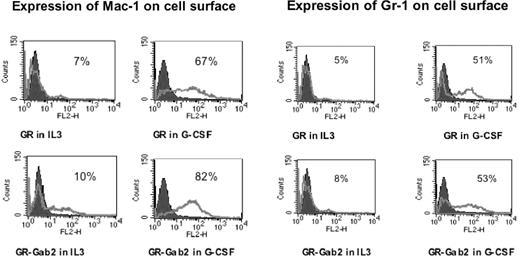
Morphologic differentiation was accompanied by up-regulation of myeloid-specific surface markers Mac-1 and Gr-1. To further demonstrate the effects of Gab2 on myeloid differentiation, we analyzed by flow cytometry the surface expression of myeloid cell markers Mac-1 and Gr-1. Parental Ba/F3 cells were negative for both Mac-1 and Gr-1. Stable transfectants were treated with or without 100 ng/mL G-CSF for 48 hours. Cells were then incubated with PE-conjugated monoclonal antibodies against Mac-1 and Gr-1 or an isotype-control antibody. In untreated Ba/F3-GR cells and untreated Ba/F3-GR cells with Gab2 overexpression, Gr-1 was detected in 5.5% ± 0.8% and 8.6% ± 1.1%, respectively, and Mac-1 in 7.4% ± 1.2% and 10.2% ± 1.6%, respectively. With G-CSF treatment, Gr-1 positivity increased to 51.2% ± 4.2% and 53.3% ± 5.1% in Ba/F3-GR cells and Ba/F3-GR cells with Gab2 overexpression, respectively (Figure 8). G-CSF treatment also increased Mac-1 expression: Mac-1 positivity increased to 67.5% ± 5.6% for Ba/F3GR cells and to 82.4% ± 6.8% for Ba/F3-GR cells with Gab2 overexpression. In contrast, after G-CSF stimulation, Ba/F3-GRprox cells showed less than 5% positivity for either Gr-1 or Mac-1 expression. However, Gab2-overexpressing Ba/F3GRprox cells showed a 29% ± 2% Gr-1 positivity and 31% ± 3% for Mac-1 positivity. In Ba/F3-GRY764F, Mac-1 and Gr-1 positivity were 44.65% ± 4.5% and 12.52% ± 2.3%, respectively. Compared with wild-type G-CSFR, the Y764F mutant showed impaired expression of myeloid differentiation markers.
Expression of Mac-1 and Gr-1. Mac-1 expression is shown in the left panel, and Gr-1 expression in the right panel. Flow cytometric analysis was performed on G-CSFR-transfected Ba/F3 and Ba/F3GR cells, which overexpressed Gab2 in the presence of IL-3 or G-CSF for 48 hours. In each panel, the fluorescence with isotype control is shown as a black histogram and the specific antibody as a solid gray line along the x-axis (log scale), and the relative number of cells is shown on the y-axis.
Expression of Mac-1 and Gr-1. Mac-1 expression is shown in the left panel, and Gr-1 expression in the right panel. Flow cytometric analysis was performed on G-CSFR-transfected Ba/F3 and Ba/F3GR cells, which overexpressed Gab2 in the presence of IL-3 or G-CSF for 48 hours. In each panel, the fluorescence with isotype control is shown as a black histogram and the specific antibody as a solid gray line along the x-axis (log scale), and the relative number of cells is shown on the y-axis.
Discussion
Tyrosine phosphorylation plays an essential role in controlling blood cell growth, differentiation, and function. A major challenge is to determine how the initial activation of protein tyrosine kinases by extracellular stimuli triggers multiple downstream signaling cascades, which ultimately elicit diverse cellular responses. Recent studies reveal that members of the Gab/DOS subfamily of scaffolding adaptor proteins play a crucial role in transmitting key signals that control cell growth and differentiation for multiple receptors. Little is known about their role in hematopoietin/cytokine receptors such as G-CSFR. To determine the role of Gab2 in G-CSF signaling, we correlated the tyrosine phosphorylation of Gab2 with responses associated with wild-type and mutant forms of human G-CSFR in Ba/F3 cells.
G-CSF stimulation of its receptor results in the tyrosine phosphorylation of Gab2, a novel adapter molecule associated with both proliferation and differentiation. Using cell lines lacking active Lyn owing to genetic ablation or pharmacologic inhibition, we found that Gab2 phosphorylation required that the Src family kinase Lyn, activated by the G-CSF, may directly phosphorylate Gab2. Tyrosine phosphorylation of Gab2 is also dependent on Y764 in the C-terminal portion of the G-CSFR. Y764 can be phosphorylated by Lyn or Jak2 (data not shown), thus creating a docking site for the SH2 domain of Grb2.42 Grb2 and Gab2 can interact via the SH3 domain of Grb2 and the proline-rich motif found in Gab2.13,22 As shown in Figure 3D, the association of Grb2 and Gab2 appears to be enhanced under the same conditions in which Gab2 is tyrosine phosphorylated after treatment by G-CSF. G-CSF stimulation of Gab2 may involve both Y764 phosphorylation to enhance Grb2 interaction with the receptor and thereby localize Gab2 to the receptor, and Gab2 phosphorylation by Lyn to enhance its interaction with Grb2 and other signaling proteins.
Importantly, tyrosine phosphorylation of Gab2 did not correlate with the G-CSFR growth/survival response. Notably, cells expressing the truncated receptor (GRprox) show enhanced G-CSF-induced cell growth, but without Gab2 tyrosine phosphorylation. Overexpression of Gab2, in fact, blunted G-CSF-induced growth for either full-length G-CSFR or truncated GRprox. This may be due to negative growth pathways triggered by Gab2 or by its sequestering of biochemical mediators of G-CSF-induced growth, such as Akt or ERK. Another interesting finding from our studies is that overexpression of Gab2 in Ba/F3 cells not only inhibited the G-CSF-mediated proliferation signaling, but also inhibited IL-3-mediated proliferation signaling. Cell cycle showed G2/M arrest in these cell lines (data not shown). The difference between our findings and those by Gu et al,43 who observed no IL-3 growth inhibition, may be due to greater overexpression of Gab2 in our transfected cell lines. Overexpressed Gab2 may sequester biochemical molecules that mediate cytokine-induced growth. Besides reduced growth, overexpression of Gab2 promoted myeloid differentiation. Unlike cells with the full-length G-CSFR, GRprox-expressing cells do not differentiate in response to G-CSF unless Gab2 is overexpressed. Thus, it appears that Gab2 complements signals generated by the distal domain of the G-CSFR.
Gab2 appears to stimulate G-CSF-induced PI3-kinase activity, as detected by the increase in phosphorylated Akt with Gab2 overexpression. Therefore, PI3-kinase (and Akt) activation, previously implicated in G-CSF-induced proliferation, does not strictly correlate with proliferation. In other situations, end-cell function may be promoted, as in the case of Gab2-associated PI3 kinase and subsequent Akt activation in FcγR-mediated phagocytosis.44 Enhanced Akt can be associated with differentiation.45-48 A Lyn-PI3-kinase-Akt pathway for differentiation has been previously described in trophoblasts.49
ERK activation by G-CSF is maintained in GRprox-expressing cells when Gab2 is overexpressed. Modulation of ERK and other MAP kinases, such as p38, may provide a critical event that directs a cell to either cytokine-stimulated proliferation or differentiation.50-55 All together, these data demonstrate that G-CSF-induced tyrosine phosphorylation of Gab2 requires Lyn and Y764 position of the G-CSFR and contributes to the differentiation response by modulating signals mediated by Akt and perhaps ERK.
Prepublished online as Blood First Edition Paper, December 4, 2003; DOI 10.1182/blood-2003-06-1861.
Supported by grants from the American Cancer Society and the National Institutes of Health; S.J.C. is a recipient of the National Institutes of Health (NIH) Independent Scientist Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal