Abstract
The activity of recombinant human growth hormone (rhGH) in enhancing CD34+ cell mobilization elicited by chemotherapy plus recombinant human granulocyte colony-stimulating factor (rhG-CSF) was evaluated in 16 hard-to-mobilize patients, that is, those achieving a peak of circulating CD34+ cells 10/μL or less, or a collection of CD34+ cells equal to or less than 2 × 106/kg. Patients who had failed a first mobilization attempt with chemotherapy plus rhG-CSF (5 μg/kg/d) were remobilized with chemotherapy plus rhG-CSF and rhGH (100 μg/kg/d). As compared with rhG-CSF, the combined rhGH/rhG-CSF treatment induced significantly higher (P ≤ .05) median peak values for CD34+ cells/μL (7 versus 29), colony-forming cells (CFCs)/mL (2154 versus 28 510), and long-term culture-initiating cells (LTC-ICs)/mL (25 versus 511). Following rhG-CSF and rhGH/rhG-CSF, the median yields of CD34+ cells per leukapheresis were 1.1 × 106/kg and 2.3 × 106/kg (P ≤ .008), respectively; the median total collections of CD34+ cells were 1.1 × 106/kg and 6 × 106/kg (P ≤ .008), respectively. No specific side effect could be ascribed to rhGH, except a transient hyperglycemia occurring in 2 patients. Reinfusion of rhGH/rhG-CSF-mobilized cells following myeloablative therapy resulted in prompt hematopoietic recovery. In conclusion, our data demonstrate that in poor mobilizers addition of rhGH to rhG-CSF allows the patients to efficiently mobilize and collect CD34+ cells with maintained functional properties. (Blood. 2004;103: 3287-3295)
Introduction
Mobilized peripheral blood progenitor cells (PBPCs) have an established role in the management of patients with non-Hodgkin lymphoma (NHL),1,2 relapsed Hodgkin lymphoma (HL),3 or multiple myeloma (MM)4 who are eligible for high-dose sequential chemotherapy and autologous stem cell transplantation (ASCT). Because the number of infused CD34+ cells correlates with the rate of hematopoietic reconstitution, the availability of adequate amounts of PBPCs is a prerequisite for the feasibility of high-dose chemotherapy and ASCT.5 There is a general consensus that patients receiving PBPC autografts containing less than or equal to 2 × 106 CD34+ cells/kg are at risk for delayed hematopoietic recovery, increased procedure-related morbidity and mortality, engraftment failure, and myelodysplasia, whereas those receiving 5 × 106 or more CD34+ cells/kg experience prompt and durable hematopoietic engraftment.6 PBPCs are mobilized efficiently by the administration of short courses of recombinant human (rh) granulocyte colony-stimulating factor (G-CSF) alone or during recovery from cytotoxic chemotherapy.7-9 Indeed, due to prior chemoradiotherapy, disease stage, or disease-intrinsic factors, a substantial proportion of cancer patients (10%-30%) mobilize suboptimal amounts of CD34+ cells (ie, ≤ 2 × 106 CD34+ cells/kg).10-12 The lack of autologous stem cells raises important issues for the clinical management of patients for whom ASCT has proved to be clinically beneficial.
PBPC mobilization might be improved by molecules capable of interfering with the mechanisms regulating hematopoietic stem cell trafficking.13-16 An increase of CD34+ cell mobilization might also be achieved by combinations of cytokines, such as granulocyte-macrophage colony-stimulating factor (rhGM-CSF) plus rhG-CSF,17 interleukin-3 (rhIL-3) plus rhG-CSF or rhGM-CSF,18 and PIXY-321.19 Additionally, PBPC mobilization may be enhanced by incorporating in the standard mobilization regimen early-acting cytokines, such as stem cell factor (rh-SCF)20-22 or flt-323 ligand. So far, substitutes or adjuncts to rhG-CSF either failed to substantially improve the mobilization of blood progenitors achieved with rhG-CSF alone or resulted in a limited improvement.24,25
Growth hormone (GH) is a pleiotropic cytokine targeting a variety of nonhematopoietic and hematopoietic cells by binding to a specific receptor.26,27 In vitro, rhGH significantly increases colony formation by human myeloid (granulocyte-macrophage colony-forming unit [CFU-GM]) and erythroid (erythroid burst-forming unit [BFU-E]) progenitors.28-30 In vivo, a 7-day course of rhGH induces a significant increase of marrow and spleen CFU-GMs and BFU-Es in both normal and azidothymidine-treated mice.31 Following syngeneic marrow transplantation, rhGH significantly hastens multilineage hematopoietic recovery in mice.32 When given for 4 weeks, rhGH restores the age-associated decline of marrow cellularity in rats,33 as well as the stem cell mobilization capacity in mice.34 Collectively, these data suggest that bone marrow is an important target for the action of rhGH and allow us to hypothesize that rhGH might enhance rhG-CSF-induced mobilization of CD34+ cells by increasing the numbers of marrow stem cells susceptible to be released on a subsequent or concomitant mobilization stimulus.
Based on these findings, we conducted a pilot study aimed at investigating the feasibility and efficacy of rhGH administration as an adjunct to chemotherapy plus rhG-CSF for enhancing stem cell mobilization. Included in this study were 16 consecutive patients with relapsed or refractory hematologic malignancies who had failed a first mobilization attempt with chemotherapy plus rhG-CSF. Patients were then remobilized with chemotherapy plus rhG-CSF and rhGH. Mobilization failure was defined as a peak value of circulating CD34+ cells equal to or less than 10/μL, or a collection of CD34+ cells equal to or less than 2 × 106/kg. To eliminate the interpatient variability induced by the considerable heterogeneity in patient characteristics and responses to a given mobilization regimen, we prospectively compared the number of PBPCs mobilized into blood after 2 consecutive cycles of the same chemotherapy regimen administered in the same patient. The objectives of the study were to: (1) assess the activity of rhGH in increasing rhG-CSF-induced mobilization and harvesting of CD34+ cells, committed colony-forming cells (CFCs), as well as the more primitive long-term culture-initiating cells (LTC-ICs), and (2) assess the safety and tolerability of rhGH, given in combination with rhG-CSF. Our data indicate that in the great majority of poor mobilizers addition of rhGH to rhG-CSF allows efficient mobilization and collection of CD34+ cells with maintained functional properties.
Patients, materials, and methods
Patients
Between September 2000 and December 2002, 16 hard-to-mobilize consecutive patients with relapsed or refractory hematologic malignancies who were eligible for ASCT were enrolled in this study. Demographic and disease characteristics for these patients at the time of study entry are shown in Table 1. Patients (11 women, 5 men) ranged in age from 19 to 67 years (median, 52 years). Previous chemotherapy was to be completed at least 3 weeks before study entry. Patients were required to have a Karnofsky performance status of 80% or greater, left ventricular ejection fraction more than 50% at rest by echocardiography assessment, and a diffusing capacity of the lung for carbon monoxide (DLCO) more than 50%. Criteria for exclusion were: (1) renal or hepatic insufficiency or severe central nervous system or psychiatric diseases, (2) hepatitis B or C, or HIV tests positive, and (3) pregnancy. The study protocol was approved by Institutional Ethical Committee and written informed consent was obtained from each patient. Patients mobilized with rhGH/rhG-CSF were retrospectively compared with an historical group of hard-to-mobilize patients who were treated at our institution between January 1999 and August 2000, before the present study was conducted. The historical group of hard-to-mobilize patients included 14 transplantation-eligible patients (9 women, 5 men) with a median age of 50 years (range, 28-67 years).
Clinical characteristics of patients at time of study
Case . | Age/sex . | Diagnosis . | Stage . | Disease status . | Bone marrow involvement . | Previous chemotherapy . | Previous radiotherapy . | Splenectomy . | Time from last treatment, mo . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 25/F | HL | IIA | Relapse | No | EBVD × 3 | STNI | No | 6 |
| 2 | 34/F | HL | IIBx | Relapse | No | VEBEP × 8 | Mantle | No | 24 |
| 3 | 57/M | HL | IVB | Relapse | Yes | VEBEP × 8 | STNI | No | 36 |
| 4 | 32/M | HL | IBx | Relapse | No | MOPP/ABVD × 6 | Mediastinum | No | 18 |
| 5 | 42/F | HL | IIBx | Refractory | No | ABVD × 7 | — | No | 1 |
| 6 | 57/F | HL | IVAx | Refractory | Yes | EBVD × 8 | — | No | 1 |
| 7 | 45/F | HL | IVA | Relapse | No | ABVD × 8 | Para-aortic | No | 12 |
| 8 | 19/F | HL | IIB | Relapse | No | ABVD × 7 | Mantle | No | 12 |
| 9 | 60/F | FL | IVA | Relapse | Yes | Fludarabine × 6 DHAP × 1 | Para-aortic | No | 1 |
| 10 | 47/F | FL | IVA | Relapse | No | CVP × 8 2CdA × 7 | — | Yes | 1 |
| 11 | 59/M | DLBCL | IVA | Relapse | No | Fludarabine × 10 | — | No | 10 |
| 12 | 57/F | DLBCL | IVB | Relapse | No | CHOP × 6 | — | No | 8 |
| 13 | 67/M | MM | IIIA | PR | Yes | VAD × 2 DT-PACE × 1 | — | No | 1 |
| 14 | 52/M | t-AML | — | CR | No | BEP × 3 FLAG-Ida × 1 | Para-aortic | No | 2 |
| 15 | 53/F | t-AML | — | CR | No | MOPP/ABVD × 8 VEBEP × 8 CBDCA × 8 FLAG-Ida × 1 | STNI | Yes | 2 |
| 16 | 58/F | t-AML | — | CR | No | FLAG-Ida × 1 | STNI | Yes | 2 |
Case . | Age/sex . | Diagnosis . | Stage . | Disease status . | Bone marrow involvement . | Previous chemotherapy . | Previous radiotherapy . | Splenectomy . | Time from last treatment, mo . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 25/F | HL | IIA | Relapse | No | EBVD × 3 | STNI | No | 6 |
| 2 | 34/F | HL | IIBx | Relapse | No | VEBEP × 8 | Mantle | No | 24 |
| 3 | 57/M | HL | IVB | Relapse | Yes | VEBEP × 8 | STNI | No | 36 |
| 4 | 32/M | HL | IBx | Relapse | No | MOPP/ABVD × 6 | Mediastinum | No | 18 |
| 5 | 42/F | HL | IIBx | Refractory | No | ABVD × 7 | — | No | 1 |
| 6 | 57/F | HL | IVAx | Refractory | Yes | EBVD × 8 | — | No | 1 |
| 7 | 45/F | HL | IVA | Relapse | No | ABVD × 8 | Para-aortic | No | 12 |
| 8 | 19/F | HL | IIB | Relapse | No | ABVD × 7 | Mantle | No | 12 |
| 9 | 60/F | FL | IVA | Relapse | Yes | Fludarabine × 6 DHAP × 1 | Para-aortic | No | 1 |
| 10 | 47/F | FL | IVA | Relapse | No | CVP × 8 2CdA × 7 | — | Yes | 1 |
| 11 | 59/M | DLBCL | IVA | Relapse | No | Fludarabine × 10 | — | No | 10 |
| 12 | 57/F | DLBCL | IVB | Relapse | No | CHOP × 6 | — | No | 8 |
| 13 | 67/M | MM | IIIA | PR | Yes | VAD × 2 DT-PACE × 1 | — | No | 1 |
| 14 | 52/M | t-AML | — | CR | No | BEP × 3 FLAG-Ida × 1 | Para-aortic | No | 2 |
| 15 | 53/F | t-AML | — | CR | No | MOPP/ABVD × 8 VEBEP × 8 CBDCA × 8 FLAG-Ida × 1 | STNI | Yes | 2 |
| 16 | 58/F | t-AML | — | CR | No | FLAG-Ida × 1 | STNI | Yes | 2 |
HL indicates Hodgkin lymphoma; EBVD, etoposide, BCNU, vincristine, dexamethasone; STNI, subtotal nodal irradiation; VEBEP, etoposide, epirubicin, bleomycin, cyclophosphamide, prednisone; MOPP, mechlorethamine, vincristine, procarbazine, prednisone; ABVD, adriamycin, bleomycin, vinblastine, dexamethasone; FL, follicular lymphoma; DHAP, cisplatin, cytarabine, dexamethasone; CVP, cyclophosphamide, vincristine, prednisone; 2CdA, cladribine; DLBCL, diffuse large B-cell lymphoma; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; MM, multiple mycloma; PR, partial remission; VAD, vincristine, doxorubicin, dexamethasone; DT-PACE, dexamethasone, thalidomide, cisplatin, cyclophosphamide, doxorubicin, etoposide; t-AML, therapy-related acute myeloid leukemia; CR, complete remission; BEP, bleomycin, etoposide, cisplatin; FLAG-Ida, fludarabine, cytarabine, granulocyte colony-stimulating factor, idarubicin; CBDCA, carboplatin; STNI, subtotal nodal irradiation; and—, patient underwent no previous radiotherapy.
Study design
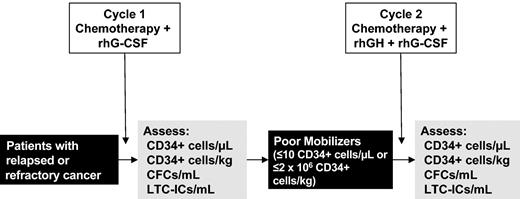
Studied were patients eligible to receive 2 consecutive cycles of the same chemotherapy regimen. An overview of the study is shown in Figure 1. Following the administration of a first chemotherapy cycle supported by rhG-CSF (5 μg/kg/d subcutaneously), patients identified as poor mobilizers (ie, those with a peak value of circulating CD34+ cells < 10/μL, or collecting ≤ 2 × 106 CD34+ cells/kg) were remobilized with the same chemotherapy regimen supported by rhGH (100 μg/kg/d, subcutaneously; maximum daily dose of 6 mg) plus rhG-CSF (5 μg/kg/d, subcutaneously). Thus, according to the study design, the kinetics of PBPC mobilization achieved following cycle 1 served as intrapatient control to assess the mobilization achieved following cycle 2. Despite the fact that no prospective control group was envisaged for this pilot study, patients mobilized with rhGH/rhG-CSF were retrospectively compared with an historical group of hard-to-mobilize patients who had failed PBSC mobilization after standard-dose chemotherapy plus rhG-CSF (5 μg/kg/d) and were remobilized with the same chemotherapy plus a higher dose of rhG-CSF (15 μg/kg/d; Table 6). Mobilization treatments were started 48 hours after stopping chemotherapy and administered until the completion of CD34+ cell harvest. After each mobilization cycle, absolute numbers of circulating CD34+ cells and committed and primitive hematopoietic progenitors were monitored on a daily basis starting when the white blood cell (WBC) counts were 1000/μL or higher and until completion of leukapheresis.
Study design. Schematic representation of the study. Details on treatment and cytokine dosages are reported in “Patients, materials, and methods.”
Study design. Schematic representation of the study. Details on treatment and cytokine dosages are reported in “Patients, materials, and methods.”
CD34+ cell mobilization and collection in hard-to-mobilize historical controls receiving 2 consecutive cycles of the same chemotherapy regimen plus rhG-CSF at either 5 μg/kg/d (first cycle) or 15 μg/kg/d (second cycle)
. | . | . | WBCs × 109/L, peak value . | . | CD34+ cells/μL, peak value . | . | Leukapheresis, no. . | . | CD34+ cells/leukapheresis, 106/kg . | . | Total CD34+ cells, 106/kg . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case . | Diagnosis . | Chemotherapy . | First cycle . | Second cycle . | First cycle . | Second cycle . | First cycle . | Second cycle . | First cycle . | Second cycle . | First cycle . | Second cycle . | |||||
| 1 | HL | IFO-VNB | 18 | 23 | 2 | 17 | — | 2 | — | 2.15 | — | 4.3 | |||||
| 2 | HL | IFO-VNB | 19 | 17 | 10 | 6 | 1 | — | 1.5 | — | 1.5 | — | |||||
| 3 | FL | DHAP | 34 | 47 | 4 | 5 | — | — | — | — | — | — | |||||
| 4 | MM | D-PACE | 25 | 45 | 5 | 10 | — | 1 | — | 0.5 | — | 0.5 | |||||
| 5 | HL | IFO-VNB | 17 | 21 | 5 | 30 | — | 4 | — | 3 | — | 12 | |||||
| 6 | MCL | DHAP | 50 | 52 | 5 | 4 | — | — | — | — | — | — | |||||
| 7 | FL | DHAP | 32 | 65 | 17 | 10 | 1 | 1 | 1.2 | 2.2 | 1.2 | 2.2 | |||||
| 8 | DLBCL | DHAP | 14 | 36 | 10 | 33 | 1 | 3 | 1.5 | 1.6 | 1.5 | 4.7 | |||||
| 9 | HL | IFO-VNB | 39 | 65 | 10 | 28 | 1 | 4 | 0.3 | 1.5 | 0.3 | 6 | |||||
| 10 | DLBCL | DHAP | 20 | 29 | 2 | 2 | — | — | — | — | — | — | |||||
| 11 | FL | DHAP | 12 | 43 | 25 | 11 | 1 | 1 | 1.9 | 1 | 1.9 | 1 | |||||
| 12 | MM | D-PACE | 27 | 35 | 2 | 5 | — | — | — | — | — | — | |||||
| 13 | MM | D-PACE | 28 | 23 | 9 | 11 | — | 1 | — | 0.6 | — | 0.6 | |||||
| 14 | FL | DHAP | 29 | 34 | 17 | 12 | 1 | 1 | 1.4 | 0.7 | 1.4 | 0.7 | |||||
. | . | . | WBCs × 109/L, peak value . | . | CD34+ cells/μL, peak value . | . | Leukapheresis, no. . | . | CD34+ cells/leukapheresis, 106/kg . | . | Total CD34+ cells, 106/kg . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case . | Diagnosis . | Chemotherapy . | First cycle . | Second cycle . | First cycle . | Second cycle . | First cycle . | Second cycle . | First cycle . | Second cycle . | First cycle . | Second cycle . | |||||
| 1 | HL | IFO-VNB | 18 | 23 | 2 | 17 | — | 2 | — | 2.15 | — | 4.3 | |||||
| 2 | HL | IFO-VNB | 19 | 17 | 10 | 6 | 1 | — | 1.5 | — | 1.5 | — | |||||
| 3 | FL | DHAP | 34 | 47 | 4 | 5 | — | — | — | — | — | — | |||||
| 4 | MM | D-PACE | 25 | 45 | 5 | 10 | — | 1 | — | 0.5 | — | 0.5 | |||||
| 5 | HL | IFO-VNB | 17 | 21 | 5 | 30 | — | 4 | — | 3 | — | 12 | |||||
| 6 | MCL | DHAP | 50 | 52 | 5 | 4 | — | — | — | — | — | — | |||||
| 7 | FL | DHAP | 32 | 65 | 17 | 10 | 1 | 1 | 1.2 | 2.2 | 1.2 | 2.2 | |||||
| 8 | DLBCL | DHAP | 14 | 36 | 10 | 33 | 1 | 3 | 1.5 | 1.6 | 1.5 | 4.7 | |||||
| 9 | HL | IFO-VNB | 39 | 65 | 10 | 28 | 1 | 4 | 0.3 | 1.5 | 0.3 | 6 | |||||
| 10 | DLBCL | DHAP | 20 | 29 | 2 | 2 | — | — | — | — | — | — | |||||
| 11 | FL | DHAP | 12 | 43 | 25 | 11 | 1 | 1 | 1.9 | 1 | 1.9 | 1 | |||||
| 12 | MM | D-PACE | 27 | 35 | 2 | 5 | — | — | — | — | — | — | |||||
| 13 | MM | D-PACE | 28 | 23 | 9 | 11 | — | 1 | — | 0.6 | — | 0.6 | |||||
| 14 | FL | DHAP | 29 | 34 | 17 | 12 | 1 | 1 | 1.4 | 0.7 | 1.4 | 0.7 | |||||
Chemotherapy and cytokines
Standard-dose chemotherapy regimens administered to study patients are listed in Table 2. rhG-CSF (filgrastim) was from Roche (Milan, Italy) and rhGH (somatropin) was from Serono (Milan, Italy). Both rhG-CSF and rhGH were kept refrigerated at 2 to 8°C until the time of injection. Lyophilized rhGH was reconstituted with sterile water for injection before subcutaneous administration.
Chemotherapy regimens used in the study patients at the first and second mobilization attempts
Case . | Chemotherapy regimen . |
|---|---|
| 1-8 | IFO-VNB |
| 9-12 | DHAP |
| 13 | DT-PACE |
| 14-16 | FLAG-Ida |
Case . | Chemotherapy regimen . |
|---|---|
| 1-8 | IFO-VNB |
| 9-12 | DHAP |
| 13 | DT-PACE |
| 14-16 | FLAG-Ida |
IFO-VNB is ifosfamide (3 g/m2, intravenously [IV], days 1-4) and vinorelbine (25 mg/m2, IV, days 1 and 5).
DHAP is cisplatin (CDDP [cisplatin], 100 mg/m2, continuous IV infusion, day 1), cytarabine (ara-C, 2 g/m2, IV, every 12 hours, day 2), and dexamethasone (40 mg, IV, day 1-4).
DT-PACE is dexamethasone (40 mg, IV, day 1-4), thalidomide (100 mg, by mouth, CDDP (10 mg/m2, IV, day 1-4), cyclophosphamide (400 mg/m2, IV, day 1-4), doxorubicin (10 mg/m2, IV, day 1-4), and etoposide (40 mg/m2, IV, day 1-4).
FLAG-Ida is fludarabine (30 mg/m2, IV, day 1-5), ara-C (2 g/m2, IV, day 1-5), rhG-CSF (200 μg, subcutaneously, day 0-5), and idarubicin (10 mg/m2, IV, day 1-3).
Collection of PBPCs
PBPC collection was started when equal to or more than 10 CD34+ cells/μL blood were detected. If the number of circulating CD34+ cells remained 10/μL or less, leukapheresis procedures were continued daily until the completion of CD34+ cell harvest (target cell dose was ≥ 5 × 106 CD34+ cells/kg). Each leukapheresis processed approximately 2.5-fold the total blood volume using a Cobe Spectra apparatus (Gambro BCT, Lakewood, CO).
Flow cytometry
Samples were analyzed for expression of CD34 on a FACScalibur flow cytometry system (Becton Dickinson, San Jose, CA) equipped with a Macintosh PowerMac G4 personal computer (Apple Computer, Cupertino, CA) using Cell Quest (Becton Dickinson) software. Briefly, cells (1 × 106) were labeled with either phycoerythrin (PE)-conjugated anti-CD34 (HPCA-2; Becton Dickinson) or with a mouse IgG1-PE antibody (Becton Dickinson) as negative control and analyzed for FL2 and low side scatter. A gate was established from the analysis of forward and light scatter to include all WBCs but to exclude platelets, red blood cells, and debris. CD34+ cells were assessed by analysis of a minimum of 50 000 events.
CFU-Mix, BFU-E, CFU-GM assay
The assay for committed CFCs, including CFU-GMs, BFU-Es, and multilineage progenitors (CFU-Mix), was carried out as previously described.35 Briefly, 1 to 5 × 104 nucleated cells from mobilized blood or leukapheresis were plated in 35-mm Petri dishes in methylcellulose-based medium (HCC-4100; StemCell Technologies, Vancouver, BC, Canada) supplemented with rhSCF (50 ng/mL; StemCell Technologies), rhIL-3 (10 ng/mL; StemCell Technologies), rhG-CSF (10 ng/mL, StemCell Technologies), rhGM-CSF (10 ng/mL, StemCell Technologies), and erythropoietin (rhEpo, 3 U/mL; R&D Systems, Abingdon, United Kingdom). Progenitor cell growth was evaluated after 14 to 18 days of incubation (37°C, 5% CO2) in a humidified atmosphere.
LTC-IC assay
LTC-ICs were assayed as previously described.36 Briefly, test cells were resuspended in complete medium consisting of α-medium (Cambrex, Verviers, Belgium) supplemented with fetal bovine serum (12.5%; Stem-Cell Technologies), horse serum (12.5%; StemCell Technologies), L-glutamine (2 mM), 2-mercaptoethanol (10-4 M), inositol (0.2 mM), folic acid (20 μM), and freshly dissolved hydrocortisone (10-6 M). Test cell (5-8 × 106 nucleated cells) suspension was seeded into cultures containing a feeder layer of irradiated (8000 cGy) murine M2-10B4 cells (3 × 104/cm2, kindly provided by Dr C. Eaves, Terry Fox Laboratory, Vancouver, BC, Canada) engineered by retroviral gene transfer to produce human IL-3 and G-CSF.37 After 5 weeks in culture, nonadherent cells and adherent cells harvested by trypsinization were pooled, washed, and assayed together for clonogenic cells in methylcellulose culture. The total number of clonogenic cells (ie, CFU-Mix plus BFU-Es plus CFU-GMs) present in 5-week-old LTCs provides a relative measure of the number of LTC-ICs originally present in the test suspension. Absolute LTC-IC values were calculated by dividing the total number of clonogenic cells by 4, which is the average output of clonogenic cells per LTC-IC.38
Patient evaluation
Patients with histologic bone marrow involvement at study entry were evaluated by bone marrow biopsies after each mobilization attempt; patients with molecular or cytogenetic markers had their leukapheresis results analyzed using either cytogenetic analysis or polymerase chain reaction (PCR) analysis for immunoglobulin complementarity determining region 3 (CDR3) or Bcl-2 (see “Consensus IgH and Bcl-2 PCR”). All lymphoma patients were evaluated using computed tomography (CT) or 67Ga scanning.
Consensus IgH and Bcl-2 PCR
PBPC contamination by occult lymphoma cells was studied using CDR3 analysis. Samples of frozen or paraffin-embedded diagnostic lymph node biopsy specimens were obtained at the time of diagnosis. After mobilization treatments, aliquots of harvested PBPCs were saved for PCR analysis and comparison with the above samples. Tumor DNA was amplified using consensus VH.D and JH.D primers (FR1 primers).39 Amplified DNA was directly sequenced using VH.D and JH.D primers and VH-, D-, JH-regions and N-inserts were identified by sequence comparison. The CDR3 region was identified as the junction of these 3 regions including the N-inserts. The 20mer antisense allele-specific oligonucleotide (ASO) primers were designed from the CDR3 regions including N-insert. DNA from the patient samples was amplified by semi-nested PCR. The first amplification used the relevant VH.D family and JH.D consensus primers, whereas the second amplification was performed with the same VH.D primer and the designed ASO antisense primer. Amplified DNAs were analyzed by electrophoresis on 1.5% agarose gels containing ethidium bromide and visualized by UV light. Bcl-2/IgH PCR was used if there was no predominant CDR3 clone. For bcl-2/IgH translocation, PCR amplification of major (MBR) and minor (mcr) was performed using oligonucleotide primers originally designed by Gribben et al.40
Statistical analysis
To test the probability of significantly different means or medians at the first and second mobilization attempt, the Student t test for paired data (2-tail) or the Wilcoxon matched pairs test was used, as appropriate. Differences were considered significant if P was less than or equal to .05. Statistical analysis was performed with the statistical package Prism 4.0 (GraphPad Software, San Diego, CA) run on a Macintosh G4 personal computer (Apple Computer).
Results
Study patients
Between September 2000 and December 2002, 16 transplantation-eligible consecutive patients who failed a first mobilization attempt with chemotherapy plus rhG-CSF were remobilized with the same chemotherapy regimen followed by rhGH/rhG-CSF (Figure 1; Table 2). Study patients were defined as poor mobilizers if the peak value of circulating CD34+ cells was less than 10/μL or the collection was 2 × 106 CD34+ cells/kg or less. As shown in Table 1, the median number of cycles of chemotherapy prior to study entry was 7 (range, 1-25), with 10 of 16 patients (62%) having also received prior radiotherapy. At the time of the study, bone marrow involvement was detected in 4 of 16 patients who showed less than 10% infiltration. The median period from last treatment and inclusion in this study was 4 months (range, 1-36 months). The median interval between the 2 mobilization attempts was 1 month (range, 1-2 months). No chemotherapy or radiotherapy was given during the interval between the 2 mobilization procedures.
PBPC mobilization
After both mobilization attempts, WBCs, circulating CD34+ cells, CFCs, and LTC-ICs were monitored on a daily basis starting when the recovery WBC count first exceeded 1 × 109/L (typically on days 10-12). The median duration of cytokine administration was 13 days (range, 9-22 days) and 15 days (range, 10-22 days) after rhG-CSF and rhGH/rhG-CSF, respectively.
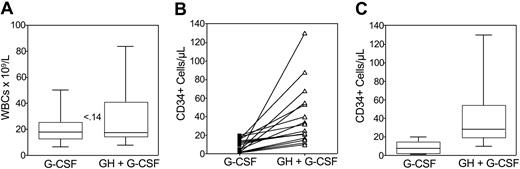
The median peak values of WBC counts were 17.8 × 109/L (range, 6.6-50 × 109/L) and 17.5 × 109/L (range, 8.0-84 × 109/L; P ≤ .14) after rhG-CSF and rhGH/rhG-CSF, respectively (Figure 2A). The duration of leukocytosis was related to the duration of cytokine administration and no patient experienced any clinical sequelae in association with leukocytosis.
WBC counts and circulating CD34+cells. Box plots of peripheral WBC counts (A), peak values (B), and box plots (C) of CD34+ cells/μL blood in poor mobilizers receiving rhG-CSF (n = 16) or rhGH/rhG-CSF (n = 16). The peak of CD34+ cells was defined as the maximum number of CD34+ cells detected in the peripheral blood during mobilization. The boxes extend from the 25th percentile to the 75th percentile, the lines indicate the median values, and the whiskers indicate the range of values. Statistical difference was evaluated using the Wilcoxon matched pairs test (2-tail). Number in panel (A) indicates P value.
WBC counts and circulating CD34+cells. Box plots of peripheral WBC counts (A), peak values (B), and box plots (C) of CD34+ cells/μL blood in poor mobilizers receiving rhG-CSF (n = 16) or rhGH/rhG-CSF (n = 16). The peak of CD34+ cells was defined as the maximum number of CD34+ cells detected in the peripheral blood during mobilization. The boxes extend from the 25th percentile to the 75th percentile, the lines indicate the median values, and the whiskers indicate the range of values. Statistical difference was evaluated using the Wilcoxon matched pairs test (2-tail). Number in panel (A) indicates P value.
Figure 2B shows the peak values of circulating CD34+ cells detected in each patient at the first and second mobilization cycle. In all cases, rhGH/rhG-CSF administration resulted in a higher peak of CD34+ cells as compared with rhG-CSF administration. Median peak values of CD34+ cells/μL after rhG-CSF and rhGH/rhG-CSF were 7 (range, 1-20) and 29 (range, 10-130; P ≤ .0005), respectively, with a median 4-fold increase (range, 2-65; Figure 2C). By comparing the median days of peak value of CD34+ cells at the first and second mobilization attempts (12 versus 13, P ≥ .05), it is evident that addition of rhGH in the mobilization regimen does not alter the kinetics of rhG-CSF mobilization.
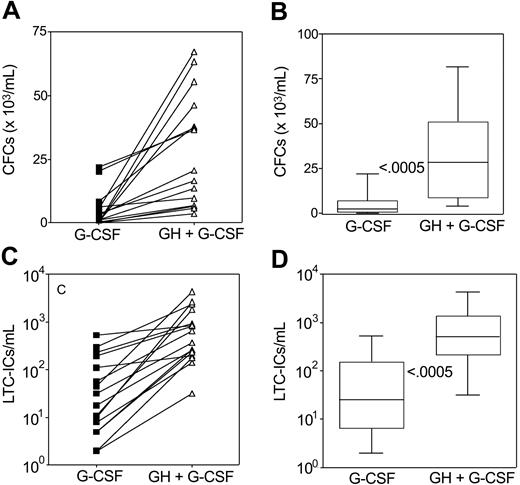
Table 3 summarizes the median peak values of circulating CFU-Mix, BFU-Es, and CFU-GMs per milliliter blood. As compared with rhG-CSF administration, the combined rhGH/rhG-CSF treatment was associated with a median 9-fold (range, 1-828; P ≤ .0005), 8-fold (range, 2-389; P ≤ .0005), and 15-fold (range, 1-261; P ≤ .0005) increase of circulating CFU-Mix, BFU-Es, and CFU-GMs, respectively (Table 3). Peak values of total CFCs measured in each patient following rhGH/rhG-CSF were higher than those following rhG-CSF (Figure 3A). Median peak values of total CFCs/mL blood after rhG-CSF and rhGH/rhG-CSF were 2154 (range, 76-22 000) and 28 510 (range, 3600-81 600; P ≤ .0005), respectively, with a median 13-fold increase (range, 2-528; Figure 3B).
Median peak values of circulating CFU-Mix, BFU-Es, and CFU-GMs per milliliter blood at the first and second mobilization cycles
. | Chemotherapy + rhG-CSF, n = 16 (range) . | Chemotherapy + rhGH/rhG-CSF, n = 16 (range) . | P . |
|---|---|---|---|
| CFU-Mix/mL | 48 (1-430) | 450 (56-1656) | ≤ .0005* |
| BFU-E/mL | 795 (1-6462) | 6 774 (389-34 100) | ≤ .0005 |
| CFU-GM/mL | 1 196 (74-15 110) | 18 090 (3016-48 070) | ≤ .0005 |
. | Chemotherapy + rhG-CSF, n = 16 (range) . | Chemotherapy + rhGH/rhG-CSF, n = 16 (range) . | P . |
|---|---|---|---|
| CFU-Mix/mL | 48 (1-430) | 450 (56-1656) | ≤ .0005* |
| BFU-E/mL | 795 (1-6462) | 6 774 (389-34 100) | ≤ .0005 |
| CFU-GM/mL | 1 196 (74-15 110) | 18 090 (3016-48 070) | ≤ .0005 |
Wilcoxon matched pairs test (2-tail).
Circulating CFCs and LTC-ICs. Peak values (A,C) and box plots (B,D) of CFCs and LTC-ICs per milliliter of blood in poor mobilizers receiving rhG-CSF (n = 16) or rhGH/rhG-CSF (n = 16). CFCs include CFU-GMs, BFU-Es, and CFU-Mix. Peaks of CFCs and LTC-ICs were defined as the maximum numbers of CFCs or LTC-ICs detected in the peripheral blood during mobilization. The boxes extend from the 25th percentile to the 75th percentile, the lines indicate the median values, and the whiskers indicate the range of values. Statistical difference was evaluated using the Wilcoxon matched pairs test (2-tail). Numbers in the panels (B,D) indicate P values.
Circulating CFCs and LTC-ICs. Peak values (A,C) and box plots (B,D) of CFCs and LTC-ICs per milliliter of blood in poor mobilizers receiving rhG-CSF (n = 16) or rhGH/rhG-CSF (n = 16). CFCs include CFU-GMs, BFU-Es, and CFU-Mix. Peaks of CFCs and LTC-ICs were defined as the maximum numbers of CFCs or LTC-ICs detected in the peripheral blood during mobilization. The boxes extend from the 25th percentile to the 75th percentile, the lines indicate the median values, and the whiskers indicate the range of values. Statistical difference was evaluated using the Wilcoxon matched pairs test (2-tail). Numbers in the panels (B,D) indicate P values.
The combined rhGH/rhG-CSF administration resulted in significantly higher peaks of circulating LTC-ICs as compared with rhG-CSF administration (Figure 3C). Median peak values of LTC-ICs/mL blood after rhG-CSF and rhGH/rhG-CSF were 25 (range, 2-528) and 511 (range, 32-4303; P ≤ .0005), respectively, with a median 20-fold increase (range, 2-262; Figure 3D).
PBPC harvest
PBPC harvesting was started when at least 10 CD34+ cells/μL blood were detected. Such a value of CD34+ cells occurred in 8 of 16 patients after rhG-CSF and 16 of 16 patients after rhGH/rhG-CSF. Mobilization with rhG-CSF alone resulted in a short duration of CD34+ cell release, which allowed us to perform only one leukapheresis in 7 of 8 patients, and 2 leukapheresis procedures in one patient. In striking contrast, after the combined rhGH/rhG-CSF mobilization a sustained CD34+ cell release was observed, which allowed us to perform a median of 3 leukapheresis procedures (range, 2-4) in 15 of 16 patients. Patient no. 16 asked to be withdrawn from the study and cell samples were not collected.
Following rhGH/rhG-CSF as compared with rhG-CSF alone, significantly higher median yields per leukapheresis were detected for CD34+ cells (2.3 × 106/kg versus 1.1 × 106/kg; P ≤ .008), CFCs (6.3 × 105/kg versus 3.3 × 105/kg; P ≤ .01), and LTC-ICs (1.6 × 104/kg versus 0.5 × 104/kg; P ≤ .01; Table 4). The median total collection of CD34+ cells/kg body weight was 1.1 × 106 (range, 0.8-2 × 106) following rhG-CS, and 6 × 106 (range, 2.4-15 × 106) following rhGH/rhG-CSF (P ≤ .008; Figure 4B). Following rhG-CSF alone, no patient could collect the target cell dose of CD34+ cells (ie, ≥ 5 × 106/kg body weight), whereas following mobilization with rhGH/rhG-CSF 13 of 15 (87%) patients could collect the target cell dose of CD34+ cells, with 2 remaining patients collecting 2.4 and 2.5 × 106 CD34+ cells/kg, respectively.
Yields of TNCs, CD34+ cells, CFCs, and LTC-ICs per leukapheresis following chemotherapy plus either rhG-CSF (first cycle) or rhGH/rhG-CSF (second cycle)
. | TNCs, × 108/kg . | . | CD34+ cells, × 106/kg . | . | CFCs, × 105/kg . | . | LTC-Ics, × 104/kg . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case . | First cycle . | Second cycle . | First cycle . | Second cycle . | First cycle . | Second cycle . | First cycle . | Second cycle . | ||||
| 1 | 2.7 | 5.7 | 1.1 | 5.0 | 3.6 | 10 | 0.5 | 1.7 | ||||
| 2 | — | 3.5 | — | 1.7 | — | 4.3 | — | 0.8 | ||||
| 3 | 3.3 | 4.5 | 0.8 | 2.3 | 3.2 | 12 | 0.1 | 0.8 | ||||
| 4 | 2.0 | 1.8 | 1.1 | 1.5 | 3.0 | 5.0 | 1.6 | 1.2 | ||||
| 5 | 2.7 | 2.7 | 1.1 | 2.3 | 6.5 | 6.3 | 0.7 | 3.2 | ||||
| 6 | 3.8 | 4.5 | 0.6 | 0.8 | 3.4 | 5.9 | 0.2 | 2.5 | ||||
| 7 | 2.9 | 12 | 1.5 | 1.7 | 6.3 | 7.2 | 0.5 | 2.3 | ||||
| 8 | — | 7.0 | — | 2.5 | — | 6.2 | — | 1.8 | ||||
| 9 | — | 3.7 | — | 2.5 | — | 3.7 | — | 2.0 | ||||
| 10 | — | 6.9 | — | 2.2 | — | 7.5 | — | 0.8 | ||||
| 11 | — | 4.2 | — | 3.8 | — | 28 | — | 7.9 | ||||
| 12 | 4.0 | 5.5 | 0.9 | 1.2 | 1.3 | 3.1 | 0.2 | 1.0 | ||||
| 13 | 3.5 | 3.4 | 2.0 | 3.7 | 2.7 | 8.2 | 0.5 | 1.6 | ||||
| 14 | — | 4.8 | — | 1.9 | — | 5.4 | — | 0.5 | ||||
| 15 | — | 3.8 | — | 2.6 | — | 9.8 | — | 1.5 | ||||
| 16 | — | — | — | — | — | — | — | — | ||||
| Median(range) | 3.1 (2-4) | 4.5* (1.8-12) | 1.1 (0.6-2) | 2.3† (0.8-5) | 3.3 (1.3-6.5) | 6.3‡ (3.1-28) | 0.5 (0.1-1.6) | 1.6‡ (0.5-7.9) | ||||
. | TNCs, × 108/kg . | . | CD34+ cells, × 106/kg . | . | CFCs, × 105/kg . | . | LTC-Ics, × 104/kg . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case . | First cycle . | Second cycle . | First cycle . | Second cycle . | First cycle . | Second cycle . | First cycle . | Second cycle . | ||||
| 1 | 2.7 | 5.7 | 1.1 | 5.0 | 3.6 | 10 | 0.5 | 1.7 | ||||
| 2 | — | 3.5 | — | 1.7 | — | 4.3 | — | 0.8 | ||||
| 3 | 3.3 | 4.5 | 0.8 | 2.3 | 3.2 | 12 | 0.1 | 0.8 | ||||
| 4 | 2.0 | 1.8 | 1.1 | 1.5 | 3.0 | 5.0 | 1.6 | 1.2 | ||||
| 5 | 2.7 | 2.7 | 1.1 | 2.3 | 6.5 | 6.3 | 0.7 | 3.2 | ||||
| 6 | 3.8 | 4.5 | 0.6 | 0.8 | 3.4 | 5.9 | 0.2 | 2.5 | ||||
| 7 | 2.9 | 12 | 1.5 | 1.7 | 6.3 | 7.2 | 0.5 | 2.3 | ||||
| 8 | — | 7.0 | — | 2.5 | — | 6.2 | — | 1.8 | ||||
| 9 | — | 3.7 | — | 2.5 | — | 3.7 | — | 2.0 | ||||
| 10 | — | 6.9 | — | 2.2 | — | 7.5 | — | 0.8 | ||||
| 11 | — | 4.2 | — | 3.8 | — | 28 | — | 7.9 | ||||
| 12 | 4.0 | 5.5 | 0.9 | 1.2 | 1.3 | 3.1 | 0.2 | 1.0 | ||||
| 13 | 3.5 | 3.4 | 2.0 | 3.7 | 2.7 | 8.2 | 0.5 | 1.6 | ||||
| 14 | — | 4.8 | — | 1.9 | — | 5.4 | — | 0.5 | ||||
| 15 | — | 3.8 | — | 2.6 | — | 9.8 | — | 1.5 | ||||
| 16 | — | — | — | — | — | — | — | — | ||||
| Median(range) | 3.1 (2-4) | 4.5* (1.8-12) | 1.1 (0.6-2) | 2.3† (0.8-5) | 3.3 (1.3-6.5) | 6.3‡ (3.1-28) | 0.5 (0.1-1.6) | 1.6‡ (0.5-7.9) | ||||
P values derived as compared with the first cycle by Wilcoxon matched pairs test (2-tail):
P ≤ 08.
P ≤ 008.
P ≤ 01.
Total yields of TNCs, CD34+cells, CFCs, and LTC-ICs. Box plots of total yields of total nucleated cells (TNCs; A), CD34+ cells (B), CFCs (C), and LTC-ICs (D) in poor mobilizers receiving rhG-CSF (n = 8) or rhGH/rhG-CSF (n = 15). The boxes extend from the 25th percentile to the 75th percentile, the lines indicate the median values, and the whiskers indicate the range of values. Statistical difference was evaluated using the Wilcoxon matched pairs test (2-tail). P values are shown in the panels.
Total yields of TNCs, CD34+cells, CFCs, and LTC-ICs. Box plots of total yields of total nucleated cells (TNCs; A), CD34+ cells (B), CFCs (C), and LTC-ICs (D) in poor mobilizers receiving rhG-CSF (n = 8) or rhGH/rhG-CSF (n = 15). The boxes extend from the 25th percentile to the 75th percentile, the lines indicate the median values, and the whiskers indicate the range of values. Statistical difference was evaluated using the Wilcoxon matched pairs test (2-tail). P values are shown in the panels.
Following rhG-CSF alone and rhGH/rhG-CSF, the median total collections of CFCs/kg were 3.4 × 105 (range, 1.3-6.9 × 105) and 19 × 105 (range, 6.3-57 × 105; P ≤ .008), respectively (Figure 4C); the median total collections of LTC-ICs/kg were 0.5 × 104 (range, 0.1-1.6 × 104) and 4.1 × 104 (range, 1.6-16 × 104; P ≤ .008), respectively (Figure 4D).
Toxicity
Toxicities occurring during rhGH/rhG-CSF administration were generally consistent with those observed during rhG-CSF administration. During injection of rhGH plus rhG-CSF, 2 of 16 patients experienced a transient hyperglycemia requiring insulin therapy, but not preventing the completion of stem cell mobilization and collection. There were no additional hematologic or extrahematologic toxicities considered possibly, probably, or definitely related to rhGH therapy.
Stimulation of tumor cell growth
In 4 patients (nos. 3, 6, 9, and 13) with histologic bone marrow involvement, analysis of bone marrow biopsies failed to reveal any evidence of increased tumor cell infiltration following rhGH/rhG-CSF therapy. Five patients with a molecular or cytogenetic marker had their leukapheresis products analyzed to identify contaminating tumor cells. As shown in Table 5, in no instance could the presence of tumor cells contaminating the leukapheresis products be demonstrated. All patients with nodal masses detectable by CT or 67Ga scan were evaluated for disease progression after rhGH/rhG-CSF injection, and in no instance could signs of disease progression be detected.
Cytogenetic and molecular analysis of diagnostic samples and leukapheresis performed after rhGH/rhG-CSF administration
Patient . | Cytogenetic/molecular marker at diagnosis . | Cytogenetic/molecular marker in leukapheresis . |
|---|---|---|
| 9 | Bcl-2 | Negative |
| 10 | IgH | Negative |
| 12 | IgH | Negative |
| 15 | 45,XX;−18; del(5q); del(20q) | 46,XX |
| 16 | 46,XY; del(5q) | 46,XY |
Patient . | Cytogenetic/molecular marker at diagnosis . | Cytogenetic/molecular marker in leukapheresis . |
|---|---|---|
| 9 | Bcl-2 | Negative |
| 10 | IgH | Negative |
| 12 | IgH | Negative |
| 15 | 45,XX;−18; del(5q); del(20q) | 46,XX |
| 16 | 46,XY; del(5q) | 46,XY |
Engraftment and survival
Following conditioning with either BEAM (n = 10; BCNU [bischloroethylnitrosourea], etoposide, ara-C [cytarabine], melphalan) or high-dose melphalan (n = 1), 11 of 16 patients underwent ASCT with rhGH/rhG-CSF-mobilized stem cells. Of the remaining patients, 3 (nos. 14, 15, and 16) received an allogeneic stem cell transplant from an unrelated marrow donor, whereas 2 (nos. 6 and 12) who had collected less than or equal to 5 × 106 CD34+ cells/kg after rhGH/rhG-CSF were reinfused with both rhG-CSF-and rhGH/rhG-CSF-mobilized stem cells and are not considered for engraftment analysis. In patients given autografts with rhGH/rhG-CSF-mobilized PBPCs, the median number of days to achieve absolute neutrophil counts equal to or more than 0.5 × 109/L and equal to or more than 1 × 109/L were 9 (range, 8-12) and 10 (range, 9-13), respectively. The median number of days to achieve platelet counts 20 × 109/L or higher and 50 × 109/L or higher were 10 (range, 9-16) and 14 (range, 12-18), respectively. After transplantation, all patients required a median of 3 (range, 0-6) platelet transfusions and one (range, 0-6) red blood cell transfusion. No patient experienced early or late graft failure.
Historical controls
Patients mobilized with rhGH/rhG-CSF were retrospectively compared with an historical control group of poor mobilizers who were treated at our institution between January 1999 and August 2000. This cohort includes 14 transplantation-eligible patients who had failed a first mobilization attempt with standard-dose chemotherapy plus rhG-CSF at 5 μg/kg/d and had been remobilized with the same chemotherapy regimen plus a higher dose of rhG-CSF (15 μg/kg/d; Table 6). The median number of cycles of chemotherapy prior to mobilization was 8 (range, 1-27), with 8 of 14 patients (57%) having also received prior radiotherapy. Bone marrow involvement was detected in 2 of 14 patients who showed less than 10% infiltration. The median interval between the 2 mobilization attempts was 1 month (range, 1-2 months). No chemotherapy or radiotherapy was given during the interval between the 2 mobilization procedures.
The median duration of cytokine administration was 14 (range, 10-24 days) and 16 days (range, 12-25 days) at the first and the second mobilization attempts, respectively. The median peak values of WBC counts were 26 × 109/L (range, 12-50 × 109/L) and 36 × 109/L (range, 17-65 × 109/L; P ≤ .001) after rhG-CSF at 5 and 15 μg/kg/d, respectively (Table 6). After rhG-CSF at 5 and 15 μg/kg/d, the median peak values of CD34+ cells/μL were 7 (range, 2-25) and 11 (range, 2-33; P = .30), respectively (Table 6). Six (43%) and 9 (64%) patients fulfilled harvesting criteria following rhG-CSF at 5 and 15 μg/kg/d, respectively. The first and second mobilization attempts resulted in similar median yields of CD34+ cells per leukapheresis (1.4 × 106/kg, range, 0.3-1.9 × 106/kg versus 1.5 × 106/kg; range, 0.5-3 × 106/kg; P = .62) and median total CD34+ cell collections (1.4 × 106/kg, range, 0.3-1.9 × 106/kg versus 2.2 × 106/kg, range, 0.5-12 × 106/kg; P = .31). None of the patients receiving 5 μg/kg/d rhG-CSF could collect the target CD34+ cell dose, whereas 2 of 14 patients (14%) receiving rhG-CSF at 15 μg/kg/d collected the target CD34+ cell dose. As compared with historical controls receiving rhG-CSF at 15 μg/kg/d, the combined rhGH/rhG-CSF mobilization was associated with a significantly higher number of patients who could collect the target dose of CD34+ cells (P ≤ .05, by Fisher exact test).
Discussion
Data reported in this pilot study clearly demonstrate that the concomitant administration of rhGH and rhG-CSF to poor mobilizers significantly enhances mobilization of CD34+ cells, committed (CFU-Mix, BFU-E, CFU-GM) as well as primitive (LTC-IC) progenitors, thus allowing the collection of adequate amounts of functionally competent stem cells. In addition, we show that rhGH given for mobilization purposes at 100 μg/kg/d for up to 22 days is well-tolerated and devoid of short-term adverse events.
Included in this study were patients identified as poor mobilizers following a first mobilization attempt with chemotherapy plus rhG-CSF, that is, those with a peak value of circulating CD34+ cells of 10/μL or less or a collection of CD34+ cells of 2 × 106/kg or less. These patients were therefore remobilized with the same chemotherapy regimen plus the combined rhGH/rhG-CSF therapy. After mobilization with rhG-CSF alone, only 50% of the patients were eligible for leukapheresis, but in no instance could the target cell dose of 5 × 106 CD34+ cells/kg be collected. In striking contrast, the use of rhGH plus rhG-CSF was associated with a sustained mobilization of CD34+ cells, which allowed 100% of the patients to undergo repeated stem cell collections, and 87% of them to receive the target CD34+ cell dose with a median of 3 leukapheresis proceudres. Thus, the combined rhGH/rhG-CSF treatment allows patients to achieve a clinically significant increase of the median collection of CD34+ cells (ie, from 1.1 × 106/kg up to 6 × 106/kg). CD34+ cells mobilized under rhGH/rhG-CSF had a maintained functional activity not only in vitro, but also in vivo, as suggested by the fast hematopoietic engraftment observed in our patients following myeloablative therapy.
Chemotherapy-induced reduction of massive bone marrow infiltration by tumor cells may result at subsequent mobilization attempts in an improvement of PBPC mobilization. In our series, bone marrow involvement was detected in 4 of 16 patients who showed less than 10% involvement, thus ruling out the possibility that the improved PBPC mobilization achieved under rhGH/rhG-CSF may have resulted from chemotherapy-induced reduction of bone marrow disease.
The intervals between mobilization and remobilization may affect CD34+ cell release. Given the well-known cumulative toxicity of repeated chemotherapy cycles on marrow progenitors, a reduced PBPC mobilization at the second as compared with the first chemotherapy cycle is usually observed when consecutive chemotherapy courses are administered at 4-week intervals. Limited increases in the CD34+ cell yield at the second mobilization attempt have been reported in poor mobilizers who had failed the first mobilization attempt.41 However, in our experience, administration of repeated chemotherapy cycles at 3-to 4-week intervals is associated with a decreased CD34+ cell mobilization (data not shown).
Increasing the dose of rhG-CSF up to 16 μg/kg/d at the second mobilization attempt in patients who had failed an initial mobilization has been reported to double the median collection of CD34+ cells (ie, from 0.51 × 106/kg using rhG-CSF at 5 μg/kg/d up to 1.1 × 106/kg using rhG-CSF at 10-16 μg/kg/d).42 A dose-dependent mobilization response to rhG-CSF has also been achieved by increasing the dose of rhG-CSF as late as the third week of mobilization (0.07 × 106/kg using rhG-CSF at 5 μg/kg/d versus 2.27 × 106/kg using rhG-CSF at 10 μg/kg/d).43 In our hard-to-mobilize historical controls who had failed an initial mobilization with chemotherapy plus rhG-CSF at 5 μg/kg/d, increasing the dose of rhG-CSF up to 15 μg/kg/d at the subsequent chemotherapy cycle resulted in a limited increase of the total CD34+ cell collection with a modest percentage of patients (14%) being able to collect an optimal target cell dose of CD34+ cells.
GH acts on hematopoietic progenitors either directly by binding to specific membrane receptors or indirectly by stimulating the production of insulin-like growth factor I (IGF-I), or interacting with hematopoietic cytokines.44 The exact mechanism by which rhGH is able to restore the stem cell mobilization capacity in heavily pretreated patients with relapsed or refractory cancers remains a matter of hypothesis. Based on the in vivo capacity of rhGH to expand marrow and spleen hematopoietic progenitors in either normal or hematologically suppressed mice,31 as well as to reverse age-associated loss of bone marrow progenitor cells in aged rats33 and mice,34 it is likely that rhGH-enhanced mobilization is related to the in vivo expansion of primitive or committed marrow stem/progenitor cells that become susceptible to being released on a subsequent or concomitant mobilization stimulus, such as rhG-CSF infusion.
The stimulation of tumor cells might represent a major concern for the in vivo use of rhGH.45 Despite the fact that rhGH raises serum levels of IGF-I, which are associated with an increased risk of epithelial cancers, the role of rhGH in carcinogenesis is unclear,46 and several studies have shown that there is no increase in cancer risk in patients receiving prolonged replacement therapy with rhGH. In a large epidemiologic study, the long-term treatment of GH deficiency has established health benefits without any evidence that rhGH replacement increases cancer risk.47 Recently, the risk of relapse of acute lymphoblastic leukemia (ALL) and that of a second malignancy was compared in long-term survivors (n = 47) who received rhGH for a median of 4.5 years with those survivors (n = 860) who had not.48 By landmark analysis, there was no statistical evidence that rhGH replacement therapy was associated with relapse of ALL or second malignancy. Moreover, no evidence of tumor progression has been reported in patients with AIDS treated with 100 μg/kg/d rhGH for 12 weeks.49
The potential risk of exposing our patients to a short rhGH treatment, which did not imply a chronic stimulation, was adequately outweighed by the clinical benefit deriving from ASCT. In fact, for all patients included in the present study, a high-dose chemotherapy program including autografting of hematopoietic progenitor cells was the only chance of cure, and no better alternative existed. Due to mobilization failure and the lack of autologous stem cells, all these patients should have been excluded from high-dose chemotherapy. Our patients bearing nodal masses were carefully monitored by means of CT or 67Ga scan, whereas patients with therapy-related acute myelogenous leukemia (t-AML) and NHL were evaluated at the cytogenetic or molecular level. In no instance could radiographic signs as well as cytogenetic or molecular evidence suggesting a tumor growth stimulation be detected. However, a longer observation period and larger patient numbers are required before the concerns of tumor growth stimulation induced by rhGH can be ruled out.
The dose of rhGH used in this pilot study (ie, 100 μg/kg/d) is the highest dose approved for clinical use and was empirically selected as the dose potentially allowing the highest chance to detect an effect on stem cell mobilization by means of a short treatment. Because our study has provided consistent evidence on the mobilization efficacy of the rhGH/rhG-CSF regimen, a dose-finding study will be required to address the issue of the optimal dose of rhGH to be used for PBPC mobilization.
According to a recently reported Gruppo Italiano Trapianto Midollo Osseo (GITMO) study, poor mobilizers account for 15% to 20% of chemotherapy-naïve patients and 30% to 40% of patients with relapsed or refractory cancers.50 No reliable laboratory tests exist for identifying poor mobilizers, and no reliable and effective treatment exists to enhance mobilization in poor mobilizers. Based on our data, the combined rhGH/rhG-CSF therapy represents a very effective and safe strategy that allows efficient mobilizing and collecting of CD34+ cells with maintained functional properties in nearly 90% of poor mobilizers. These findings will require confirmation from a larger appropriately controlled study including a more homogeneous cohort of patients.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-07-2428.
Supported in part by grants from Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR, Rome, Italy), Associazione Italiana per la Ricerca sul Cancro (AIRC, Milano, Italy), Ministero della Salute (Rome, Italy), and Michelangelo Foundation for Advances in Cancer Research and Treatment (Milano, Italy).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal