Abstract
The breast cancer susceptibility gene BRCA2 has recently been identified as identical to the Fanconi anemia (FA) gene FANCD1. Here we expand the clinical implications of this discovery. Notably, we identified 6 children in 5 kindreds exhibiting the co-occurrence of BRCA2 mutations, FA, and early onset acute leukemia. Leukemia occurred at a median of 2.2 years of age in the BRCA2 patients in contrast to a median onset of 13.4 years in all other FA patients in the International Fanconi Anemia Registry (IFAR; P < .0001). Breast cancer was noted in 4 of the 5 kindreds. Of the 6 children with leukemia, 4 were treated with bone marrow transplantation and 2 are alive at 3 and 9 months after treatment. Our results suggest that BRCA2 testing should be considered in all patients with FA in whom the complementation group cannot be defined or in whom leukemia is diagnosed at or before 5 years of age. (Blood. 2004;103:3226-3229)
Introduction
Fanconi anemia (FA) is a genetically and phenotypically heterogeneous autosomal recessive disorder defined by cellular hypersensitivity to DNA cross-linking agents and characterized by congenital malformations, progressive marrow failure, and marked predisposition to malignancy. Based on patient outcome data as reported to the International Fanconi Anemia Registry (IFAR), the cumulative incidence of bone marrow failure by age 40 years is 90% with median time to onset of 7 years.1 In contrast, the cumulative incidence of hematologic malignancy, defined as the onset of acute leukemia or myelodysplastic syndrome (MDS), by 40 years of age is 33% with no significant difference between the FA complementation groups A, C, and G.1
Eight of at least 11 FA genes have been cloned (FANCA, FANCC, FANCD1, FANCD2, FANCE, FANCF, FANCG, and FANCL) and the intracellular interactions between the FA proteins have been elucidated.2-5 The identification of BRCA2 mutations in FA-D1 cell lines6 suggests that these patients will have an FA-like phenotype with congenital malformations and defective hematopoiesis and marked cancer susceptibility in both patients and family members. We report here the genetic, hematologic, and clinical findings in 7 children in 5 kindreds with segregating mutations in BRCA2 and demonstrate the presence of unique characteristics in patients and their families that may alter current monitoring and treatment practices for this specific patient cohort.
Patients and methods
Patients
The diagnosis of FA was made in all cases by study of chromosomal breakage induced by diepoxybutane (DEB) in phytohemagglutinin (PHA)–stimulated peripheral blood lymphocytes as reported elsewhere.7 The kindreds described in this report were all enrolled in the IFAR with clinical information provided by the treating physician (5 from the University of Minnesota).1 After confirmation of the diagnosis at Rockefeller University, cell lines were established and DNA was prepared using standard methodologies.8 Complementation group assignment for IFAR patients1 was made by mutation screening8-10 or by correction of cross-link hypersensitivity by transduction with retroviral vectors containing the normal FANCA, FANCC, FANCD2, FANCE, FANCF, or FANCG cDNAs.11 Cellular testing to rule out other complementation groups was successfully performed prior to BRCA2 mutation testing for the kindreds reported here, except for kindred 4; 3 independently established B-cell lines from patient IFAR 800/1 in this kindred were DEB resistant, and no fibroblasts were available for study. Approval for these studies was obtained from the Institutional Review Boards of both the University of Minnesota and Rockefeller University. Informed consent for clinical data collection and complementation group analyses was obtained from the parents of each subject.
BRCA2 testing
Analysis of the coding regions and intron/exon junctions of BRCA2 was performed on genomic DNA (gDNA), using direct DNA sequencing (Myriad Genetic Laboratories, Salt Lake City, UT). Nucleotide sequencing used dye-primer chemistry (ABI PRISMÆ BigDye Primer Sequencing; Applied Biosystems, Foster City, CA) detected on automated instruments (ABI PRISM 377 DNA Sequencer Model 377; Applied Biosystems). Reverse transcription–polymerase chain reaction (RT-PCR) was performed to evaluate the integrity of the BRCA2 mRNA for kindreds 2 and 5 in which only a single protein-truncating BRCA2 mutation was found. Commercially available kits were used to extract RNA from Epstein-Barr virus (EBV)–transformed lymphocyte cell lines (RNAeasy; Qiagen, Chatsworth, CA, and Superscript Preamplification Kit; Gibco BRL, Gaithersburg, MD), which was subsequently used for cDNA synthesis. The primers used delineate the abnormal transcript in kindred 5 contained the following designations and gene-specific sequences (all numeric base-pair designations conform to GenBank submission U43746): exon1F 5′-CTTCTGAAACTAGGCGGCAGA (bases 12-32); exon10R 5′-TTCCAGATATTGCCTGCTTTACTG (bases 1699-1676); exon11R 5′-AGGCATGACAGAGAATCAGCTTCT (bases 2363-2340). All primers carried M13 tails with the sequences 5′-GTTTTCCCAGTCACGACG for forward primers (designated “F”) and 5′-AGGAAACAGCTATGACCAT for reverse primers (designated “R”). All PCRs used TaqPlus Long polymerase in the accompanying high-salt buffer (Stratagene, La Jolla, CA) with primers at 1 pg each and target gDNA or cDNA at a concentration of approximately 20 ng and 10 ng, respectively. Following the initial denaturation step, thermal cycling proceeded using 35 cycles at 94°C for 20 seconds, 62°C for 30 seconds, and 72°C for 1 to 3 minutes, depending on product length.
Statistical considerations
Hematologic malignancy was defined as acute leukemia or refractory anemia with excess blasts in transformation. The time to hematologic malignancy (acute myelocytic leukemia [AML] or acute lymphocytic leukemia [ALL]) was defined as the time elapsed (in months) between the date of birth and the date of diagnosis of hematologic malignancy or the last follow-up date. The cumulative incidence of hematologic malignancy was estimated after adjusting for competing risk due to death for patients with BRCA2 mutations (n = 14) and compared to that observed for the rest of the IFAR patients (n = 746).12
Results
Patient and cellular characteristics
Data on 7 patients in 5 kindreds with mutations in BRCA2 and hematologic abnormalities are described in Table 1. The clinical phenotype in the 7 patients with BRCA2 mutations was typical for FA. Diagnosis of FA was made at age 0.1 to 5.2 years; DEB sensitivity in 6 patients was within the established FA range (range, ∼1-24 mean breaks/cell; median, 8.7 breaks/cell).3 The single patient (IFAR 900/1) with an exceptionally high number of spontaneous and DEB-induced breaks per cell was tested only after exposure to induction chemotherapy for the treatment of leukemia. All 7 patients presented with intrauterine growth retardation (IUGR) and were noted to have failure to thrive or short stature in the early childhood period. Other physical anomalies included café au lait spots, microcephaly, and imperforate anus (Table 1). Notably, all 5 kindreds had a cancer history with 4 having breast cancer in 1 to 3 female and male family members (Table 1).
Genotypes and phenotypes of FA patients with BRCA2 mutations
. | . | Breaks/cell . | . | . | . | . | BRCA2 mutations, exon . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kindred . | IFAR no. . | Spon . | DEB . | Clinical manifestations . | Hematologic manifestations . | Survival at last follow-up, mo . | Paternal . | Maternal . | Family cancer history, relation and age . | ||
| 1* | 129/1 | 0.84 | 8.0 | IUGR, imperforate anus, café au lait, microcephaly, FTT | AML at 26 mo, chemotherapy refractory | 36 | IVS7 + 2T>G, IVS7 | IVS7 + 2T>G, IVS7 | Breast: PGM 40 y, MA 45 y; Pancreatic: MU 51 y | ||
| 2 | 357/1 | 0.12 | 3.7 | IUGR, imperforate anus, hypoplastic thumb, FTT | AML at 23 mo, chemotherapy refractory | 9 | W2626C† 8106G>C, 17 | 2041 insA, 10 | Breast: MGM 46 y, MGGM 46 y, MGGA 45 y; Prostate: MGF 48 y; Bladder: MGGF 51 y | ||
| 3 | 632/1 | 1.0 | 13.9 | IUGR, short stature, café au lait, bilateral hip dysplasia | AML at 36 mo, chemotherapy refractory | 48 | IVS7 + 1G>A, IVS7 | Y1894X 5910C>G, 11 | Breast: M 38 y, MGF 60 y, PGM 48 y | ||
| 632/2 | — | 7.7 | IUGR, short stature | AML at 21 mo, chemotherapy refractory | 29+, alive/well | ||||||
| 4 | 800/1 | 0.40 | 14.0 | IUGR, microcephaly, FTT, micropenis, café au lait | AML at 11 mo, chemotherapy refractory | 26 | IVS7+2T>G, IVS7 | 5164del4, 11 | Brain tumor: MGGF 50 y | ||
| 800/2 | 1.1 | 11.1 | IUGR, microcephaly, café au lait, FTT | Neutropenia at 5 mo, Wilms tumor at 9 mo | 9+, alive/well | ||||||
| 5 | 900/1 | 6.6‡ | 25.9‡ | IUGR, FTT, café au lait | T-cell ALL at 62 mo, complete remission after chemotherapy | 90+, alive/well | 2816insA, 11 | Abnormal RNA transcript§ | Breast: MGM 29 y | ||
. | . | Breaks/cell . | . | . | . | . | BRCA2 mutations, exon . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kindred . | IFAR no. . | Spon . | DEB . | Clinical manifestations . | Hematologic manifestations . | Survival at last follow-up, mo . | Paternal . | Maternal . | Family cancer history, relation and age . | ||
| 1* | 129/1 | 0.84 | 8.0 | IUGR, imperforate anus, café au lait, microcephaly, FTT | AML at 26 mo, chemotherapy refractory | 36 | IVS7 + 2T>G, IVS7 | IVS7 + 2T>G, IVS7 | Breast: PGM 40 y, MA 45 y; Pancreatic: MU 51 y | ||
| 2 | 357/1 | 0.12 | 3.7 | IUGR, imperforate anus, hypoplastic thumb, FTT | AML at 23 mo, chemotherapy refractory | 9 | W2626C† 8106G>C, 17 | 2041 insA, 10 | Breast: MGM 46 y, MGGM 46 y, MGGA 45 y; Prostate: MGF 48 y; Bladder: MGGF 51 y | ||
| 3 | 632/1 | 1.0 | 13.9 | IUGR, short stature, café au lait, bilateral hip dysplasia | AML at 36 mo, chemotherapy refractory | 48 | IVS7 + 1G>A, IVS7 | Y1894X 5910C>G, 11 | Breast: M 38 y, MGF 60 y, PGM 48 y | ||
| 632/2 | — | 7.7 | IUGR, short stature | AML at 21 mo, chemotherapy refractory | 29+, alive/well | ||||||
| 4 | 800/1 | 0.40 | 14.0 | IUGR, microcephaly, FTT, micropenis, café au lait | AML at 11 mo, chemotherapy refractory | 26 | IVS7+2T>G, IVS7 | 5164del4, 11 | Brain tumor: MGGF 50 y | ||
| 800/2 | 1.1 | 11.1 | IUGR, microcephaly, café au lait, FTT | Neutropenia at 5 mo, Wilms tumor at 9 mo | 9+, alive/well | ||||||
| 5 | 900/1 | 6.6‡ | 25.9‡ | IUGR, FTT, café au lait | T-cell ALL at 62 mo, complete remission after chemotherapy | 90+, alive/well | 2816insA, 11 | Abnormal RNA transcript§ | Breast: MGM 29 y | ||
Spon indicates mean number of spontaneous breaks per cells; DEB, mean number of DEB induced (0.1 μg/mL, DEB) per cell; FTT, failure to thrive; PGM, paternal grandmother; MA, maternal aunt; MU, maternal uncle; MGM, maternal grandmother; MGGM, maternal great grandmother; MGGA, maternal great grand aunt; MGF, maternal grandfather; MGGF, maternal great grandfather; M, mother; and —, not done.
Parents are second cousins, and patient IFAR 129/1 is homozygous by descent for BRCA2*IVS7+2T>G.
Classified as a variant of unknown significance in the BIC database.
Chromosomal breakage evaluation performed on cells after induction chemotherapy.
DNA analysis revealed an unidentified mutation causing aberrant splicing upstream of exon 3.
Hematologic disease progression
All 7 BRCA2 patients have demonstrated hematologic abnormalities, with 6 having leukemia (Table 1). Four patients presented with marrow failure and 3 presented with leukemia without prior known marrow abnormality. Five were diagnosed with AML and one with ALL at a median age of 23 months (range, 11-62 months). Three of 4 patients who received induction chemotherapy failed to achieve remission, with 2 (1 with persistent disease and 1 in complete remission) subsequently treated by bone marrow transplantation (BMT). Two others were treated by BMT alone. Of the 4 patients undergoing transplantation (IFAR 632/1, 632/2, 800/1, and 900/1), 2 are alive and well 4 months (HLA-identical sibling donor) and 10 months (HLA I antigen–mismatched maternal donor) after BMT. One patient (IFAR 800/2) was found to have neutropenia and marrow hypoplasia at 6 months of age and an occult stage I Wilms tumor (favorable histology) as part of a scheduled screening examination. Treatment consisted of surgical resection without further intervention.
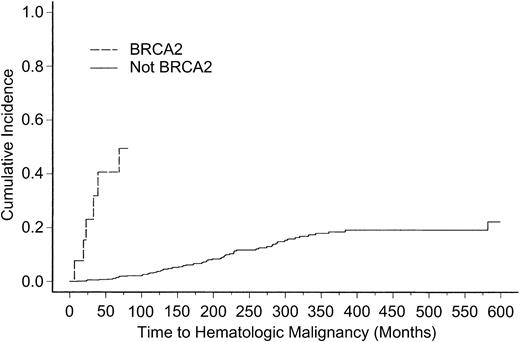
Importantly, the cumulative incidence of hematologic malignancy among FA patients with BRCA2 mutations is 41% (95% confidence interval [CI], 20%-85%) at 5 years of age. This contrasts markedly with the incidence of hematologic malignancy in non-BRCA2 FA patients in the IFAR registry (P < .0001) whose cumulative incidence at 5 years is 1% (95% CI, 0.5%-2.1%; Figure 1).
Cumulative incidence of hematologic malignancy. Cumulative incidence of hematologic malignancy among BRCA2 mutation carriers (14 patients) versus all other complementation groups in the IFAR (746 patients).
Cumulative incidence of hematologic malignancy. Cumulative incidence of hematologic malignancy among BRCA2 mutation carriers (14 patients) versus all other complementation groups in the IFAR (746 patients).
BRCA2 mutations and family cancer history
Results of paternal and maternal BRCA2 mutation analyses are detailed in Table 1. Complete sequencing of gDNA from kindreds 2 and 5, however, revealed only a single protein-truncating mutation. To determine whether the other BRCA2 allele carried a protein-trucating mutation undetected by genomic sequencing, cDNA from cultured cells was evaluated by single-nucleotide polymorphism (SNP) haplotype analysis. Loss of transcript was not detected for kindred 2; however, a maternal transcript anomaly affecting splicing upstream from exon 3 was detected for kindred 5. As postulated, a significant history of breast cancer was observed, particularly in kindreds 1, 2, and 3. Although breast cancer was noted in the maternal grandmother of IFAR 900/1, the father's family cancer history is unknown (adoption).
Discussion
The present study demonstrates that biallelic mutations in BRCA2 result in (1) the classic FA phenotype including short stature and failure to thrive, café au lait spots, and hypersensitivity to clastogenic agents like DEB; (2) exceptionally high risk of acute leukemia at an early age compared with non-BRCA2 FA patients; and (3) family history of breast cancer typical of known BRCA2 kindreds. The data also suggest that biallelic pathogenic mutations in BRCA2 do not preclude fetal survival as previously considered.3 In this regard, kindreds 1, 3, and 4 are particularly interesting. The probands in these 3 kindreds all have a mutation that alters the conserved sequence of the intron 7 donor splice site. The proband in kindred 1 was homozygous by descent for BRCA2*IVS7+2T>G, whereas in kindreds 3 and 4, BRCA2*IVS7+1G>A and BRCA2*IVS7+2T>G, respectively, are in trans with protein-truncating mutations in exon 11 (Table 1). Pyne and colleagues13 have shown by analysis of RNA from a breast cancer patient with BRCA2*IVS7+2T>G that this allele results in a deletion of exon 7 and does not result in a productive message. Furthermore, this mutation is associated with a high incidence of cancer among carrier families. We hypothesize that the presence of minor alternative RNA species, commonly generated by the cellular splicing machinery, enable these embryos to survive. Complete sequencing of gDNA from kindreds 2 and 5 revealed a single protein-truncating mutation found in trans with BRCA2*W2626C (exon 17) classified as a variant of unknown significance in the Breast Cancer Information Core (BIC) database,14 and BRCA2*H372N (exon 10) a common BRCA2 variant,15 respectively. However, results provide convincing evidence for a maternal transcript anomaly for kindred 5 but not kindred 2. W2626C, observed in kindred 2, has previously been seen in 9 breast cancer kindreds tested at a single reference laboratory in Salt Lake City. W2626C may be disease causing in our patient or an unidentified mutation remains to be identified by other methodology. On Western blotting (data not shown), protein lysate from a lymphoblastoid cell line derived from the FA patient in kindred 2 consistently showed both FANCD2-S and FANCD2-L, providing further evidence that this patient is in group FA-D1/BRCA2.3
A single case of biallelic BRCA2 mutations (BRCA2*K2729N and BRCA2*S2835X) in a 2-year-old Japanese boy with FA and AML has previously been reported.16 As in our series, FA was suspected on the basis of physical findings and confirmed by documenting hypersensitivity to DEB. Although chemotherapy and BMT was well tolerated in this patient, relapse occurred 5 months after transplantation and the patient eventually died from refractory disease.
Taken together, these data suggest that FA patients with biallelic mutations in BRCA2 are at markedly high risk of acute leukemia during the first 5 years of life. Presence of biallelic BRCA2 mutations clearly mandates more intensive surveillance of the marrow and possibly “prophylactic” hematopoietic stem cell (HSC) transplantation prior to the development of myelodysplasia and AML. Further, FA patients who are compound heterozygotes for BRCA2 mutations are at high risk of solid tumors.17,18 Of the 14 patients with biallelic BRCA2 mutations in the IFAR database, 5 died of early onset brain tumors, primarily medulloblastomas,17 and 3 developed Wilms tumors,18 including patient 632/2 in this report. Therefore, screening for solid tumors of the brain and kidney before and after HSC transplantation is also warranted. Importantly, genetic counseling should be provided for parents of children with biallelic BRCA2 mutations. Such counseling should address the risks of breast cancer and other solid tumors for both men and women who carry heterozygous BRCA2 mutations, the availability of a number of important family planning options for these couples,19 and BRCA2 testing as part of the preventive medical care of other family members.20
In summary, testing for BRCA2 must be considered for FA patients for whom complementation group assignment has failed for FA-A, C, D2, E, F, and G or the onset of malignancy is at 5 years of age or younger. The finding of a Wilms tumor at 7.5 months of age in the sibling of a patient who died of AML at 19 months of age (Table 1, kindred 4) is evidence that particular BRCA2 mutations can lead to both acute leukemia and solid tumors in FA patients.17,18 Although further experience is required to know whether children who have survived acute leukemia are likely to develop solid tumors, surveillance for brain and renal tumors should be considered. At this point, remaining clinical issues include: (1) advisability of using sibling HSC donors with one pathogenic BRCA2 mutation, and (2) optimal treatment strategies for patients with biallelic mutations in BRCA2 (eg, “prophylactic” BMT). Answers to these questions will only come from close monitoring and reporting of FA patients with BRCA2 mutations to understand the natural history of the disease.
Supported in part by the Fanconi Anemia Research Fund, Inc (J.E.W., J.T., M.L.M.), Children's Cancer Research Fund, Inc (J.E.W., J.T., M.L.M.), the Elterninitiative Kinderkrebsklinik e. V. and the Kinderstern e. V. (H.H.), the National Institutes of Health (R37 HL32987; A.D.A.), and Research Center grant M01-RR00102 to the Rockefeller University Hospital General Clinical Research Center, National Institutes of Health, Department of Health and Human Services.
T.S. and A.D. are employed by Myriad Genetics Laboratories Inc, whose product (BRCAnalysis test) was used in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, January 8, 2004; DOI 10.1182/blood-2003-09-3138.
We thank Dr Marianne Berwick for expert advice on epidemiologic aspects of this study, Dr Kenneth Offit for critical review of the manuscript, and Toni Smith for Western blotting for FANCD2.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal