Abstract
Chemoresistance is a major problem in the treatment of patients with multiple myeloma (MM). Because of the central role of the nuclear transcription factors nuclear factor–κB (NF-κB) and signal transducer and activator of transcription 3 (STAT3) in chemoresistance, cell survival, and proliferation, we investigated whether MM cells derived from patients express activated NF-κB and STAT3 and if their suppression induces apoptosis. We assayed CD138+ cells from the bone marrow of 22 MM patients and checked for the activated forms of NF-κB and STAT3 by immunocytochemistry. We found that MM cells from all the patients expressed the activated forms of NF-κB and STAT3 but to a variable degree (NF-κB: low, 3 of 22; moderate, 5 of 22; or high, 14 of 22; STAT3: none, 1 of 22; low, 3 of 22; moderate, 5 of 22; or high, 14 of 22). Constitutive activation of NF-κB was in some cases also independently confirmed by electrophoretic mobility gel shift assay. In contrast to MM patients, activated forms of NF-κB and STAT3 were absent in cells from healthy individuals. Suppression of NF-κB and STAT3 activation in MM cells by ex vivo treatment with curcumin (diferuloylmethane) resulted in a decrease in adhesion to bone marrow stromal cells, cytokine secretion, and in the viability of cells. When compared with curcumin, dexamethasone was less effective in suppression of NF-κB activation and induction of apoptosis in myeloma cells. Overall, our results indicate that fresh cells from MM patients express constitutively active NF-κB and STAT3, and suppression of these transcription factors inhibits the survival of the cells. (Blood. 2004;103:3175-3184)
Introduction
Multiple myeloma (MM) is a B-cell malignancy characterized by the latent accumulation in bone marrow of secretory plasma cells with a low proliferative index and an extended life span.1 MM accounts for 1% of all cancers and more than 10% of all hematologic cancers. Various agents used for the treatment of myeloma include combinations of vincristine, 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU), melphalan, cyclophosphamide, adriamycin, and prednisone or dexamethasone.2 Usually, patients younger than 65 years old are treated with high-dose melphalan with autologous stem cell support, and older patients who are generally unable to tolerate such intensive treatment receive standard-dose oral melphalan and prednisone. Despite these treatments, the complete remission rate is only 5% and a median survival only 30 to 36 months.3,4
The dysregulation of the apoptotic mechanism in plasma cells is considered a major underlying factor in the pathogenesis and subsequent chemoresistance in MM. Expression of Bcl-xL, an antiapoptotic protein, has been correlated with chemoresistance in MM patients, with response rates of 83% to 87% in non-Bcl-xL–expressing cases and 20% to 31% in Bcl-xL–expressing cases.5 It is established that interleukin-6 (IL-6), produced in either an autocrine or paracrine manner, has an essential role in the malignant progression of MM by regulating the growth and survival of tumor cells.6,7 The presence of IL-6 leads to constitutive activation of signal transducer and activator of transcription 3 (STAT3), which in turn results in expression of high levels of the antiapoptotic protein Bcl-xL.8 Bcl-2 overexpression, another important characteristic of most MM cell lines,9 rescues these tumor cells from glucocorticoid-induced apoptosis.4 Additionally, cell lines resistant to doxorubicin, such as RPMI 8226 dox-40, are known to overexpress Bcl-xL.5
Besides STAT3, another transcription factor, nuclear factor–κB (NF-κB), has also been shown to be constitutively active in MM cells.10,11 The role of NF-κB in chemoresistance of a variety of tumors is well established.12,13 Constitutive NF-κB activation in MM cells can lead to the expression of Bcl-xL and IL-6.5,10,11 Furthermore, paracrine exposure of MM cells to tumor necrosis factor (TNF) can also activate NF-κB, resulting in the secretion of IL-6 and expression of adhesion molecules.14 MM cells have been shown to express the ligand for the receptor that activates NF-κB, RANKL, a member of the TNF superfamily, which may also lead to NF-κB activation.15-17
Both NF-κB and STAT3 when activated are translocated from the cytoplasm to the nucleus. Several of the studies cited above suggest that constitutive activation of NF-κB and STAT3 could mediate chemoresistance and thus might be exploited as a target for the treatment of MM. Work from our laboratory has shown that human MM cell lines express constitutively active NF-κB, but only 1 of 4 lines tested had constitutively active STAT3. Very little is known about the frequency of constitutive expression of NF-κB and STAT3 in fresh cells from MM patients, however. Therefore, in the present report we investigated the status of NF-κB and STAT3 in freshly obtained CD138+ cells from MM patients. We also investigated the effect of down-regulation of NF-κB and STAT3 on the survival of these cells.
Patients, materials, and methods
Materials
The human MM cell lines U266 (ATCC TIB-196) and RPMI 8226 (CCL-155) were obtained from the American Type Culture Collection (Rockville, MD). Dox-6 and LR-5, doxorubicin-resistant and melphalan-resistant clones of RPMI 8226, respectively, were kindly provided by Dr William S. Dalton (H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL). MM.1R, a dexamethasone-resistant variant of MM.1, was kindly provided by Dr Steve T. Rosen of Northwestern University Medical School (Chicago, IL). Anti-CD138 microbeads and anti-CD138 conjugated with phycoerythrin (PE) were purchased from Miltenyi Biotec (Auburn, CA). Rabbit polyclonal antibodies to NF-κB p65 and STAT3 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Goat antirabbit–Alexa 594 was purchased from Molecular Probes (Eugene, OR). Curcumin was purchased from LKT Laboratories (St Paul, MN) and was prepared as a 20-mM solution in dimethyl sulfoxide and then further diluted in cell culture medium. Hoechst 33342, dexamethasone, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) were from Sigma-Aldrich Chemicals (St Louis, MO), and Ficoll-Paque was from Mediatech (Herndon, VA). RPMI 1640, Iscove modified Dulbecco medium (IMDM), deoxyribonuclease (Dnase) I, fetal bovine serum (FBS), 0.4% trypan blue vital stain, and 100 × antibiotic-antimycotic mixture were obtained from Life Technologies (Grand Island, NY). γ32P–adenosine triphosphate (γ32P-ATP) was from ICN Pharmaceuticals (Costa Mesa, CA). All other reagents were of analytical grade.
Clinical samples
Marrow samples were obtained from 22 MM patients who gave informed consent and underwent treatment at The University of Texas M. D. Anderson Cancer Center (Houston). Table 1 describes the clinical characteristics of these patients. Bone marrow samples from patients with sarcoma who were chemonaive and had a normal bone marrow (no marrow involvement) were used as controls.
Clinical characteristics of multiple myeloma patients
Patient no. . | Patient age, y/sex . | MM type . | Hgb level, g/L . | WBC count, × 109/L . | Platelets, × 109/L . | Serum paraproteins, g . | Urine paraproteins, g . | Site . |
|---|---|---|---|---|---|---|---|---|
| 1 | 67/M | lgG | 103 | 4.2 | 203 | 6.4 | 0.60 | Bone (diffuse) |
| 2 | 40/F | lgG | 86 | 5 | 49 | 9.3 | 3.40 | (−) bone survey |
| 3 | 57/F | lgG | 102 | 5.3 | 217 | 6.7 | 4.25 | Bone (diffuse) |
| 4 | 50/M | lgA | 111 | 4.9 | 184 | 5.7 | 0.00 | Bone (diffuse) |
| 5 | 52/M | lgG | 121 | 2.9 | 257 | 4.4 | 0.12 | (−) bone survey |
| 6 | 56/F | lgG | 80 | 4 | 33 | 8.4 | 1.68 | (−) bone survey |
| 7 | 63/F | lgG | 100 | 5.5 | 336 | 9.9 | 0.36 | (−) bone survey |
| 8 | 63/M | lgG | 123 | 4.6 | 151 | (−) | 0.02 | Bone (diffuse) |
| 9 | 64/M | lgG | 105 | 4.2 | 219 | 3.5 | 0.01 | T11, clavicle |
| 10 | 35/F | lgA | 91 | 2.6 | 85 | 1.5 | 0.21 | (−) bone survey |
| 11 | 52/M | lgA | 104 | 3.3 | 54 | 0.8 | 17.00 | (−) bone survey |
| 12 | 45/M | lgG | 102 | 11.8 | 338 | 0.1 | 4.273 | Bone (diffuse) |
| 13 | 54/M | lgG | 141 | 4.5 | 262 | 4.7 | 0.00 | (−) bone survey |
| 14 | 66/M | lgA | 102 | 5.9 | 154 | 3.4 | 0.10 | Bone (diffuse) |
| 15 | 50/F | lgG | 119 | 7.1 | 364 | 3.7 | 0.00 | Bone (diffuse) |
| 16 | 40/F | lgG | 103 | 6.2 | 335 | (−) | 0.00 | Skull, apex |
| 17 | 58/M | lgA | 139 | 5.1 | 205 | 4.3 | 0.00 | (−) bone survey |
| 18 | 67/M | lgG | 89 | 8.8 | 52 | 0.4 | 3.90 | (−) bone survey |
| 19 | 56/M | lgG | 133 | 7.8 | 233 | 4.6 | 0.02 | Ribs |
| 20 | 65/M | lgG | 133 | 4.8 | 227 | 2.8 | 0.32 | C2 |
| 21 | 57/M | lgA | 60 | 9.6 | 219 | 3.1 | 4.80 | (−) bone survey |
| 22 | 67/F | lgG | 128 | 5 | 249 | 1.6 | 0.00 | (−) bone survey |
Patient no. . | Patient age, y/sex . | MM type . | Hgb level, g/L . | WBC count, × 109/L . | Platelets, × 109/L . | Serum paraproteins, g . | Urine paraproteins, g . | Site . |
|---|---|---|---|---|---|---|---|---|
| 1 | 67/M | lgG | 103 | 4.2 | 203 | 6.4 | 0.60 | Bone (diffuse) |
| 2 | 40/F | lgG | 86 | 5 | 49 | 9.3 | 3.40 | (−) bone survey |
| 3 | 57/F | lgG | 102 | 5.3 | 217 | 6.7 | 4.25 | Bone (diffuse) |
| 4 | 50/M | lgA | 111 | 4.9 | 184 | 5.7 | 0.00 | Bone (diffuse) |
| 5 | 52/M | lgG | 121 | 2.9 | 257 | 4.4 | 0.12 | (−) bone survey |
| 6 | 56/F | lgG | 80 | 4 | 33 | 8.4 | 1.68 | (−) bone survey |
| 7 | 63/F | lgG | 100 | 5.5 | 336 | 9.9 | 0.36 | (−) bone survey |
| 8 | 63/M | lgG | 123 | 4.6 | 151 | (−) | 0.02 | Bone (diffuse) |
| 9 | 64/M | lgG | 105 | 4.2 | 219 | 3.5 | 0.01 | T11, clavicle |
| 10 | 35/F | lgA | 91 | 2.6 | 85 | 1.5 | 0.21 | (−) bone survey |
| 11 | 52/M | lgA | 104 | 3.3 | 54 | 0.8 | 17.00 | (−) bone survey |
| 12 | 45/M | lgG | 102 | 11.8 | 338 | 0.1 | 4.273 | Bone (diffuse) |
| 13 | 54/M | lgG | 141 | 4.5 | 262 | 4.7 | 0.00 | (−) bone survey |
| 14 | 66/M | lgA | 102 | 5.9 | 154 | 3.4 | 0.10 | Bone (diffuse) |
| 15 | 50/F | lgG | 119 | 7.1 | 364 | 3.7 | 0.00 | Bone (diffuse) |
| 16 | 40/F | lgG | 103 | 6.2 | 335 | (−) | 0.00 | Skull, apex |
| 17 | 58/M | lgA | 139 | 5.1 | 205 | 4.3 | 0.00 | (−) bone survey |
| 18 | 67/M | lgG | 89 | 8.8 | 52 | 0.4 | 3.90 | (−) bone survey |
| 19 | 56/M | lgG | 133 | 7.8 | 233 | 4.6 | 0.02 | Ribs |
| 20 | 65/M | lgG | 133 | 4.8 | 227 | 2.8 | 0.32 | C2 |
| 21 | 57/M | lgA | 60 | 9.6 | 219 | 3.1 | 4.80 | (−) bone survey |
| 22 | 67/F | lgG | 128 | 5 | 249 | 1.6 | 0.00 | (−) bone survey |
MM indicates multiple myeloma; Hgb; hemoglobin; WBC, white blood cell;/F, female;/M, male; and (−), not available.
Isolation of plasma cells from bone marrow of patients with MM
CD138 antigen, also known as syndecan-1, is expressed on normal and malignant plasma cells but not on circulating B cells, T cells, and monocytes.18-20 Anti-CD138 microbeads were used for positive selection of CD138+ cells from bone marrow derived from MM patients. A 2- to 10-mL bone marrow sample was aspirated from the upper iliac crest or sternum and diluted in an equal volume of HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–buffered IMDM, supplemented with heparin at a concentration of 100 U/mL, and mixed gently. To prevent the cells from clumping, the dilute marrow was suspended in IMDM containing 100 units of deoxyribonuclease (Dnase) I per milliliter and shaken gently at room temperature for an additional 30 minutes. Next, 30 mL dilute bone marrow cell suspension was layered over 20 mL Ficoll-Paque in 50-mL conical tubes and spun at 400g for 30 minutes to isolate mononuclear cells (MNCs). Thereafter, the MNC layer at the interface was harvested and washed twice with phosphate-buffered saline (PBS) containing 2 mM EDTA (ethylenediaminetetraacetic acid) for 10 minutes at 300g at room temperature.
The MNC concentration was adjusted to 107 per 80 μL running buffer (PBS with 2 mM EDTA plus 0.5 mM bovine serum albumin [BSA]). For every 107 MNCs in 80 μL running buffer, 20 μL anti-CD138 microbeads was added and the cell suspension incubated at 4°C to 8°C for 15 minutes. Then, the cell suspension was diluted with up to 1 mL cold running buffer and centrifuged at 300g for 10 minutes in a refrigerated centrifuge at 4°C. The supernatant was discarded, and the cell pellet was suspended in 1 mL running buffer and loaded onto the magnetic column of the AutoMACS system (Miltenyi Biotec) placed in a laminar flow hood. Anti-CD138+ cells were isolated by positive selection. The purity of the isolated CD138+ plasma cell population was determined by treating 105 CD138+ cells with 10 μL anti-CD138 conjugated with phycoerythrin (PE) and incubated in the dark in the refrigerator at 6°C to 12°C. The cells were washed twice with cold PBS, fixed with 1% paraformaldehyde, and analyzed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). The purity of the CD138+ cells is indicated in Table 2.
Status of NF-κB and STAT3 activation in CD138+ cells from bone marrow aspirates of multiple myeloma patients
. | . | . | . | . | . | . | Nuclear* . | . | |
|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | % plasma cells in BM differential . | MNC count, × 106 . | CD138+ MNCs, % . | Enriched CD138+, % . | Viability, % . | CD138+ count, × 106 . | NF-κB . | STAT3 . | |
| 1 | NA | NA | NA | NA | NA | NA | +++ | + | |
| 2 | 8 | 14 | NA | 93 | 90 | 1.1 | +++ | ++ | |
| 3 | 47 | 26 | NA | NA | 70 | NA | + | + | |
| 4 | 40 | 36 | NA | 98 | 95 | 5.6 | +++ | + | |
| 5 | 35 | 24 | NA | NA | 95 | 0.8 | ++ | +++ | |
| 6 | 96 | 40 | NA | 97 | 99 | 0.5 | +++ | ++ | |
| 7 | 45 | 21 | NA | 95 | 99 | 0.5 | +++ | + | |
| 8 | 18 | 12 | NA | NA | 95 | 0.2 | +++ | + | |
| 9 | 30 | 21 | NA | 92 | 98 | 2 | +++ | +++ | |
| 10 | 25 | 18 | NA | 95 | 95 | 1 | ++ | ++ | |
| 11 | 66 | 10 | NA | NA | 95 | 0.3 | ++ | +++ | |
| 12 | 63 | 38 | NA | 98 | 98 | 22 | ++ | +++ | |
| 13 | 14 | 6.6 | NA | 80 | 98 | 1 | +++ | +++ | |
| 14 | 44 | 9 | 3.59 | 60 | 98 | 0.6 | +++ | +++ | |
| 15 | 50 | 29 | 3.64 | 52 | 60 | 1.2 | ++ | +++ | |
| 16 | 22 | 6 | 7.68 | 87 | 90 | 0.6 | +++ | +++ | |
| 17 | 3 | 18 | 5.29 | NA | 98 | 0.3 | + | − | |
| 18 | 22 | 21 | NA | 72 | 50 | 1.1 | +++ | +++ | |
| 19 | 25 | 12 | NA | 98 | 95 | 1.5 | +++ | +++ | |
| 20 | 49 | 17 | 24.7 | 97 | 95 | 1.7 | +++ | +++ | |
| 21 | 46 | 10.2 | NA | 98 | 98 | 1.2 | +++ | +++ | |
| 22 | 14 | 5.4 | NA | 85 | 98 | 1.2 | + | ++ | |
. | . | . | . | . | . | . | Nuclear* . | . | |
|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | % plasma cells in BM differential . | MNC count, × 106 . | CD138+ MNCs, % . | Enriched CD138+, % . | Viability, % . | CD138+ count, × 106 . | NF-κB . | STAT3 . | |
| 1 | NA | NA | NA | NA | NA | NA | +++ | + | |
| 2 | 8 | 14 | NA | 93 | 90 | 1.1 | +++ | ++ | |
| 3 | 47 | 26 | NA | NA | 70 | NA | + | + | |
| 4 | 40 | 36 | NA | 98 | 95 | 5.6 | +++ | + | |
| 5 | 35 | 24 | NA | NA | 95 | 0.8 | ++ | +++ | |
| 6 | 96 | 40 | NA | 97 | 99 | 0.5 | +++ | ++ | |
| 7 | 45 | 21 | NA | 95 | 99 | 0.5 | +++ | + | |
| 8 | 18 | 12 | NA | NA | 95 | 0.2 | +++ | + | |
| 9 | 30 | 21 | NA | 92 | 98 | 2 | +++ | +++ | |
| 10 | 25 | 18 | NA | 95 | 95 | 1 | ++ | ++ | |
| 11 | 66 | 10 | NA | NA | 95 | 0.3 | ++ | +++ | |
| 12 | 63 | 38 | NA | 98 | 98 | 22 | ++ | +++ | |
| 13 | 14 | 6.6 | NA | 80 | 98 | 1 | +++ | +++ | |
| 14 | 44 | 9 | 3.59 | 60 | 98 | 0.6 | +++ | +++ | |
| 15 | 50 | 29 | 3.64 | 52 | 60 | 1.2 | ++ | +++ | |
| 16 | 22 | 6 | 7.68 | 87 | 90 | 0.6 | +++ | +++ | |
| 17 | 3 | 18 | 5.29 | NA | 98 | 0.3 | + | − | |
| 18 | 22 | 21 | NA | 72 | 50 | 1.1 | +++ | +++ | |
| 19 | 25 | 12 | NA | 98 | 95 | 1.5 | +++ | +++ | |
| 20 | 49 | 17 | 24.7 | 97 | 95 | 1.7 | +++ | +++ | |
| 21 | 46 | 10.2 | NA | 98 | 98 | 1.2 | +++ | +++ | |
| 22 | 14 | 5.4 | NA | 85 | 98 | 1.2 | + | ++ | |
A total of 2 to 10 mL bone marrow was aspirated from the upper iliac crest or sternum and was layered over Ficoll-Paque to isolate MNCs. The cell suspension was then incubated with anti-CD138 microbeads. The cell suspension was centrifuged, and the cell pellet was loaded onto the magnetic column of the AutoMACS system. Anti-CD138+ cells were isolated by positive selection. The purity of the isolated CD138+ plasma cell population was determined by FACS analysis using anti-CD 138–PE antibody. NF-κB and STAT3 activation status was determined by fixing these cells on slides by cytospin followed by immunocytochemistry for NF-κB (p65) and STAT3 as described in “Patients, materials, and methods.”
MNC indicates mononuclear cells; STAT3, signal transducer and activator of transcription 3; NA, not available.
Grading: — indicates 0% cells with nuclear positivity; +, less than 10% cells with nuclear positivity; ++, 10% to 50% cells with nuclear positivity; +++, more than 51% cells with nuclear positivity.
Cell culture
All the CD138+ cells from bone marrow of human MM patients, cell lines U266, RPMI 8226, MM.1, MM.1R, and peripheral blood mononuclear cells (PBMCs) from healthy volunteers were cultured in RPMI 1640 medium containing 1 × antibiotic-antimycotic with 10% FBS. Occasionally cells were tested by Hoechst staining and by custom polymerase chain reaction (PCR) for mycoplasma contamination. The human bone marrow stromal cell line KM102 was established from normal bone marrow.21 These cells (5 × 104 per well) were cultured in flat-bottom 96-well plate microtiter wells.
Preparation of nuclear extracts for NF-κB
The nuclear extracts were prepared according to Bharti et al.11 Briefly, 2 × 106 cells were washed with cold PBS and suspended in 0.4 mL hypotonic lysis buffer containing protease inhibitors for 30 minutes. The cells were then lysed with 12.5 μL of 10% Nonidet P-40. The homogenate was centrifuged and the supernatant discarded. The nuclear pellet was resuspended in 25 μL ice-cold nuclear extraction buffer. After 30 minutes of intermittent mixing, the extract was centrifuged, and supernatants containing nuclear extracts were secured. The protein content was measured by the Bradford method.
Electrophoretic mobility shift assay for NF-κB
NF-κB activation was analyzed by electrophoretic mobility gel shift assay (EMSA) as described previously.22 In brief, 8-μg nuclear extracts prepared from treated or untreated cells were incubated with 32P–end-labeled 45-mer double-stranded NF-κB oligonucleotide from human immunodeficiency virus-1 long terminal repeat (5′-TTGTTACAAGGGACTTTCCGCTGGGGACTTTCCAGGGAGGCGTGG-3′) for 15 minutes at 37°C and the DNA-protein complex resolved in a 6.6% native polyacrylamide gel. The radioactive bands from the dried gels were visualized and quantitated by a PhosphorImager (Molecular Dynamics, Sunnyvale, CA) using ImageQuant software (Amersham Biosciences, Piscataway, NJ).
Immunocytochemistry for NF-κB p65 and STAT3 localization
Untreated or treated MM cells were plated on a poly-l-lysine–coated glass slide by centrifugation using a Cytospin 4 (Thermoshandon, Pittsburgh, PA), air-dried for 1 hour at room temperature, and fixed with cold acetone. After a brief washing in PBS, slides were blocked with 5% normal goat serum for 1 hour and then incubated either with rabbit polyclonal antihuman NF-κB p65 antibody (SC-109; dilution, 1:100) or with antihuman STAT3 antibody (SC-482; dilution, 1:100). After overnight incubation, the slides were washed and then incubated with goat antirabbit immunoglobulin G (IgG)–Alexa 594 (A-11037; dilution, 1:100) for 1 hour and counterstained for nuclei with Hoechst (50 ng/mL) for 5 minutes. Stained slides were mounted with mounting medium (Sigma-Aldrich) and analyzed under an epifluorescence microscope (Labophot-2; Nikon, Tokyo, Japan). Pictures were captured using a Photometrics Coolsnap CF color camera (Nikon, Lewisville, TX) and MetaMorph version 4.6.5 software (Universal Imaging, Downingtown, PA). Cells with nuclear staining of NF-κB p65 or of STAT3 were counted separately. One hundred cells were counted for each patient, and the sample was graded on the basis of a 4-point scale: –, no nuclear positive cells (0%); +, low number of nuclear positive cells (less than 10%); ++, moderate number of nuclear positive cells (10%-50%); +++, high number of nuclear positive cells (more than 50%).
Western blot
Thirty to 50 μg of cytoplasmic protein extracts, prepared as described,11 were resolved on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel. After electrophoresis, the proteins were electrotransferred to a nitrocellulose membrane, blocked with 5% nonfat milk, and probed with antibodies against either phospho-IκBα, Bcl-2, Bcl-xL, or cyclin D1 (1:3000) for 1 hour. For detection of phospho-STAT3, whole-cell extracts were prepared by lysing the cells in lysis buffer (20 mM Tris [tris(hydroxymethyl)aminomethane] [pH 7.4], 250 mM NaCl, 2 mM EDTA [pH 8.0], 0.1% Triton X-100, 0.01 mg/mL aprotinin, 0.005 mg/mL leupeptin, 0.4 mM phenylmethylsulfonyl fluoride [PMSF], and 4 mM NaVO4). Lysates were then spun at 14 000 rpm for 10 minutes to remove insoluble material. Lysates were resolved on 10% gel and probed with phospho-STAT3 antibodies. Thereafter, the blot was washed, exposed to horseradish peroxidase (HRP)–conjugated secondary antibodies for 1 hour, and finally detected by chemiluminescence (ECL; Amersham Pharmacia Biotech. Arlington Heights, IL).
Cell adhesion assay
Cell adhesion assays were performed as described earlier,23 with some modifications. Briefly, 5 × 104 bone marrow stromal cells (BMSCs) were seeded in 96-well plates and cultured for 24 hours. After confirming the development of a confluent adherent monolayer, curcumin (0, 1, or 10 μM) was added to each well, and then after 12 hours TNF (5 ng/mL) was added. After 2 hours, culture media were replaced and multiple myeloma cells (5 × 104 in 0.1 mL) labeled with 3H-thymidine added. After 1 hour of incubation, each well was washed twice with media, cells were harvested on glass fiber filters, and the binding of cells was monitored by counting 3H-thymidine using a Matrix-9600 β-counter (Packard Instruments, Downers Grove, IL).
Determination of IL-6 protein
U266 multiple myeloma cells (5 × 104 per well) cultured together with BMSC monolayers were treated with curcumin (0, 1, and 10 μM) in 96-well plates for 24 hours. Cell-free supernatants were collected, and 100 μL aliquots were analyzed for IL-6 levels by enzyme-linked immunosorbent assay (ELISA) (Biosource International, Camarillo, CA).
MTT assay
The antiproliferative effects of curcumin against different MM cell lines were determined by the MTT dye uptake method as described earlier.24 Briefly, the cells (5000 per well) were incubated in triplicate in a 96-well plate in the presence or absence of indicated test samples in a final volume of 0.1 mL for 24 hour at 37°C. Thereafter, 0.025 mL MTT solution (5 mg/mL in PBS) was added to each well. After a 2-hour incubation at 37°C, 0.1 mL extraction buffer (20% SDS, 50% dimethylformamide) was added, incubation was continued overnight at 37°C, and then the optical density (OD) at 590 nm was measured using a 96-well multiscanner autoreader (Dynatech MR 5000; Dynex Technologies, Chantilly, VA), with the extraction buffer used as the blank. We expressed cell viability as a percentage: OD of the experiment samples/OD of the control × 100.
Results
In this report we investigated whether NF-κB and STAT3 are constitutively active in fresh cells from MM patients and whether suppression of NF-κB and STAT3 activation leads to a decrease in cell survival. PBMCs from healthy subjects were used as a control. For most experiments immunocytochemistry was used because it allowed us to assess the heterogeneity of the cell population. Both NF-κB and STAT3 when activated are translocated from the cytoplasm to the nucleus. To suppress NF-κB and STAT3, we used curcumin (diferuloylmethane) because it has been shown to be quite effective.11
NF-κB is constitutively active in CD138+ cells from MM patients
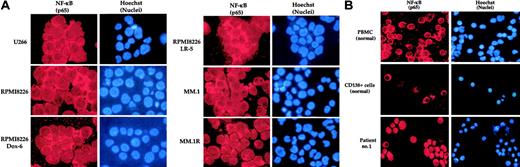
We first determined the NF-κB status in various MM cell lines. Figure 1A shows that all the cell lines expressed the nuclear form of NF-κB, suggesting the constitutively active form of NF-κB. Cells that had developed resistance to doxorubicin, melphalan, or dexamethasone also showed activated NF-κB. In contrast, PBMCs and CD138+ cells from healthy volunteers expressed cytoplasmic NF-κB, which is the inactive form of NF-κB (Figure 1B, upper and middle panels). Like MM cell lines, MM cells from patient no. 1 expressed only the nuclear form of NF-κB (Figure 1B, lower panel).
Immunocytochemical localization of NF-κB. Localization in human multiple myeloma cell lines (A), peripheral blood mononuclear cells (PBMCs) and CD138+ cells from healthy volunteers, and bone marrow CD138+ multiple myeloma cells from patient no. 1 (B). PBMCs were collected from the blood of a healthy subject by Ficoll-Paque density gradient centrifugation. CD138+ cells were enriched from bone marrow aspirates of a healthy volunteer and a multiple myeloma patient (patient no. 1), enriched by magnetic bead separation method, and immunostained for NF-κB (p65) as described in “Patients, materials, and methods.” Red stain indicates specific staining for NF-κB as indicated, whereas blue stain indicates the relative position of the nuclei in the corresponding view.
Immunocytochemical localization of NF-κB. Localization in human multiple myeloma cell lines (A), peripheral blood mononuclear cells (PBMCs) and CD138+ cells from healthy volunteers, and bone marrow CD138+ multiple myeloma cells from patient no. 1 (B). PBMCs were collected from the blood of a healthy subject by Ficoll-Paque density gradient centrifugation. CD138+ cells were enriched from bone marrow aspirates of a healthy volunteer and a multiple myeloma patient (patient no. 1), enriched by magnetic bead separation method, and immunostained for NF-κB (p65) as described in “Patients, materials, and methods.” Red stain indicates specific staining for NF-κB as indicated, whereas blue stain indicates the relative position of the nuclei in the corresponding view.
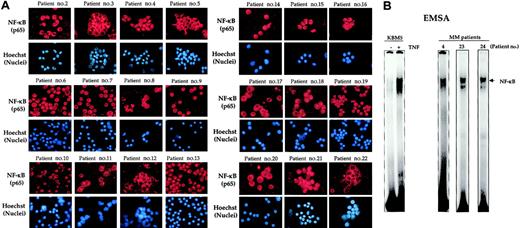
We then examined 21 different MM patient samples for NF-κB activation by immunocytochemistry. Almost every one of the 22 patients showed expression of NF-κB protein (p65) in the nucleus, indicating constitutive activation (Figure 2A). The extent of activation, however, was quite variable (Table 2). Three patients showed low, 5 showed moderate, and 14 showed high expression of constitutive NF-κB.
Nuclear localization of NF-κB in bone marrow CD138+ multiple myeloma cells from patients. (A) Enriched CD138+ cells from bone marrow aspirates of different multiple myeloma patients were immunostained for NF-κB (p65) as described in “Patients, materials, and methods.” Red stain indicates the specific staining for NF-κB, whereas blue stain indicates a relative position of the nuclei in the corresponding view. Original magnification, × 200 (B) Enriched CD138+ cells (2 × 106 cells) from bone marrow aspirates of multiple myeloma patients were tested for NF-κB activity in the nuclei by electrophoretic mobility shift assay as described in “Patients, materials, and methods.” Untreated or TNF-treated KBM-5 cells (TNF, 1 nM, 30 minutes) were used as negative and positive controls, respectively.
Nuclear localization of NF-κB in bone marrow CD138+ multiple myeloma cells from patients. (A) Enriched CD138+ cells from bone marrow aspirates of different multiple myeloma patients were immunostained for NF-κB (p65) as described in “Patients, materials, and methods.” Red stain indicates the specific staining for NF-κB, whereas blue stain indicates a relative position of the nuclei in the corresponding view. Original magnification, × 200 (B) Enriched CD138+ cells (2 × 106 cells) from bone marrow aspirates of multiple myeloma patients were tested for NF-κB activity in the nuclei by electrophoretic mobility shift assay as described in “Patients, materials, and methods.” Untreated or TNF-treated KBM-5 cells (TNF, 1 nM, 30 minutes) were used as negative and positive controls, respectively.
We also independently confirmed the constitutive activation of NF-κB by EMSA. As shown in Figure 2B, as a control NF-κB in KBM-5, a myeloid cell line that has no constitutive NF-κB, was activated by TNF. In the sample from patients, which showed constitutive NF-κB activation by immunocytochemistry, also showed activation by EMSA (Figure 2B).
STAT3 is constitutively active in CD138+ cells from MM patients
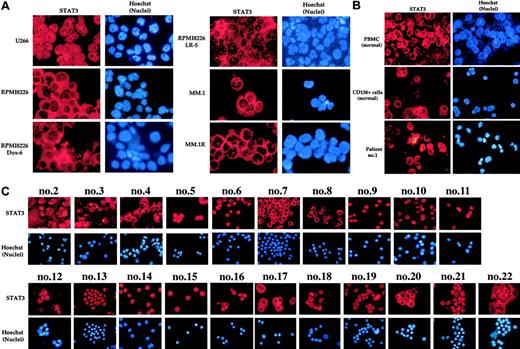
We next investigated the status of STAT3 in MM cells. Among all the MM cell lines tested, only U266 cells expressed STAT3 in nuclei (Figure 3A), suggesting that U266 cells express the constitutively active form of STAT3. All other cell lines expressed cytoplasmic STAT3 only. Likewise, PBMCs and CD138+ cells from healthy subjects expressed STAT3, but it was all cytoplasmic (Figure 3B, upper and middle panels). In contrast, MM cells from patient no. 1 expressed the nuclear form of STAT3. This suggests that the fresh cells from this patient express constitutively active form of STAT3 (Figure 3B, lower panel).
Nuclear localization of STAT3. Localization in MM cell lines (A) and PBMCs and bone marrow CD138+ cells from healthy volunteers and from multiple myeloma patients (B-C). Enriched CD138+ cells from bone marrow aspirates of healthy volunteers and multiple myeloma patients were immunostained for STAT3 as described in “Patients, materials, and methods.” Red stain indicates the specific staining for STAT3, whereas blue stain indicates the relative position of the nuclei in the corresponding view. Patient's numbers are indicated above each panel in C. Original magnification, × 200.
Nuclear localization of STAT3. Localization in MM cell lines (A) and PBMCs and bone marrow CD138+ cells from healthy volunteers and from multiple myeloma patients (B-C). Enriched CD138+ cells from bone marrow aspirates of healthy volunteers and multiple myeloma patients were immunostained for STAT3 as described in “Patients, materials, and methods.” Red stain indicates the specific staining for STAT3, whereas blue stain indicates the relative position of the nuclei in the corresponding view. Patient's numbers are indicated above each panel in C. Original magnification, × 200.
We then examined 21 different MM patient samples for STAT3 activation by immunocytochemistry. Like NF-κB, most patients showed expression of STAT3 protein in the nucleus (Figure 3C). The extent of nuclear STAT3 was quite variable (Table 2). One patient had none, 5 had low, 4 had moderate, and 12 patients showed high expression of constitutive STAT3.
Curcumin down-regulates the constitutive NF-κB and STAT3 activation in CD138+ cells from MM patients
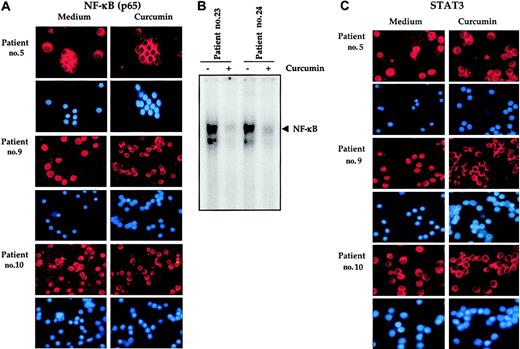
Our results, so far, indicated that CD138+ cells from most MM patients expressed constitutively active NF-κB and STAT3. We next investigated whether curcumin suppressed the constitutive activation of NF-κB and STAT3 in fresh cells from MM patients. To determine this, CD138+ cells from MM patients were exposed to 50 μM curcumin for 2 hours and then examined for STAT3 and NF-κB expression. Figure 4A indicates that NF-κB was constitutively active in patient nos. 5, 9, and 10 (the only patients tested) and exposure to curcumin down-regulated NF-κB. DNA-binding studies further confirmed that NF-κB was constitutively active in CD138+ cells from multiple myeloma patients and curcumin down-regulated the constitutive expression of NF-κB (Figure 4B). Results in Figure 4C indicate that STAT3 was likewise constitutively active in patient nos. 5, 9, and 10, and exposure to curcumin down-regulated this transcription factor.
Curcumin prevents nuclear localization of NF-κB and STAT3 in bone marrow CD138+ multiple myeloma cells. Enriched CD138+ cells (1 × 105/0.1 mL) from bone marrow aspirates of multiple myeloma patients were cultured in the absence or presence of curcumin (50 μM) for 2 hours, fixed on slides by cytospin centrifugation, and immunostained for NF-κB (A). Enriched CD138+ cells (2 × 106 cells) from bone marrow aspirates of multiple myeloma patients as indicated were cultured in absence or presence of curcumin (50 μM) for 2 hours and then tested for NF-κB activity in the nuclei by electrophoretic mobility shift assay as described in “Patients, materials, and methods” (B). Enriched CD138+ cells (1 × 105/0.1 mL) from bone marrow aspirates of multiple myeloma patients were cultured in the absence or presence of curcumin (50 μM) for 1 hour and then fixed on slides by cytospin centrifugation and immunostained for STAT3 as described in “Patients, materials, and methods” (C). Red stain indicates the specific staining for NF-κB or STAT3 as indicated, whereas blue stain indicates the relative position of the nuclei in the corresponding view. Original magnification, × 200.
Curcumin prevents nuclear localization of NF-κB and STAT3 in bone marrow CD138+ multiple myeloma cells. Enriched CD138+ cells (1 × 105/0.1 mL) from bone marrow aspirates of multiple myeloma patients were cultured in the absence or presence of curcumin (50 μM) for 2 hours, fixed on slides by cytospin centrifugation, and immunostained for NF-κB (A). Enriched CD138+ cells (2 × 106 cells) from bone marrow aspirates of multiple myeloma patients as indicated were cultured in absence or presence of curcumin (50 μM) for 2 hours and then tested for NF-κB activity in the nuclei by electrophoretic mobility shift assay as described in “Patients, materials, and methods” (B). Enriched CD138+ cells (1 × 105/0.1 mL) from bone marrow aspirates of multiple myeloma patients were cultured in the absence or presence of curcumin (50 μM) for 1 hour and then fixed on slides by cytospin centrifugation and immunostained for STAT3 as described in “Patients, materials, and methods” (C). Red stain indicates the specific staining for NF-κB or STAT3 as indicated, whereas blue stain indicates the relative position of the nuclei in the corresponding view. Original magnification, × 200.
Curcumin down-regulates the survival of CD138+ cells from MM patients
Because NF-κB and STAT3 activation have been implicated in cell survival and curcumin down-regulated these transcription factors in CD138+ cells from MM patients, we next investigated whether this down-regulation leads to a decrease in cell viability. Cells were exposed to different concentrations of curcumin and then examined for cell viability by the MTT method. As shown in Figure 5, curcumin treatment of U266 cells or fresh cells from MM patient nos. 7, 9, and 10 decreased cell survival in a dose-dependent manner. Results in Figure 4B indicate that STAT3 was also constitutively active in patient nos. 5, 9, and 10 and that exposure to curcumin down-regulated this transcription factor. These results suggest that constitutive activation of NF-κB and STAT3 are cell survival factor for CD138+ cells from MM patients.
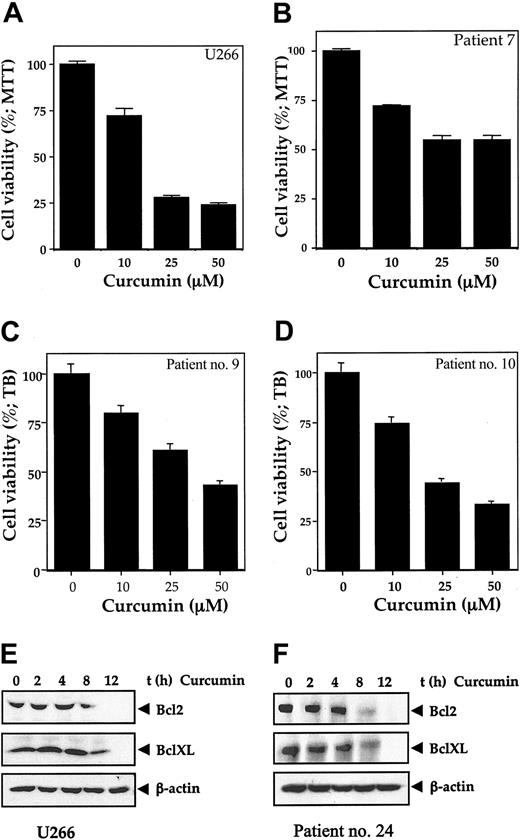
Curcumin activity. Curcumin inhibits the growth/viability of human multiple myeloma cell line U266 and bone marrow CD138+ multiple myeloma cells (A-D). Cell line U266 (A) or enriched CD138+ cells (2 × 104/0.1 mL) from bone marrow aspirates of multiple myeloma patients (nos. 7, 9, and 10) (B-D) were cultured in the absence or presence of the indicated concentrations of curcumin for 24 hours, and cell viability was measured by MTT assay (A-B) or standard trypan blue dye exclusion method (C,D) as described. Curcumin inhibits the expression of Bcl-2 and Bcl-XL proteins in human multiple myeloma cell line U266 (E) and bone marrow CD138+ multiple myeloma cells (F). A total of 2 × 106 U266 cells (E) or CD138+ multiple myeloma cells (F) were treated with curcumin (50 μM) for the indicated times, prepared the cytoplasmic extracts, resolved the 50 μg cytoplasmic extracts on 10% SDS-PAGE gel, electrotransferred on a nitrocellulose membrane, and probed for Bcl-2 and Bcl-XL by Western blot analysis. β-actin was used as a loading control. Values represent the mean ± SD of triplicate cultures.
Curcumin activity. Curcumin inhibits the growth/viability of human multiple myeloma cell line U266 and bone marrow CD138+ multiple myeloma cells (A-D). Cell line U266 (A) or enriched CD138+ cells (2 × 104/0.1 mL) from bone marrow aspirates of multiple myeloma patients (nos. 7, 9, and 10) (B-D) were cultured in the absence or presence of the indicated concentrations of curcumin for 24 hours, and cell viability was measured by MTT assay (A-B) or standard trypan blue dye exclusion method (C,D) as described. Curcumin inhibits the expression of Bcl-2 and Bcl-XL proteins in human multiple myeloma cell line U266 (E) and bone marrow CD138+ multiple myeloma cells (F). A total of 2 × 106 U266 cells (E) or CD138+ multiple myeloma cells (F) were treated with curcumin (50 μM) for the indicated times, prepared the cytoplasmic extracts, resolved the 50 μg cytoplasmic extracts on 10% SDS-PAGE gel, electrotransferred on a nitrocellulose membrane, and probed for Bcl-2 and Bcl-XL by Western blot analysis. β-actin was used as a loading control. Values represent the mean ± SD of triplicate cultures.
Because cell survival genes bcl-2 and bcl-xL are regulated by NF-κB and STAT3, we examined whether these genes are expressed and if they are also down-regulated by curcumin in MM cells. Our results show that Bcl-2 and Bcl-xL are constitutively expressed in multiple myeloma cells (Figure 5E-F). We examined the effect of curcumin on the expression of Bcl-2 and Bcl-xL in both U266 multiple myeloma cells and CD138+ cells from patients. As shown in Figure 5E-F, curcumin down-regulated the expression of Bcl-2 and Bcl-xL in both U266 and patient CD138+ cells.
Dexamethasone down-regulates the constitutive NF-κB and STAT3 activation in CD138+ cells from MM patients
Currently, dexamethasone is used as a standard therapy for MM patients. Whether dexamethasone also affects NF-κB and STAT3 in cells from MM patients was investigated. Results in Figure 6 indicate that dexamethasone down-regulated the constitutive activation of both NF-κB (Figure 6A) and STAT3 (Figure 6D) in cells from an MM patient.
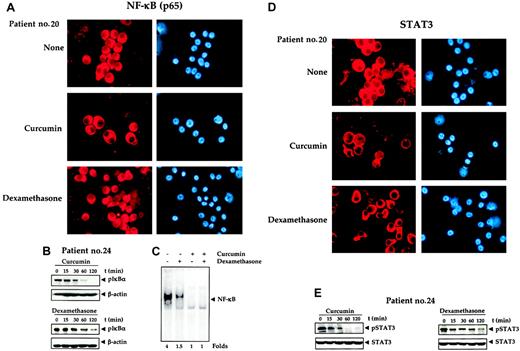
Effects of curcumin and dexamethasone. (A) Effect of curcumin and dexamethasone on nuclear localization of NF-κB. Enriched CD138+ cells (1 × 105 cells/0.1 mL) from bone marrow aspirates of multiple myeloma patient no. 20 were cultured in the absence or presence of curcumin or dexamethasone (50 μM each) for 2 hours, fixed the cells on slides by cytospin centrifugation, and immunostained for NF-κB, as described in “Patients, materials, and methods.” Original magnification, × 200. (B) Effect of curcumin and dexamethasone on phosphorylation of IκBα. A total of 2 × 106 CD138+ multiple myeloma cells were treated with curcumin (50 μM) or dexamethasone (50 μM) for the indicated times, and prepared the cytoplasmic extracts, resolved 30 μg cytoplasmic extracts on 10% SDS-PAGE gel, electrotransferred on a nitrocellulose membrane, and probed for phosphorylated IκBα by Western blot analysis. β-actin was used as a loading control. (C) Enriched CD138+ cells (2 × 106 cells) from bone marrow aspirates of multiple myeloma patients were treated with curcumin (50 μM), dexamethasone (50 μM) alone, or in combination for 3 hours and then tested for NF-κB activity in the nuclei by electrophoretic mobility shift assay as described in “Patients, materials, and methods.” (D) Effect of curcumin and dexamethasone on nuclear localization of STAT3. Enriched CD138+ cells (1 × 105/0.1 mL) from bone marrow aspirates of multiple myeloma patient no. 20 were cultured in the absence or presence of curcumin or dexamethasone (50 μM each) for 2 hours, then fixed the cells on slides by cytospin centrifugation and immunostained for STAT3, as described in “Patients, materials, and methods.” Original magnification, × 200. (E) Effect of curcumin and dexamethasone on phosphorylation of STAT3. A total of 2 × 106 CD138+ multiple myeloma cells were treated with curcumin (50 μM) or dexamethasone (50 μM) for the indicated times, prepared the whole cell extracts, resolved the 30 μg whole cell extracts on 10% SDS-PAGE gel, electrotransferred on a nitrocellulose membrane, and probed for phosphorylated STAT3 by Western blot analysis. STAT3 was used as a loading control.
Effects of curcumin and dexamethasone. (A) Effect of curcumin and dexamethasone on nuclear localization of NF-κB. Enriched CD138+ cells (1 × 105 cells/0.1 mL) from bone marrow aspirates of multiple myeloma patient no. 20 were cultured in the absence or presence of curcumin or dexamethasone (50 μM each) for 2 hours, fixed the cells on slides by cytospin centrifugation, and immunostained for NF-κB, as described in “Patients, materials, and methods.” Original magnification, × 200. (B) Effect of curcumin and dexamethasone on phosphorylation of IκBα. A total of 2 × 106 CD138+ multiple myeloma cells were treated with curcumin (50 μM) or dexamethasone (50 μM) for the indicated times, and prepared the cytoplasmic extracts, resolved 30 μg cytoplasmic extracts on 10% SDS-PAGE gel, electrotransferred on a nitrocellulose membrane, and probed for phosphorylated IκBα by Western blot analysis. β-actin was used as a loading control. (C) Enriched CD138+ cells (2 × 106 cells) from bone marrow aspirates of multiple myeloma patients were treated with curcumin (50 μM), dexamethasone (50 μM) alone, or in combination for 3 hours and then tested for NF-κB activity in the nuclei by electrophoretic mobility shift assay as described in “Patients, materials, and methods.” (D) Effect of curcumin and dexamethasone on nuclear localization of STAT3. Enriched CD138+ cells (1 × 105/0.1 mL) from bone marrow aspirates of multiple myeloma patient no. 20 were cultured in the absence or presence of curcumin or dexamethasone (50 μM each) for 2 hours, then fixed the cells on slides by cytospin centrifugation and immunostained for STAT3, as described in “Patients, materials, and methods.” Original magnification, × 200. (E) Effect of curcumin and dexamethasone on phosphorylation of STAT3. A total of 2 × 106 CD138+ multiple myeloma cells were treated with curcumin (50 μM) or dexamethasone (50 μM) for the indicated times, prepared the whole cell extracts, resolved the 30 μg whole cell extracts on 10% SDS-PAGE gel, electrotransferred on a nitrocellulose membrane, and probed for phosphorylated STAT3 by Western blot analysis. STAT3 was used as a loading control.
The effect of curcumin and dexamethasone on phosphorylation of IκBα (inhibitory subunit of NF-κB) and STAT3 was also examined in cells from patients (Figure 6B,E). Both curcumin and dexamethasone down-regulated the phosphorylation of IκBα and STAT3. Dexamethasone was, however, less effective than curcumin in down-regulating the phosphorylation of IκBα and STAT3.
How the effect of curcumin compares with dexamethasone in down-regulating the activation of NF-κB in CD138+ cells was also examined by EMSA. It was found that curcumin was more effective than dexamethasone in down-regulating the activation of NF-κB (Figure 6C). In agreement with these results, curcumin was also found to be more potent than dexamethasone in inhibiting IκBα phosphorylation (Figure 6B) and STAT3 phosphorylation (Figure 6E).
Curcumin inhibits TNF-induced adhesion of multiple myeloma cells to BMSCs
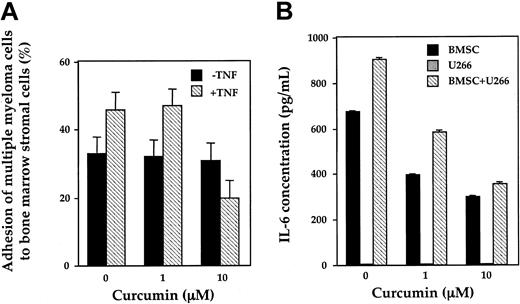
It has been reported previously that TNF increases the expression of adhesion molecules on both multiple myeloma cells and BMSCs via activation of NF-κB, resulting in increased adhesion of multiple myeloma cell to BMSCs.14 As shown in Figure 7A, TNF did indeed increase the adhesion of MM cells to BMSCs and curcumin suppressed the increased binding of MM cells to BMSCs. Curcumin, however, had no effect on the constitutive binding of MM cells to BMSCs.
Inhibition by curcumin. (A) Curcumin inhibits TNF-induced adhesion of multiple myeloma cells to bone marrow stromal cells (BMSCs). BMSCs were incubated with curcumin (0, 1, and 10 μM) for 12 hours followed by treatment with TNF (5 ng/mL) for 2 hours. After replacing the culture media, tritiated thymidine-labeled U266 cells were added and, after 1 hour, the percentage of adherent cells evaluated. (B) Curcumin inhibits constitutive and MM cell–induced IL-6 production from BMSCs. BMSCs and U266 cells were cultured alone or together in the presence or absence of curcumin (1 to 10 μM). After 24 hours, culture supernatants were collected and measured IL-6 by ELISA. Values represent the mean ± SD of triplicate cultures.
Inhibition by curcumin. (A) Curcumin inhibits TNF-induced adhesion of multiple myeloma cells to bone marrow stromal cells (BMSCs). BMSCs were incubated with curcumin (0, 1, and 10 μM) for 12 hours followed by treatment with TNF (5 ng/mL) for 2 hours. After replacing the culture media, tritiated thymidine-labeled U266 cells were added and, after 1 hour, the percentage of adherent cells evaluated. (B) Curcumin inhibits constitutive and MM cell–induced IL-6 production from BMSCs. BMSCs and U266 cells were cultured alone or together in the presence or absence of curcumin (1 to 10 μM). After 24 hours, culture supernatants were collected and measured IL-6 by ELISA. Values represent the mean ± SD of triplicate cultures.
Curcumin inhibits both constitutive and MM cell–induced IL-6 production from BMSCs
Adhesion of multiple myeloma cells to BMSCs increases the secretion of IL-6,25 mediating proliferation and survival of the cells as well as angiogenesis in the BM milieu. Our results show that BMSCs constitutively produce a significant amount of IL-6 (Figure 7B). Binding of MM cells to BMSCs induced significant increases in IL-6 secretion compared with BMSCs alone. The cytokine secretion by multiple myeloma cells alone was negligible. Treatment of cells with curcumin reduced both constitutive and MM cell–induced secretion of IL-6 from BMSCs.
Dexamethasone down-regulates the survival of CD138+ cells from MM patients
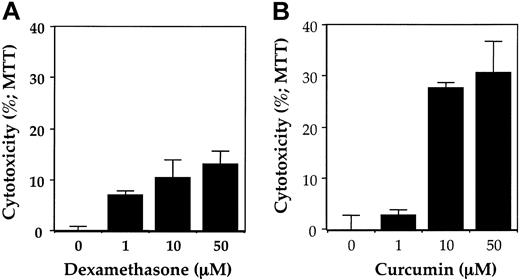
Whether dexamethasone also affects survival of cells from MM patients was investigated. Results in Figure 8A indicate that dexamethasone decreased the survival of cells from an MM patient. The dexamethasone, however, was much less effective than curcumin (Figure 8B).
Effect of curcumin and dexamethasone on growth/viability of bone marrow CD138+ multiple myeloma cells. Enriched CD138+ cells (2 × 105/0.1 mL) from bone marrow aspirates of multiple myeloma patient no. 20 were cultured in the absence or presence of indicated concentrations of dexamethasone (A) or curcumin (B) for 24 hours, and cell viability was measured by MTT assay as described in “Patients, materials, and methods.” Values represent the mean ± SD of triplicate cultures.
Effect of curcumin and dexamethasone on growth/viability of bone marrow CD138+ multiple myeloma cells. Enriched CD138+ cells (2 × 105/0.1 mL) from bone marrow aspirates of multiple myeloma patient no. 20 were cultured in the absence or presence of indicated concentrations of dexamethasone (A) or curcumin (B) for 24 hours, and cell viability was measured by MTT assay as described in “Patients, materials, and methods.” Values represent the mean ± SD of triplicate cultures.
Discussion
Because of the central role of the nuclear transcription factors NF-κB and STAT3 in chemoresistance, cell survival, and proliferation, we investigated whether multiple myeloma cells derived from patients expressed activated NF-κB and STAT3 and if their suppression decreases cell viability. We found that MM cells from all the patients expressed the activated form of NF-κB and STAT3, although to a variable degree, whereas cells from healthy individuals did not. Suppression of the activation of NF-κB and STAT3 resulted in a decrease in the viability of cells. Dexamethasone was found to be less effective than curcumin in suppressing NF-κB and STAT3 activation and in suppression of survival of MM cells.
Fresh samples from all 24 MM patients expressed constitutively active NF-κB. These results are consistent with a report of Ni et al, who examined 13 MM patients for NF-κB expression by immunohistochemical staining and found that all of them expressed activated NF-κB.10 We found that PBMCs from healthy donors were negative for the NF-κB activation. Due to lack of availability of samples, we could not examine the NF-κB status in nonneoplastic plasma cells. Ni et al, however, did show that nonneoplastic plasma cells had a lesser intensity of nuclear staining than neoplastic plasma cells. Why MM cells express constitutive NF-κB is not understood. Because NF-κB activation by most agents is transient, it indicates that MM cells are continuously being exposed to a cytokine that leads to constitutive NF-κB. TNF-α, lymphotoxin (LT), IL-1, and RANKL have been shown to be produced by MM cells,17,26-29 and these can lead to NF-κB activation in an autocrine manner. Alternatively, like the Hodgkin disease cell line L428, constitutive NF-κB activation in MM cells from patients may be due to mutated IκBα.30,31 Ma et al have indeed shown frequent alteration of the IκBα gene in MM patients.32
Our results also show that CD138+ plasma cells from most patients express constitutively active STAT3. Our results are in agreement with Catlett-Falcone et al.8 These workers examined 24 MM patients and found, by DNA-binding assay, a dramatic activation of STAT3 in one third of these patients.8 We have found that only 1 (U266) of 6 MM cell lines was capable of expressing constitutive STAT3. The frequency of constitutively active STAT3 in MM patient samples was quite higher than that observed in the cell lines.
Why STAT3 is constitutively active in MM cells is not clear. IL-6 is a potent activator of STAT3 and is required for mediating the cell growth, differentiation, and survival signals relayed through IL-6.33,34 The expression of this cytokine is regulated by NF-κB.35 We have shown that only 1 (U266) of 4 MM cell lines is capable of constitutively producing IL-6.11 Whether constitutively active STAT3 in the cells from MM patients is also due to autocrine production of IL-6 6,36 is not clear at present. Besides IL-6, several other cytokines can activate STAT3. These include epidermal growth factor (EGF), oncostatin M, and leukemia inhibitory factor (LIF).33,37-39 Whether fresh cells from MM patients express these cytokines remains to be determined.
How might activation of STAT3 and NF-κB contribute to chemoresistance in MM cells? Both STAT3 and NF-κB activation have been implicated in cell survival. For instance, expression of bcl-xL, a cell survival gene, has been shown to be regulated by both STAT3 40 and NF-κB.41 This gene has been shown to be overexpressed in MM cells.5 Bcl-xL can block cell death induced by variety of chemotherapeutic agents,42 and expression of Bcl-xL has been correlated with chemoresistance in MM patients, with response rates of 83% to 87% in non-Bcl-xL–expressing cases and 20% to 31% in Bcl-xL–expressing cases.5 Our results indeed show that curcumin down-regulates the expression of Bcl-2 and Bcl-xL in both MM cell lines and in cells from MM patients.
Our results suggest that down-regulation of NF-κB and STAT3 activation in MM cells leading to suppression of Bcl-2 and Bcl-xL expression by curcumin caused the decrease in cell viability of MM cells. Previously, we have shown that curcumin could down-regulate the expression of Bcl-xL and IL-6 in MM cell lines.11 Thus, it is possible that the curcumin-induced decrease in survival of MM cells was directly linked to sequential down-regulation of NF-κB, IL-6, STAT3, and Bcl-xL. Curcumin has been shown to inhibit IκBα kinase needed for NF-κB activation.11,43
We also showed that dexamethasone could partially inhibit NF-κB and STAT3 activation in MM cells. That dexamethasone can suppress NF-κB activation has been previously reported,4 but our study is the first to show the effect of dexamethasone on STAT3. Dexamethasone was much less potent than curcumin in down-regulating STAT3 and NF-κB. This correlated with suppression of cell survival. Curcumin was much more effective in inhibiting the survival of MM cells than dexamethasone (Figure 7). Previously, we have shown that the effect of curcumin is additive to that of dexamethasone in suppressing the survival of multiple myeloma cells.44 The established pharmacologic safety of curcumin and its ability to down-regulate expression of a large number of genes involved in cell survival and chemoresistance45 provides sufficient rationale to combine curcumin with dexamethasone for the treatment of MM patients. Recently, a proteosome inhibitor (PS341, called Velcade) and an inhibitor of TNF production (thalidomide) have been approved for the treatment of MM patients.23,46 Both of these inhibitors have also been shown to suppress NF-κB activation.47,48 Our results suggest that NF-κB and STAT3 are ideal targets for drug development for the treatment of MM.
Prepublished online as Blood First Edition Paper, December 18, 2003; DOI 10.1182/blood-2003-06-2151.
Supported by the Clayton Foundation for Research and a Senior Research Investigator Award from the Multiple Myeloma Research Foundation (B.B.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mr Walter Pagel for careful proofreading and comment on the manuscript. Generous support from Herbert and Barbara Goodfriend is also acknowledged.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal